Back to Journals » Patient Preference and Adherence » Volume 19
Exploring Patient, Caregiver, and Prescriber Preferences for an Injectable Antipsychotic Administered Every 2 Months for the Maintenance Treatment of Schizophrenia: A Multicenter Qualitative Interview Study Conducted in Europe
Authors Pappa S , Yildirim M , Loomer S, Beckham C , Bates D, Micheelsen A , Harrsen K , Nag SS, Guillaume X , Oladini B, Madera-McDonough J, Such P
Received 31 January 2025
Accepted for publication 11 April 2025
Published 29 April 2025 Volume 2025:19 Pages 1179—1195
DOI https://doi.org/10.2147/PPA.S520160
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Sofia Pappa,1,2 Murat Yildirim,3 Stephanie Loomer,4 Clodagh Beckham,5 Dawn Bates,4 Arun Micheelsen,3 Kristine Harrsen,3 Soma S Nag,6 Xavier Guillaume,4 Bolu Oladini,6 Jessica Madera-McDonough,6 Pedro Such3
1Division of Psychiatry, Imperial College London, London, UK; 2West London NHS Trust, London, UK; 3H. Lundbeck A/S, Valby, Denmark; 4Oracle Life Sciences, Austin, TX, USA; 5Otsuka Pharmaceutical Europe Ltd, Windsor, UK; 6Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ, USA
Correspondence: Murat Yildirim, H. Lundbeck A/S, Ottiliavej 9, Valby, 2500, Denmark, Email [email protected]
Purpose: This qualitative study explored patient, caregiver, and prescriber preferences for long-acting injectable (LAI) dosing frequency, and factors influencing preferences for a hypothetical LAI administered once every 2 months for the treatment of schizophrenia.
Patient and Methods: This single-person interview study recruited people living with schizophrenia, caregivers, and prescribers across France, Germany, Italy, Spain, and the UK. Semi-structured interviews were conducted in which participants were asked about their treatment experiences, views on an ideal treatment, and preferences on LAI dosing frequency. A qualitative analysis of interview transcripts was performed to identify key themes.
Results: Fifteen people living with clinically stable schizophrenia, 11 caregivers, and 13 prescribers were interviewed. When talking about current treatment (a once-monthly LAI), people living with schizophrenia and caregivers expressed mixed views, with some describing treatment as “easy”, whilst others described a fear that treatment will stop working or are frustrated with the frequency of appointments. When asked about treatment goals, a common theme was wishing for the person living with schizophrenia to be clinically stable, leading to a reduction in symptoms and emotional outbursts. When asked about an LAI administered once every 2 months, people living with schizophrenia and caregivers expressed positive views, and perceived that such a treatment would be less burdensome than current treatment. Prescribers were open to recommending an LAI given once every 2 months to clinically stable patients, or those expressing a preference for a decreased dosing frequency or for LAIs in general.
Conclusion: In this qualitative study, participants expressed overall positive views on a potential transition to an LAI given once every 2 months, due to the advantages of greater freedom and less treatment burden. Selection of a specific LAI should acknowledge individual patient and caregiver preferences regarding formulation and frequency, to ensure that targeted disease management goals are met.
