Back to Journals » Journal of Inflammation Research » Volume 17
Excessive Erythrophagocytosis Accounts for Systemic Inflammation in Chronic Kidney Disease
Authors Meng Q, Yang X, Liu Z, You G, Chen W, Zhao B, Zhu H, Xu L, Zhou Y, Liu X, Zhai C, Wang R, Zhao L, Sun J
Received 26 April 2024
Accepted for publication 7 September 2024
Published 9 October 2024 Volume 2024:17 Pages 7111—7121
DOI https://doi.org/10.2147/JIR.S467136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Qian Meng,1,* Xiaowei Yang,1,* Zhongcheng Liu,2 Guoxing You,3 Wanyi Chen,3 Bing Zhao,1 Huizi Zhu,4 Liang Xu,1 Yan Zhou,1 Xiang Liu,1 Chunjuan Zhai,5 Rong Wang,1 Lian Zhao,3 Jing Sun1
1Department of Nephrology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China; 2Department of Cardiovascular, The North City Hospital of Jinan, Jinan, Shandong, 250031, People’s Republic of China; 3Academy of Military Medical Sciences, Beijing, 100850, People’s Republic of China; 4Department of Nephrology, Fuyang People’s Hospital, Fuyang, Anhui, 236000, People’s Republic of China; 5Department of Cardiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Sun, Department of Nephrology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, 324 Jingwu Road, Jinan, Shandong, 250021, People’s Republic of China, Tel +86 151 6888 6367, Email [email protected] Lian Zhao, Academy of Military Medical Sciences, 27 Taiping Road, Beijing, 100850, People’s Republic of China, Tel +8613671217095, Email [email protected]
Purpose: Chronic kidney disease (CKD) is associated with persistent systemic inflammation. Reduced red blood cell (RBC) survival in patients with CKD has been identified for several decades. The purpose of this study is to explore whether excessive erythrophagocytosis exists and contributes to systemic inflammation in CKD.
Patients and methods: A CKD rat model was induced by 5/6 nephrectomy. Erythrocyte osmotic fragility was determined with hypotonic NaCl solutions. Erythrocyte deformability was evaluated by filterability. RBC cell death was quantified using fluorescence-activated cell sorting analyses of fluorescent annexin V–bound surface phosphatidylserine (PS). Erythrophagocytosis was evaluated in vivo and in vitro. RT-qPCR and immunohistochemistry were used to determine the inflammatory effects after erythrophagocytosis.
Results: Erythrocyte osmotic fragility and deformability progressively declined, and the percentage of PS-exposing RBCs progressively increased in CKD rats. Levels of erythrophagocytosis in vivo were evaluated by autologous injection of CFSE-labeled erythrocytes. In comparison with the control group, higher fluorescence intensity of CFSE was detected in the spleen homogenates of rats with CKD. In vitro, more of erythrocytes from 5/6Nx rats were phagocytosed by peritoneal macrophages in comparison to those from control rats. Compared with macrophages phagocytosed control erythrocytes, macrophages phagocytosed CKD erythrocytes exhibited higher mRNA levels of IL-6, CXCL-10, CXCL-11, iNOS, IL-1β, ICAM-1 and MCP-1. Compared with the control group, the red pulp of rats with CKD exhibited higher levels of p-NFκB, IL-6, iNOS and CXCL-10. ELISA results showed significantly increased plasma levels of both IL-6 and CXCL-10 in patients with long-term hemodialysis compared with those in healthy controls (2.30 ± 1.38 pg/mL vs 1.33 ± 0.65 pg/mL, P=0.01; 78.11 ± 27.34 pg/mL vs 37.45 ± 7.08 pg/mL, P=0.001).
Conclusion: Our results indicated that excessive erythrophagocytosis may contribute to systemic inflammation in CKD.
Keywords: chronic kidney disease, chronic inflammation, erythrocyte, phagocytosis
Graphical Abstract:

Introduction
Chronic kidney disease (CKD) is associated with persistent systemic inflammation.1 Circulating levels of several proinflammatory cytokines, such as interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α), are significantly elevated and are independently correlated with poor clinical outcome in CKD.2–4 It was thought that dialysis- and non-dialysis-related factors including infection, intravenous iron administration, nonbiocompatible dialysis filters account for this phenomenon.5,6 However, systemic inflammation has persisted in CKD patients despite technical innovations such as less immunogenic iron preparations, biocompatible dialysis membranes, and ultrapure dialysate.1 The underlying cause for CKD–related systemic inflammation remains to be further elucidated.