Keywords: dosing frequency, long-acting injectable, healthcare professional preferences, patient preferences, caregiver preferences, shared decision-making
Introduction
Schizophrenia is a chronic and debilitating mental health disorder that is estimated to affect 23.6 million people worldwide.1 It has a typical onset in late adolescence or early adulthood,1 and is associated with significant morbidity and mortality that accrues a substantial clinical and economic burden to people living with schizophrenia and their caregivers, as well as to the healthcare systems, and society.2,3 Schizophrenia has a negative impact on levels of burden and stress experienced by caregivers and,4 in turn, this burden affects the health-related quality of life of those with schizophrenia.5
Maintenance treatment with antipsychotic therapy is recommended in schizophrenia to achieve and maintain symptom control and prevent relapse.6,7 However, adherence to oral antipsychotics is often poor, with an estimated 67% of patients not taking their treatment as prescribed.8 Non-adherence is associated with a significantly increased risk of relapse,9 which has been correlated with accelerated disease progression.10
Long-acting injectable (LAI) formulations of second-generation antipsychotics improve treatment adherence compared with daily oral treatments,11 thus reducing the rate of relapse and hospitalization, protecting people living with schizophrenia from progressive functional decline,12 and reducing mortality.13,14 In addition to their clinical benefits, LAIs offer improved convenience compared with daily oral therapies, and the introduction of LAIs with longer dosing intervals (ie, an interval longer than one month) may positively affect patients’ subjective wellbeing, quality of life, and treatment satisfaction.15,16
Despite the recognized benefits of LAIs, there is evidence that they are underutilized in people living with schizophrenia.2,17,18 While adherence is comparatively better with LAIs than with oral therapies,14 it may still be suboptimal due to interruptions in treatment and partial compliance.19–21 To improve adherence, it is important to understand factors affecting medication compliance in people living with schizophrenia, such as perceptions of the partnership with their prescriber,22 and factors influencing their willingness to try a new medication, such as treatment goals.23 Consequently, physician assessment of patient views and concerns is an important aspect of shared decision-making,24 and deriving a personalized treatment plan to support treatment continuation and improve satisfaction and outcomes.23,25
Several studies have used surveys or questionnaires to investigate the views of people living with schizophrenia, caregivers, and healthcare professionals on the use of LAIs for the management of schizophrenia.25–31 Some of these preference studies focused on understanding perceptions of a specific LAI with a dosing interval longer than one month,25,26,28–30 with several studies gathering data in a clinical trial setting.25–27,31 These studies demonstrated that an antipsychotic treatment with a dosing interval longer than one month may have several benefits for people living with schizophrenia, as well as for their caregivers, including improved flexibility and convenience,29,30 reduced treatment burden and reduced caregiver burden,25,26,29 less stigmatization,28,30 reductions in the patient’s focus on illness,29 and increased involvement in daily activities and social interactions.28,29
As the treatment landscape for schizophrenia continues to evolve, there is a need to improve our understanding of perceptions and preferences relating to disease management, and the potential role that LAIs with longer dosing intervals may play, beyond views relating to a specific treatment option. Therefore, this qualitative, single-person interview study takes a broader approach than previous studies and examines the personal perspectives of people living with schizophrenia, caregivers, and prescribers regarding treatment goals and LAI dosing frequency. Furthermore, the study (conducted in five countries across Europe) investigates preferences for a hypothetical LAI administered once every 2 months compared to an LAI given once monthly.
Materials and Methods
Study Objectives
The primary objective of the study was to explore the preferences of people living with schizophrenia regarding LAI features, including dosing frequency, and to identify factors that may influence preference for a hypothetical LAI administered once every 2 months. Secondary objectives were to understand the preferences of caregivers and prescribers with respect to LAI treatment features focusing on dosing frequency, identify factors that may influence the preference for an LAI administered every 2 months, and examine similarities and differences in preferences between all three stakeholder groups.
An additional exploratory objective was to assess how caregivers and prescribers perceived the treatment preferences of people living with schizophrenia, and gain a better understanding of the experiences of shared decision-making during the discussion of treatment choices for all participants involved in the process.
Recruitment
This study planned to recruit people living with schizophrenia, caregivers, and prescribers across France, Germany, Italy, and Spain; during recruitment, the study was expanded to include the UK. Recruitment aimed to ensure variety across age and gender among people living with schizophrenia and caregivers, and variety across practice setting and specialties for prescribers. People living with schizophrenia and caregivers were recruited via a multimodal approach involving physician referral or other channels, such as panels, social media, patient advocacy groups, and recruitment partner databases. Prescribers were recruited through recruitment partner databases. The three groups of participants were independent of each other, such that people living with schizophrenia were not being cared for or treated by the caregivers or prescribers, respectively. Participants were required to self-report their eligibility criteria (Table 1), which were developed using patient-accessible language.
 |
Table 1 Key Eligibility Criteria for People Living With Schizophrenia, Caregivers, and Prescribers |
Study Materials
A review of the relevant scientific literature, public databases, and proprietary publications was conducted to identify key concepts relevant to preferences for different dosing regimen frequencies among people living with schizophrenia and their caregivers. These findings were used to inform the development of a semi-structured interview discussion guide for each participant group. Each of the discussion guides included the same core questions to harmonize the conversation flow across participant groups and countries. Interviews were conducted by moderators with experience of healthcare research and training in qualitative methods. All moderators were given additional training in conducting interviews with vulnerable populations, including guidance and potential resources to share should participants experience distress during interviews. They were also all individually briefed on the study, including its goals and discussion guides. Some adaptations to the discussion guides were permitted, including the exact wording of questions, and the moderator could probe to explore topics further. The categories of questions that were included in the discussion guides are shown in Table 2.
 |
Table 2 Question Categories Included in the Semi-Structured Discussion Guide for Each Participant Group |
Study materials were written in English and translated into French, German, Italian, and Spanish. The study protocol and all qualitative materials were submitted to a US independent institutional review board (Pearl IRB, 29 E McCarty St #100, Indianapolis, IN 46225, US) and received exempt status on 25 April 2023. The materials were submitted to a US institutional review board to ensure the study and its’ materials met ethical standards, as this research was not eligible for ethics review in France, Germany, Italy, Spain, or the UK. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Qualitative Data Collection
All participants meeting the inclusion criteria and none of the exclusion criteria were required to complete an informed consent form prior to interview and were able to withdraw from the study at any time. Data were collected through in-depth, one-to-one interviews to allow participants to express their personal experience without the judgment or influence of others. The interviews were conducted using online or virtual platforms such as Zoom, Microsoft Teams, or Skype, and lasted 60 minutes for people living with schizophrenia and caregivers, and 45 minutes for prescribers. All interviews were completed in the local language, and were audio recorded and transcribed in English.
This study took a qualitative descriptive approach during interviews to ensure the information gathered aligned with the naturalistic description of patient and caregiver experiences and preferences, with minimal interference from the researcher.32 A qualitative descriptive approach aims to gather a straight description and comprehensive summary on specific questions and allows flexibility to interpret data in a meaningful manner.32 Reliability in applying qualitative description was established by using the concepts of descriptive and interpretive validity.33
Participants received a country-specific fair market value incentive for their time, in accordance with the Sponsor’s compliance regulations. The study was anonymized, with no personal identifying information collected for use in the analysis. A double-blind approach was used, such that the identities of the Sponsor and participants were unknown to each other before and during the interview. Participants in France, Germany, Italy, and Spain were informed of the Sponsor’s name at the end of the interview, in line with General Data Protection Regulations. Participants in the UK were also informed of the Sponsor’s name at the end of the interview, in line with the British Healthcare Business Intelligence Association guidance.
Data Analysis
Qualitative data analysis software, MAXQDA, was used to review the verbatim transcripts and code the data. A bottom-up approach to coding was used, relying on inductive coding to develop concepts and themes from the raw data. Coding was completed in layers: to begin, a member of the study team systematically read the text, formed understandings and established a code system driven by topics in the interview guide. A second level of coding further focused on the data, using thematic analysis to identify richer and more detailed findings by themes, driven by the research objectives. Throughout the coding process, the study team reviewed these codes intermittently to establish patterns, identify new concepts and make refinements to ensure central themes were relevant to the study objectives.