Reduced red blood cell (RBC) survival in patients with CKD has been identified for several decades.7–10 RBC lifespan progressively decreased from nearly normal levels in patients with stage 1 CKD to about 20% to 50% of normal survival in patients with end-stage renal disease (ESRD).11 The exact mechanism for the decrease of RBC lifespan is confounding. A plausible explanation may be the stimulation of accelerated eryptosis probably by uremic environment.12 Eryptosis is the apoptosis -like suicidal cell death of anucleate erythrocytes, characterized by loss of membrane asymmetry with consequent phosphatidylserine (PS) exposure on the outer membrane leaflet and subsequent phagocytosis by macrophages. An increase in RBC osmotic fragility, and impaired RBC deformability have also been observed in CKD patients and animal models.13,14 These mechanisms could act in concert to clear abnormal RBCs by macrophages from circulation in CKD.
RBCs are the most abundant cell in the human body, accounting for ~83% of the 30 trillion total cells, ~2×1011 RBCs are continuously cleared in the blood stream every day with an average lifespan of 120 days in circulation in a healthy adult individual.15 For the obviously shortened RBC survival, CKD macrophages were very likely confronted with highly stressed erythrophagocytosis. Recent studies have shown that in mice transfused with aged RBCs, increased erythrophagocytosis can lead to immunosuppression through heme-mediated signal transducer and activator of transcription 1 (STAT1) dysregulation and ferroptotic cell death of splenic red pulp macrophages.16,17 Arsenic retention in erythrocytes induced excessive erythrophagocytosis and led to an increased inflammatory status of splenic macrophages.18 The purpose of this study is to explore whether excessive erythrophagocytosis exists and contributes to systemic inflammation in CKD.
Materials and Methods
Rat Studies
Animal experiments were approved by the Animal Care and Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University (No. HSRF 2022–0027) and all animal experiments were conducted in accordance with welfare guidelines for experimental animals promulgated by the People’s Republic of China: Laboratory animals-General Code of Animal Welfare (GB/T 42011–2022). Six-week-old male Sprague-Dawley rats were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). Rats were housed three per cage in a controlled environment with a 12 h light/dark cycle in the Institute of Laboratory Animal Science.
Rat CKD was simulated with a 5/6 nephrectomy (5/6Nx) model.19 Briefly, rats were anesthetized with tribromoethanol, shaved, and disinfected with 70% ethanol. An incision near the left waist was performed to expose the left kidney completely. One-third of each of the upper and lower poles of the left kidney were removed with sharp scissors, and the remaining tissue was quickly compressed with a pair of hemostatic sponges for 1 min. Hemostasis was confirmed, and the remaining 1/3 of the kidney was returned to the abdominal cavity. Antibiotic-containing saline was applied before the abdominal cavity was closed carefully. After recovery from anesthesia, the rats were placed in a cage with adequate water and food. One week later, the entire right kidney was removed following a similar procedure on the other side of the abdomen. Sham surgery involved all procedures except nephrectomy.
Patients
The patient study was conducted in compliance with the Declaration of Helsinki and was approved by the local ethics committee of Shandong Provincial Hospital affiliated to Shandong First Medical University (SZRJJ: No.2022–188). Lithium-heparin blood samples were obtained from patients with long-term hemodialysis (HD) therapy (n=40) at the Shandong Provincial Hospital Dialysis Unit. Subjects with concurrent malignancy, history of autoimmune diseases, acute infective/inflammatory response were excluded. A total of 20 healthy participants were recruited to match HD participants according to age and sex. All human samples were collected after informed consent. Clinical characteristics of the patients are stated in Table 1.