Overall data saturation for people living with schizophrenia and caregivers was assessed with the goal of identifying code saturation in core themes.34 Code saturation was not assessed for prescribers as prescriber data were meant to supplement data collected from people living with schizophrenia. The study team remained open to all insights emerging from the data and all concepts were coded. To ensure data quality and limit biases, both data collection and analysis followed the Consolidated Criteria for Reporting Qualitative Research,35 and the Standards for Reporting Qualitative Research.36
Results
Fifteen people living with schizophrenia, 11 caregivers of people living with schizophrenia, and 13 prescribers participated in individual interviews as part of this study. Study participant characteristics from France, Germany, Italy, Spain, and the UK are presented in Table 3 (see Supplementary Table 1 for a breakdown of participant characteristics data by country). By the last interview, no new concepts associated with perceptions of LAIs, treatment preferences, and thoughts on an LAI given every 2 months were identified for people living with schizophrenia and caregivers, indicating that data saturation was achieved.
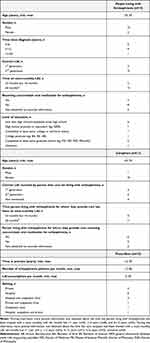 |
Table 3 Study Participant Characteristics |
People living with schizophrenia included in the study had transitioned from oral medication to an LAI for several reasons. These reasons included lack of compliance, not doing well on their current medication, reaching a crisis point, side effects, the convenience of an LAI, or general interest in an LAI compared with oral medication. People living with schizophrenia reported that they primarily learned about LAIs from their doctor or during hospitalization for an acute phase. Their doctors described the LAI as “more manageable”, “easier” and that it may result in “less worry”. Some doctors mentioned that the “drug would be the same as the oral therapy”, and that “the LAI had worked for their other patients”.
Healthcare Experiences of People Living with Schizophrenia and Caregivers
People living with schizophrenia and caregivers typically experienced limited barriers to accessing care. They reported living close to a large city or near their doctor’s office and had their cost of care covered through their country’s healthcare system. For those that experienced challenges in finding and receiving care, these included long waiting times, lack of consistent care, limited specialist availability, and time and cost spent attending appointments. Some challenges, such as doctors being dismissive of patient needs or needs not being met, led to negative experiences of seeking care. Experiences of stigma and judgement also led to hesitancy in engaging with healthcare providers, with one caregiver from Italy saying,
This illness is surrounded by stigma… it’s not easy to be understood because the problems we face are different than anyone else’s.
Caregivers described the stress and burden of providing support, which impacted their daily lives and the ability to work. The support role of caregivers included providing help with personal care, cooking, finances, and keeping appointments, in addition to encouraging treatment adherence, independence with daily tasks, and societal engagement. One caregiver from Italy noted,
Before my son’s disease, I liked going to the gym, walking in the park, and riding a bike. I absolutely cannot do it anymore because I must take care of my son.
Caregivers experienced an emotional toll from supporting people living with schizophrenia who lacked insight into their condition or did not accept their diagnosis.
When talking about current treatment (a once-monthly LAI), people living with schizophrenia and caregivers expressed mixed views. Treatment with a once-monthly LAI was described as “easy”, “balanced” and “comfortable”; receiving the injection was viewed as part of their routine, and they valued phone reminders from their doctor ahead of appointments. One person living with schizophrenia from Germany said,
Well, I’m definitely glad that I no longer have to take the tablets every day, because I sometimes forgot to take them. And somehow, I feel a bit liberated from it, although, when the day comes when I have to go there, I don’t really like that either. But it’s better than taking tablets every day and at least I’ll have peace of mind for a month.
Caregivers described a fear that the medication may stop working and several people living with schizophrenia indicated they were “bored” with appointments, with one person from Spain saying,
I see a lot of waiting time… things stop for a bit and when I take the medication once a month it kind of hinders, so to speak, the rest of my responsibilities.
When asked for one thing they would improve about their current once-monthly LAI, people living with schizophrenia would reduce the frequency of administration, while caregivers prioritized a reduction in side effects and less need for oral supplementation.