 |
Table 1 General Characteristics of the Study HD Participants |
RBC Osmotic Fragility (Resistance of Erythrocytes to Hypotonic Shock)
The tubes containing 1 mL of 0–300 mOsm/L NaCl were preincubated for 20 min at 37°C after addition of 10 μL of whole blood with mixing. Vials were then centrifuged (1300 g, 10 min) at the assay temperature. Absorbance (A) at 540 nm for each supernatant was measured and converted to percentage haemolysis using the following equation:  , Authors contributed equally to this work, where Amax is the mean maximal absorbance. The dependency of percentage haemolysis on NaCl osmotic pressure was adjusted to a sigmoidal regression line according to the equation:
, Authors contributed equally to this work, where Amax is the mean maximal absorbance. The dependency of percentage haemolysis on NaCl osmotic pressure was adjusted to a sigmoidal regression line according to the equation:  , where A1 and A2 were the mean maximal and mean minimal percentage haemolysis, S was the NaCl osmotic pressure, H50 was the NaCl osmotic pressure that produced 50% haemolysis, and dS represented the change in NaCl osmotic pressure related to the lysis transition.20
, where A1 and A2 were the mean maximal and mean minimal percentage haemolysis, S was the NaCl osmotic pressure, H50 was the NaCl osmotic pressure that produced 50% haemolysis, and dS represented the change in NaCl osmotic pressure related to the lysis transition.20
RBC Deformability Measurement
The blood sample was separated, washed and left with washed and compacted RBCs. The RBC suspension samples were prepared according to the standard hematocrit of 5%. The deformability of erythrocytes is characterized by the erythrocyte filtration index (EFI): EFI = (ts − tb)/tb × 1/H, where ts is the filtration time of the RBC suspension, tb is the filtration time of the buffer (without RBCs) and H is the volume percentage of red blood cells. The EFI refers to the relative resistance that RBCs experience when passing through a nuclear pore filter compared with the same volume of buffer. Therefore, if the EFI of erythrocytes is larger, it means that the deformability of erythrocytes is worse; in contrast, the smaller the EFI, the better the deformability of erythrocytes.
Detection of Phosphatidylserine Exposure
Erythrocytes (isolated from 0.5 mL of blood) were washed and resuspended in 500 μL of binding buffer containing 5 μL FITC-annexin V (Keygen, Nanjing, China), then incubated for 15 min under protection from light. A total of 1×105 cells were analyzed by flow cytometer (BD Biosciences, CA, USA) using the FL1 channel (488/530 nm).
In vivo Phagocytosis Assay
In vivo phagocytosis assay was performed according to a previously described method with mild modification.18 In brief, erythrocytes from rats (n = 3 per group) were collected via tail vein bleeding and labeled with carboxyfluorescein diacetate succinamidyl ester (CFSE; 5 μM; MedChemExpress, Monmouth Junction, NJ, USA) at 37 ◦C for 15 min. Rats were then injected with the autologous erythrocytes via tail vein. After housing for 2 h, rats were euthanized followed by collection of spleens. Spleens were lysed and the fluorescence intensity of CSFE in lysates was measured by using a microplate reader SpectraMax M5 (Molecular Devices, San Jose, USA).
In vitro Phagocytosis Assay
To determine erythrophagocytosis in vitro, the peritoneal macrophages of rats were harvested as follows: 10 mL of RPMI medium 1640 (Gibco, Grand Island, NY) were injected into the peritoneal cavity of the rats, which were gently shaken and rotated. After 5 min, 8 mL of peritoneal fluid was withdrawn. The cells from each rat were sedimented by centrifugation of the fluid for 5 min at 800 rpm, resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS).21 Then 4×105 macrophages per well were seeded into a 12-well plate and incubated for 4–5 h at 37 °C in a humidified 5% CO2 incubator. For the erythrophagocytosis assay, PKH26-labeled rat RBCs (1×108 cells/well) were added and cocultured with the macrophages at 37 °C for 2 h. Non-phagocytosed RBCs were lysed by adding 1 mL of ice-cold 0.2% hypotonic saline for 2 min followed by the addition of 1 mL of ice-cold 1.6% hypertonic saline to restore isotonicity.22 Then the macrophages were detached from the surface with accutase and analyzed by flow cytometry.
RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
Total RNA was isolated from macrophages by using the Ultrapure RNA Kit (cwbio, Beijing, China) and a total of 500 ng isolated RNA for each sample were reversely transcribed to cDNA using PrimeScript™ RT reagent Kit (Perfect Real Time) (Takara, Beijing, China). The qPCR was performed by utilizing SYBR® Green Realtime PCR Master Mix (Toyobo Biotech Co., Ltd., Shanghai, China) and EasyPGX qPCR instrument 96 (Diatech Pharmacogenetics srl, Jesi, Ancona, Italy) under the following parameters: 95 ◦C for 2 min, 50 cycles at 95 ◦C for 10s, 60 ◦C for 30s, and 72 ◦C for 30s. The mRNA levels of target genes were normalized to the levels of GAPDH and calculated by the 2−ΔΔCT method and three biological replicates were analyzed for each group.18 The qPCR primers were synthesized from Sangon Biotech Co., Ltd. (Shanghai, China).
Immunohistochemistry
For immunohistochemical procedures, spleen sections (n = 3 per group) were air-dried, fixed, heated with citrate buffer (10 mm, pH 6.0) for antigen retrieval, and blocked with 5% normal goat serum (Yeasen, Shanghai, China). Sections were then incubated with anti-p-NFκB (AF2006; 1:100; Affinity, Changzhou, China), anti-IL6 (DF6087; 1:100; Affinity, Changzhou, China), anti-iNOS (AF0199; 1:100; Affinity, Changzhou, China), or anti-CXCL10 (DF6417; 1:100; Affinity, Changzhou, China), overnight at 4 ◦C followed by incubation with HRP conjugated secondary antibody (S0001; 1:200; Affinity, Changzhou, China) for 1 h at room temperature. Then, the target proteins in spleen sections were visualized using a DAB kit (ZSGB-Bio, Beijing, China) and the nuclei were counterstained with hematoxylin (Leagene, Beijing, China). After sealing, the slides were scanned by using a Pannoramic MIDI (3DHISTECH, Hungary).
Immunoassays for IL-6 and CXCL-10
Plasma levels of IL-6 and CXCL-10 plasma levels of patients with long-term HD were determined by ELISAs according to the manufacturer’s suggestions (Elabscience).
Statistical Analysis
Statistical analysis was performed using SPSS software (version 25.0; IBM, NY, USA) and GraphPad prism (version 9, San Diego, California, USA) was used for graphing. Data with a normal distribution were expressed as mean ± standard deviation (SD) and data with a non-normal distribution were presented as the median. The data from the two groups were evaluated by a two-tailed unpaired Student’s t-test. P < 0.05 was considered statistically significant.
Results
Changes in Erythrocyte Properties in 5/6Nx Rats
As shown in Figure 1A, serum creatinine in the model group increased gradually over time after 5/6Nx operation. Significantly increased serum creatinine concentrations were found at week 6 as compared to sham group. At the end of week 26, 5/6Nx operation resulted in more than triple increase in serum creatinine (sham, 51.9 ± 3.6 μmol/L; 5/6Nx, 172.0 ± 42.2 μmol/L; P<0.001).
Compared with the sham group, Hb levels decreased significantly at 6 weeks after 5/6Nx operation, and then progressively decreased until the end of the experimental period. At the end of week 26, Hb level was about 25% lower than the values seen in the control group (Figure 1B).
As exposure of RBCs to hypertonic extracellular conditions in vitro mimics the osmotic environment encountered in the kidney medulla, erythrocyte osmotic fragility was determined with hypotonic NaCl solutions. H50 was the NaCl osmotic pressure necessary to promote 50% red cell hemolysis. The NaCl osmotic pressure eliciting 50% hemolysis was significantly higher for 5/6Nx rats in comparison to sham group from week 10 after 5/6Nx operation to the end of the experimental period, indicating reduced erythrocyte membrane stability in CKD rats (Figure 1C).
To further investigate RBC functional changes, deformability was evaluated by filterability. The EFI did not differ significantly between experimental and control rats until 22 weeks after 5/6Nx operation. Since then, RBC deformability was significantly reduced in 5/6Nx rats, as indicated by an increased EFI (Figure 1D).
Externalization of PS on the outer leaflet of the erythrocyte membrane is an indicator of cell death and a promoter of erythrophagocytosis. RBC cell death was quantified using fluorescence-activated cell sorting analyses of fluorescent annexin V–bound surface PS. The percentage of PS-exposing erythrocytes did not differ significantly between experimental and control rats until 22 weeks after 5/6Nx operation. Since then, the percentage of PS-exposing RBCs was significantly but slightly higher in 5/6Nx rats in comparison to sham group (0.85% ±0.03% vs 0.60% ± 0.11%, P=0.01) (Figure 1E and F).
Excessive Erythrophagocytosis in CKD Rats
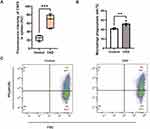
The red pulp of the spleen is the primary site where damaged or senescent erythrocytes are removed through phagocytosis by the reticuloendothelial macrophages. Levels of erythrophagocytosis in vivo were evaluated by autologous injection of CFSE-labeled erythrocytes. In comparison with the control group, higher fluorescence intensity of CFSE was detected in the spleen homogenates (Figure 2A) of rats with CKD. Data showed no significant differences in fluorescence intensity of CFSE in erythrocytes and tissues (including hearts, livers and kidneys) between groups (data not shown).
To determine erythrophagocytosis in vitro, erythrocytes from CKD and healthy rats were incubated with rat peritoneal macrophages. As expected, more of erythrocytes from 5/6Nx rats were phagocytosed by peritoneal macrophages in vitro in comparison to those from control rats (Figure 2B and C).
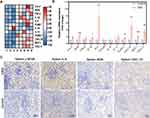
Phagocytosis of Erythrocytes from CKD Rats Induced Activation of Macrophages
To evaluate the effects of phagocytosis of CKD erythrocytes on macrophages, qPCR was employed to assess mRNA levels for inflammatory markers. Compared with macrophages phagocytosed control erythrocytes, macrophages phagocytosed CKD erythrocytes exhibited higher mRNA levels of IL-6, CXCL-10, CXCL-11, iNOS, IL-1β, ICAM-1 and MCP-1, with no significant differences in the mRNA level of COX-2, TNF-α, VCAM-1, IL-10 and IL-12 (Figure 3A and B). To further confirmed the activation of macrophages in the rats, the levels of p-NFκB, IL-6, iNOS and CXCL-10 were detected in the rat spleen sections (Figure 3C). Compared with the control group, the red pulp of rats with CKD exhibited higher levels of p-NFκB, IL-6, iNOS and CXCL-10.
Plasma Levels of IL-6 and CXCL10 in Patients with Long-Term Hemodialysis
Among the proinflammatory cytokines induced by phagocytosis of uremic rat erythrocytes, IL-6 and CXCL-10 were the two most strongly upregulated ones. Then we detected plasma concentrations of IL-6 and CXCL-10 in patients with long-term HD. ELISA results showed significantly increased plasma levels of both IL-6 and CXCL-10 in patients with long-term HD compared with those in healthy controls (2.30 ± 1.38 pg/mL vs 1.33 ± 0.65 pg/mL, P=0.01; 78.11 ± 27.34 pg/mL vs 37.45 ± 7.08 pg/mL, P=0.001) (Figure 4A and B).
Discussion
Chronic inflammation status has been recognized as an important component of CKD, being accountable for cardiovascular and all-cause mortality. In this study, we demonstrated that enhanced erythrophagocytosis existed in CKD animals in vivo and in vitro. More importantly, phagocytosis of RBCs from CKD induced significant proinflammatory cytokines secretion by macrophages. Since RBCs were the most abundant host cells, our results indicated that the persistent aberrant erythrophagocytosis might play distinct roles in the development of persistent inflammation in CKD.