Shared Decision-Making
When asked about their involvement in treatment decisions, the experiences of people living with schizophrenia varied. They commonly reported being involved in discussions but trusting their physician or caregiver for the final decision, while some worked with their physician to make the final decision. People living with schizophrenia who were not involved in decision-making also experienced having no choice in treatment selection due to the severity of their illness at the time or complying with their doctor. Caregivers responded that they often took part in treatment decision-making but placed trust in the doctor and deferred to their recommendations, or reported leaving the final decision to the person living with schizophrenia. Where caregivers did make the final treatment choice, they did their best to involve the person living with schizophrenia, and where the person was not doing well the caregiver described making the decision to ensure they would get better. Prescribers revealed that several factors affect caregiver involvement in decision-making, such as the patient’s desire for privacy, the relationship between the patient and caregiver, and the patient’s age and duration of family support.
Although people living with schizophrenia and caregivers commonly reported having no questions during treatment decisions as they trusted their doctor or viewed them as being “in charge”, they did detail the information that was most important to them when learning about treatment or LAIs specifically (Figure 1).
 |
Figure 1 Information viewed as most important by people living with schizophrenia and caregivers when learning about treatment options, specifically LAIs. |
A common concept captured in the interviews was that people living with schizophrenia and caregivers did not search for information on treatment in general, with some leaving decisions to their doctor or avoiding searching for information as they found it scary or inaccurate. Similarly, they often reported that they did not research LAIs before starting on the treatment, and instead expressed that they had confidence and trust in their doctor.
Prescribers described spending time with patients and caregivers to share information on treatment options, discuss goals, and highlight quality of life benefits. Regarding the behaviors of patients living with schizophrenia and caregivers around treatment selection, prescribers reported that they received questions on efficacy and side effects. Patients also queried whether they needed medication and the effects it might have on their personality, while caregivers questioned whether treatment would improve patients’ autonomy, including washing themselves and engaging socially. When moving from an oral to an LAI antipsychotic, patients and caregivers queried prescribers on the differences in side effects, duration of effect, and impact on having a child. Typical concerns shared with prescribers about LAIs specifically related to the amount of medication given at one time, fear of needles and injection site pain, the stigma that receiving an injectable means a more serious and chronic disease, and loss of autonomy or feeling that an LAI is punitive. One psychiatrist from Germany noted,
It’s possible that patients may be confused by how LAIs work at a lower dose over a longer period of time, but if I say that, I usually don’t tell, I draw it. I draw a curve where the concentration course of a long-term preparation, and on top, I draw the curve of an oral medication, and then patients see very quick which one has more dosage and which less.
While prescribers often described presenting various treatment options to patients and caregivers, some encouraged LAIs specifically due to the benefits they had observed in other patients. Other prescribers reported that they present only one treatment option, to increase patient confidence and reduce decision anxiety, while giving further information to allow for an open-ended discussion.
When discussing treatment options, prescribers viewed caregivers as allies, though they typically do not make the final treatment decision. One psychiatrist from the UK mentioned,
I like to involve [the family] as much as they want to be involved and as much as the patient is happy for them to be involved… It’s not just doctors working with patients, but caregivers and friends and family and society…
Prescribers emphasized the importance of patient awareness of their condition and the need for treatment and sought to establish a trusting relationship with their patients to build this understanding. One psychiatrist from Spain shared,
Schizophrenia is a disease that sounds as something severe, it sounds as something ‘very hard’, both for caregivers but also for patients when they have certain disease awareness. But we must talk about it, we must destroy the myths surrounding it, and then, from that demystification, we must make them understand that it’s a chronic disease, and as it is a chronic disease, it requires a chronic treatment.
Treatment Goals
In their responses to questions on treatment goals, participants mentioned the common themes of wishing for the person living with schizophrenia to be clinically stable leading to a reduction in symptoms and emotional outbursts, and for treatment to be effective and result in the patient feeling “balanced” (Figure 2).
 |
Figure 2 Treatment goals among people living with schizophrenia, caregivers and prescribers. |
Prescribers described their primary treatment goal as improved quality of life, which encompassed the ability to work, be productive, enjoy life, and have functional relationships with family and friends. Stability, in terms of avoiding positive or negative symptoms, was similarly important, and quality of life and stability were often described together, implying that they go hand-in-hand for patients living with schizophrenia.