Deterioration in erythrocyte quality, including accelerated eryptosis, impaired deformability, and increase in osmotic fragility, has been numerously reported in CKD patients.9,12,13,20 Animal models of CKD are important means to investigate the pathophysiology of renal anemia. To the best of our knowledge, there were only two studies to determine RBC changes in CKD animal models. Reduced erythrocyte deformability was detected in a CKD rat model induced by uninephrectomy followed by anti-Thy1.1 antibody injection.23 Circulating RBCs displayed altered morphology and diminished osmotic-sensitive deformability together with increased phosphatidylserine externalization in two mouse models with severe kidney failure (doxorubicin-induced nephropathy in 129S1/SvImJ mice and mice with inducible podcin deficiency).24 The 5/6Nx rat remnant kidney model was commonly used to study CKD. Our study demonstrated that although 5/6 Nx rats exhibited mild to moderate anemia as previously reported,25 a higher osmotic fragility of RBCs presented at the early phase of CKD and anemia, reduced membrane elasticity and increased percentage of PS-exposing RBCs were observed along with the progression of renal failure in 5/6Nx rats. Our results indicated that RBC property changes in 5/6Nx rats mimic those found in patients with CKD.
Since removal of senescent erythrocytes is considered to be a combination of reduced deformability, resulting in sequestration in the spleen, and senescence-associated changes on the cell surface, leading to recognition by macrophages,26–28 RBC property changes in CKD were supposed to promote accelerated phagocytic clearance and contribute to shorten RBC lifespan. However, there were few studies to directly evaluate the levels of erythrophagocytosis in CKD. In our study, we found that after autologous injection of CFSE-labeled erythrocytes, more RBCs were trapped in spleens of CKD rats compared with controls. When rat RBCs were incubated with rat peritoneal macrophages in vitro, macrophages phagocytosed much more uremic RBCs compared to normal RBCs. Our data provided in vivo and in vitro evidence for the existence of excessive erythrophagocytosis in 5/6Nx CKD rats.
Macrophages are essential in preserving of systemic heme-iron homeostasis during disposal of senescent RBCs. They also play important roles in immune modulation and host defense. Acute increases in erythrophagocytosis in a mouse model of transfusion induced ferroptotic cell death of splenic macrophages and immunosuppression.16,17 Cellular defects in erythrocytes and macrophages in Jak2V617F mice leaded to increased erythrophagocytosis but defective efferocytosis, inducing inflammation of macrophages in atherosclerotic plaques.29 These studies demonstrated that defective erythrophagocytosis promoted metabolic reprogramming of macrophages. In our study, activation of the NFκB signaling pathway was detected in macrophages after phagocytosis of uremic erythrocytes as evidenced by the higher mRNA levels of NFκB target genes and the higher levels of phosphorylated NFκB, IL-6 and CXCL10 in spleen sections. Moreover, higher mRNA levels of M1 markers were also detected in macrophages after phagocytosis of uremic erythrocytes and in the red pulp of spleen sections (iNOS). These preliminary results indicated that phagocytosis of uremic erythrocytes could induce activation and M1 polarization of splenic macrophages.
Among the proinflammatory cytokines induced by phagocytosis of uremic erythrocytes, IL-6 and CXCL-10 were the two most strongly upregulated ones. Plasma levels of IL-6 are known to be significantly higher in CKD patients,30 and have been shown to be independently associated with overall and cardiovascular mortality in CKD,31,32 even much more powerful in predicting mortality than CRP, TNF-α, and albumin. CXCL-10 is a chemokine playing significant roles in the recruitment of immune cells, regulating cell growth, and inhibiting angiogenesis during tissue injury, development and maintenance.33 CXCL-10 has been reported to be involved in the pathological processes of several human kidney diseases, such as mesangial proliferative glomerulonephritis and acute kidney injury.34,35 Furthermore, CXCL-10 has been identified as a major biological indicator of disease severity and may be utilized as a prognostic indicator in renal allograft dysfunction and lupus nephritis.36,37 In this study, we found plasma IL-6 and CXCL10 levels elevated in patients with long-term HD. Our results proposed that aberrant erythrophagocytosis might contribute to the systemic inflammation in CKD.
Our study has some limitations: 5/6 Nx CKD rat model presents comparatively mild anemia and alterations in erythrocyte characteristics, in contrast to those observed in ESRD patients,9 which might underestimate the conditions in patients with ESRD. This is a preliminary study, specific signaling pathways involved in the aberrant erythrophagocytosis in CKD require further investigation.