Perceptions of LAIs for People Living with Schizophrenia and Caregivers
In terms of perceptions of LAIs, people living with schizophrenia and caregivers often viewed remaining on the same medicine and having a choice of where the LAI is injected as the most important features, with other factors playing a lesser role in LAI selection (Table 4).
 |
Table 4 Perceptions of the Feature of LAIs and on the Features of an “Ideal” Treatment Among People Living With Schizophrenia and Caregivers |
Reasons Behind the Decision to Prescribe an LAI
Prescribers identified a range of patient, caregiver, physician and medication themes that were important to consider when making the decision to prescribe an LAI (Table 5). They highlighted the important role played by caregivers in keeping the physician informed of how the patient was doing, and in supporting with adherence. Patient buy-in was seen as a critical factor in treatment success, as were dosing frequency and previous treatment response due to prescriber preference for patients remaining on the same molecule when transitioning to an LAI. Prescribers also noted that the use of an LAI provided certainty that patients with advanced, severe, chronic disease had received medication.
 |
Table 5 Prescriber Views of Important Factors for Consideration When Prescribing an LAI |
Views on an LAI Administered Every 2 Months
People living with schizophrenia and caregivers generally expressed positive views about an LAI with a 2-month dosing frequency, describing it with words such as “great”, “better”, “calm”, and “free”. Participants often highlighted the perception that such a treatment would be convenient and less burdensome than their current treatment (Table 6). They indicated that visiting their psychiatrist once every 2 months would be acceptable, especially if visits aligned with injection appointments and treatment was going well. However, both people living with schizophrenia and caregivers stated that disease stability is prioritized over convenience.
 |
Table 6 Perceptions of the Advantages and Disadvantages of an LAI Administered Every 2 months Versus a Once-Monthly LAI |
Similarly, prescribers responded that an LAI given every 2 months would be received positively by patients due to convenience, less injection pain, and feeling more normal due to receiving fewer injections (Table 6). When asked about perceived disadvantages, prescribers highlighted a perceived lack of need, loss of dosing “control”, less frequent visits with patients experiencing issues, and the potential need for supplemental oral antipsychotics (Table 6).
Prescribers stated that they were open to recommending an LAI given once every 2 months to patients stable on an oral medication or the associated once-monthly LAI, or for those expressing a preference for a decreased dosing frequency or for LAIs in general. Other suitable patients included those demonstrating prior adherence to treatment, and those who may benefit due to a busy schedule (eg, with work or school).
Discussion
In previous studies that have evaluated LAIs with dosing intervals longer than one month, people living with schizophrenia and caregivers reported the benefits of improved flexibility and convenience,29,30 and reduced treatment and caregiver burden.25,26,29 A reduced dosing frequency was also reported to be less stigmatizing,28,30 reduce the patient’s focus on illness,29 increase their involvement in daily activities and social interactions,28,29 and may lead to improved quality of life and overall level of adherence.14 Further benefits were noted by physicians and nurses, who experienced improved communication with their patients.28
The present qualitative, single-person interview study reflects many of these experiences, and builds on these findings by providing insights from people living with schizophrenia, caregivers, and prescribers about a hypothetical LAI given every 2 months rather than a specific treatment. Over the course of the interviews, people living with schizophrenia and caregivers shared a range of perspectives and, by the last interview, no new concepts were identified. This suggests that the sample size was appropriate and that the interviews achieved data saturation, consistent with previous evidence suggesting that it takes 12 interviews to attain saturation.37 All three participant groups reported a general acceptance for an LAI given once every 2 months, perceiving that it would bring greater freedom and a reduced treatment burden. Caregiver burden can reduce quality of life for people living with schizophrenia5 and, for some patients and caregivers in this study, less caregiver involvement and burden were factors that may influence acceptance of an LAI given once every 2 months. However, despite the perceived benefits, people living with schizophrenia and caregivers unequivocally prioritized symptom stability over convenience. Overall, these findings indicated that dosing once every 2 months was viewed as a treatment option that would fit in with patients’ and caregivers’ everyday lives, and aligned with prescribers’ preferences for providing holistic care for their patients that encompasses improved efficacy, tolerability, self-agency, and freedom from burden.