In summary, our data provided evidence for the existence of enhanced erythrophagocytosis in CKD, and erythrophagocytosis in CKD induced significant proinflammatory cytokines secretion by macrophages. Our results indicated that excessive erythrophagocytosis might be an important source of systemic inflammation in CKD. Besides RBC quantity, RBC quality might also play essential roles in the pathogenesis and progression of CKD, and deserves more attention in clinical practice.
Funding
This work was supported by a grant from China international medical foundation [No. Z-2017-24-2037].
Disclosure
This paper has been uploaded to ResearchSquare as a preprint: https://www.researchsquare.com/article/rs-3767556/latest.
The authors report no conflicts of interest in this work.
References
1. Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease--what have we learned in 10 years?. Semin Dial. 2010;23(5):498–509. doi:10.1111/j.1525-139X.2010.00784.x
2. Amdur RL, Feldman HI, Dominic EA, et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings From the CRIC Study. Am J Kidney Dis. 2019;73(3):344–353. doi:10.1053/j.ajkd.2018.09.012
3. Zoccali C, Tripepi G, Mallamaci F. Dissecting inflammation in ESRD: do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients?. J Am Soc Nephrol. 2006;17(12 Suppl 3):S169–73. doi:10.1681/ASN.2006080910
4. Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–658. doi:10.1046/j.1523-1755.1999.00273.x
5. Nassar GM. Preventing and treating inflammation: role of dialysis access management. Semin Dial. 2013;26(1):28–30. doi:10.1111/sdi.12023
6. Vaziri ND. Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis. 2013;61(6):992–1000. doi:10.1053/j.ajkd.2012.10.027
7. Joske RA, McAlister JM, Prankerd TA. Isotope investigations of red cell production and destruction in chronic renal disease. Clin Sci. 1956;15(4):511–522.
8. Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis. 2004;44(4):715–719. doi:10.1016/S0272-6386(04)00951-5
9. Zhao B, Yang X, Li W, et al. Effect of roxadustat on red blood cell lifespan in patients with long-term haemodialysis: a single-centre, prospective, single-arm study. Clin Kidney J. 2023;16(9):1500–1507. doi:10.1093/ckj/sfad080
10. Yang X, Zhao B, Wang J, et al. Red blood cell lifespan in long-term hemodialysis patients treated with roxadustat or recombinant human erythropoietin. Ren Fail. 2021;43(1):1428–1436. doi:10.1080/0886022X.2021.1988968
11. Li JH, Luo J-F, Jiang Y, et al. Red Blood cell lifespan shortening in patients with early-stage chronic kidney disease. Kidney Blood Press Res. 2019;44(5):1158–1165. doi:10.1159/000502525
12. Abed M, Artunc F, Alzoubi K, et al. Suicidal erythrocyte death in end-stage renal disease. J Mol Med (Berl). 2014;92(8):871–879. doi:10.1007/s00109-014-1151-4
13. Wu SG, Jeng F-R, Wei S-Y, et al. Red blood cell osmotic fragility in chronically hemodialyzed patients. Nephron. 1998;78(1):28–32. doi:10.1159/000044878
14. Linde T, Sandhagen B, Wikström B, et al. The required dose of erythropoietin during renal anaemia treatment is related to the degree of impairment in erythrocyte deformability. Nephrol Dial Transplant. 1997;12(11):2375–2379. doi:10.1093/ndt/12.11.2375
15. Ballas SK. Erythrocyte concentration and volume are inversely related. Clin Chim Acta. 1987;164(2):243–244. doi:10.1016/0009-8981(87)90078-7
16. Olonisakin TF, Suber T, Gonzalez-Ferrer S, et al.. Stressed erythrophagocytosis induces immunosuppression during sepsis through heme-mediated STAT1 dysregulation. J Clin Invest. 2021;131(1). doi:10.1172/JCI137468
17. Youssef LA, Rebbaa A, Pampou S, et al. Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood. 2018;131(23):2581–2593. doi:10.1182/blood-2017-12-822619
18. Cai Z, Zhang Y, Zhang W, et al. Arsenic retention in erythrocytes and excessive erythrophagocytosis is related to low selenium status by impaired redox homeostasis. Redox Biol. 2022;52:102321. doi:10.1016/j.redox.2022.102321
19. Kujal P, Vernerová Z. Model 5/6 nefrektomie, jako experimentální model chronické renální insuficience a adaptace ledvin na redukci poctu nefronů [5/6 nephrectomy as an experimental model of chronic renal failure and adaptation to reduced nephron number]. Cesk Fysiol. 2008;57(4):104–109.