During interviews, participants from all groups indicated that a decreased frequency of clinic visits was acceptable provided symptoms remained stable, and a preferable feature of an ideal treatment for people living with schizophrenia and caregivers. Prescriber responses indicated that they saw the value of less frequent dosing in terms of allowing patients to feel normal due to receiving fewer injections and would consider this approach in clinically stable patients and those expressing a wish for reduced dosing. However, it is important that prescribers take the opportunity to consult with their patients outside of injection visits as needed, rather than appointments becoming associated purely with the administration of treatment.
Perceived disadvantages included the fear that a dosing interval of once every 2 months may mean the effects of the medication would lessen before the next injection, with no option to alter dosing and, therefore, the potential need for supplemental oral antipsychotics. It is noteworthy that, although these are concerns that are applicable to all LAIs,38 the decrease in plasma concentrations of an antipsychotic is much more gradual after the end of the dosing period with an LAI compared with oral dosing.39 Although “wearing off” of medication may be a concern, pharmacokinetic studies of antipsychotic drugs have confirmed the maintenance of stable blood levels throughout the dosing interval with LAIs,40,41 which provides the benefits of consistent bioavailability and fewer plasma peaks compared with oral antipsychotics.42 For example, evidence shows that efficacious concentrations of medication are maintained for the full dosing interval with the recently approved LAI formulation of aripiprazole monohydrate, which is administered once every 2 months compared to its once-monthly formulation.40,43 Furthermore, comparable efficacy has been demonstrated with aripiprazole administered once-monthly and once every 2 months for the maintenance treatment of schizophrenia, with all patients demonstrating clinical stability.44 Finally, a meta-analysis of 115 randomized clinical trials highlighted that some LAIs are associated with fewer side effects and better tolerability compared with oral formulations of the same antipsychotic.45
The findings of this study have revealed important insights into LAI use in the management of schizophrenia and the experiences of patients. By detailing this study’s results relating to participants’ healthcare experiences, shared decision-making, and treatment goals ahead of those relating to the primary objective, we have sought to provide the context of their general views of disease management and treatment ahead of presenting their perceptions of an LAI given every 2 months. Interview findings highlighted that people living with schizophrenia do not always share their questions and concerns about treatment with their physician. This emphasizes the importance of shared decision-making and the need for prescribers to engage the patient in conversation to establish if they have any preconceptions about LAIs, such as the perceptions that LAIs signify a greater severity of disease or the effects of the medication may lessen before the next injection. Such discussions ensure that prescribers have the opportunity to address concerns or misconceptions preemptively and identify ways to support patients with adherence. However, evidence suggests that shared decision-making is underutilized for people with psychiatric disorders.46 Consequently, interventions to promote shared decision-making, such as training for physicians and patients or use of decision aids, may improve use of this approach.46
Strengths and Limitations
A strength of this study is its qualitative research design, which allowed participant insights to be examined in depth to enable a full understanding of treatment preferences and the factors influencing these decisions. Study interviews were semi-structured, meaning participants were not restricted to specific questions but could be guided and redirected by moderators in real time as new information emerged. The study also drew from a diverse range of participants, in terms of age and geographical location, and the extensive experience of prescribers in the management of schizophrenia; however, it should be noted that the majority of people living with schizophrenia were male and caregivers were female.