20. Lee S, Lee MY, Nam JS, et al. Hemorheological approach for early detection of chronic kidney disease and diabetic nephropathy in type 2 diabetes. Diabetes Technol Ther. 2015;17(11):808–815. doi:10.1089/dia.2014.0295
21. Salman H, Bergman M, Bessler H, Alexandrova S, Djaldetti M. Ultrastructure and phagocytic activity of rat peritoneal macrophages exposed to low temperatures in vitro. Cryobiology. 2000;41(1):66–71. doi:10.1006/cryo.2000.2267
22. Gartner S. The macrophage and HIV: basic concepts and methodologies. Methods Mol Biol. 2014;1087:207–220.
23. Aizawa K, Kawasaki R, Tashiro Y, et al. Epoetin beta pegol for treatment of anemia ameliorates deterioration of erythrocyte quality associated with chronic kidney disease. BMC Nephrol. 2018;19(1):19. doi:10.1186/s12882-018-0818-4
24. Bissinger R, Nemkov T, D’Alessandro A, et al. Proteinuric chronic kidney disease is associated with altered red blood cell lifespan, deformability and metabolism. Kidney Int. 2021;100(6):1227–1239. doi:10.1016/j.kint.2021.08.024
25. Rahman A, Yamazaki D, Sufiun A, et al. A novel approach to adenine-induced chronic kidney disease associated anemia in rodents. PLoS One. 2018;13(2):e0192531. doi:10.1371/journal.pone.0192531
26. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–616. doi:10.1038/nri1669
27. Waugh RE, Narla M, Jackson CW, et al. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79(5):1351–1358. doi:10.1182/blood.V79.5.1351.1351
28. Klei TR, Meinderts SM, van den Berg TK, et al. From the cradle to the grave: the role of macrophages in erythropoiesis and erythrophagocytosis. Front Immunol. 2017;8:73. doi:10.3389/fimmu.2017.00073
29. Wang W, Liu W, Fidler T, et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 V617FMice. Circ Res. 2018;123(11):e35–e47. doi:10.1161/CIRCRESAHA.118.313283
30. Herbelin A, Ureña P, Nguyen AT, et al. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991;39(5):954–960. doi:10.1038/ki.1991.120
31. Barreto DV, Barreto FC, Liabeuf S, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77(6):550–556. doi:10.1038/ki.2009.503
32. Batra G, Lakic TG, Lindbäck J, et al. Interleukin 6 and cardiovascular outcomes in patients with chronic kidney disease and chronic coronary syndrome. JAMA Cardiol. 2021;6(12):1440–1445. doi:10.1001/jamacardio.2021.3079
33. Antonelli A, Ferrari SM, Giuggioli D, et al. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014;13(3):272–280. doi:10.1016/j.autrev.2013.10.010
34. Huang H, Zhou H, Wang W, et al. Prediction of acute kidney injury, sepsis and mortality in children with urinary CXCL10. Pediatr Res. 2022;92(2):541–548. doi:10.1038/s41390-021-01813-y
35. Gao J, Wu L, Wang Y, et al. Knockdown of cxcl10 inhibits mesangial cell proliferation in murine habu nephritis via ERK Signaling. Cell Physiol Biochem. 2017;42(5):2118–2129. doi:10.1159/000479914
36. Raza A, Firasat S, Khaliq S, et al. The association of urinary interferon-gamma inducible protein-10 (IP10/CXCL10) levels with kidney allograft rejection. Inflamm Res. 2017;66(5):425–432. doi:10.1007/s00011-017-1025-7
37. El-Gohary A, Hegazy A, Abbas M, et al. Serum and urinary interferon-gamma-inducible protein 10 in lupus nephritis. J Clin Lab Anal. 2016;30(6):1135–1138. doi:10.1002/jcla.21993
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.