Qualitative research may be associated with researcher and participant bias, which may limit its findings. To minimize this effect, moderators were briefed in the subject matter and received instruction on the use of the discussion guide. For concept elicitation research, data are collected from a relatively small number of individuals and thus findings are not intended to be generalizable to a larger population; the value of qualitative research is the representation of key values and opinions that are important to the populations being studied. These data can then be used to generate hypotheses regarding the study populations and provide direction on further investigation. Additionally, as the insight of people living with schizophrenia was not formally assessed, it was not possible to determine the extent to which schizophrenia may have impaired insight for this participant group, and the impact on their opinions and preferences for treatment. The potential impact of impaired insight was likely reduced by excluding participants with a current acute episode, when insight is usually most impacted.47 Although this does not rule out the possibility of impaired insight, understanding the patient perspective was key to the objectives of the study, regardless of level of insight. Finally, although the sample size was small, data saturation was reached for people living with schizophrenia and caregivers and, although this was not assessed for prescribers, results were concordant between all three participant groups. Concordance between the three participant groups further indicates that the views of people living with schizophrenia are unlikely to have been biased by impaired insight.
Conclusion
In this qualitative interview study participants expressed overall positive views on a potential transition to an LAI given once every 2 months. The findings indicate that there may be general acceptance of an LAI administered once every 2 months for the treatment of schizophrenia given the perceived advantages of greater freedom and less treatment burden. Treatment selection of a specific LAI should acknowledge individual patient and caregiver preferences regarding formulation and frequency, to ensure that targeted disease management goals are met. In particular, this study indicates that treatment duration and efficacy are critical factors for uptake.
This qualitative analysis is intended to be hypothesis generating, and the insights gathered will inform the development of a larger, one-time, cross-sectional, quantitative survey to evaluate preferences for an LAI given once every 2 months.
Data Sharing Statement
To submit inquiries related to Otsuka clinical research, or to request access to individual participant data (IPD) associated with any Otsuka clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data-sharing platform.
Ethics Approval and Informed Consent
The study protocol and all qualitative materials were submitted to a US independent institutional review board (Pearl IRB, 29 E McCarty St #100, Indianapolis, IN 46225, US) and received exempt status on 25 April 2023. This research was not eligible for ethics review in France, Germany, Italy, Spain, or the UK.
Acknowledgments
The authors would like to thank the participants for their contribution to this study. Medical writing support was provided by Sarah Ramsden and colleagues of Cambridge (a division of Prime, Knutsford, UK), funded by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA) and H. Lundbeck A/S (Valby, Denmark).
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Consent for Publication
Written informed consent for the publication of their anonymized statements was obtained from study participants at the beginning of the study. Participant consent was reiterated verbally prior to the initiation of participant interviews.
Funding
This work was sponsored by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA) and H. Lundbeck A/S (Valby, Denmark). The sponsors were involved in the design of the study, the collection, analysis and interpretation of data, the writing and reviewing of this article, and the decision to submit the article for publication.
Disclosure
Sofia Pappa has received research grants from Janssen and Recordati. She has also received honoraria as a speaker or consultant from Gedeon Richter, Janssen, Lundbeck, Otsuka, Recordati, ROVI Biotech, and Sunovion.
Stephanie Loomer, Dawn Bates, and Xavier Guillaume are full-time employees of Oracle Life Sciences.
Murat Yildirim, Arun Micheelsen, Kristine Harrsen, and Pedro Such are a full-time employees of H. Lundbeck A/S.
Soma S Nag is a full-time employee of Otsuka Pharmaceutical Development & Commercialization, Inc. At the time of the study Jessica Madera-McDonough and Bolu Oladini were full-time employees of Otsuka Pharmaceutical Development & Commercialization, Inc.
Clodagh Beckham is a full-time employee of Otsuka Pharmaceutical Europe Ltd.
The authors report no other conflicts of interest in this work.
Some of the data in this manuscript have previously been reported in a poster presented at the SIRS 2024 Annual Congress, April 3–7, 2024, Florence, Italy.
References
1. Solmi M, Seitidis G, Mavridis D, et al. Incidence, prevalence, and global burden of schizophrenia - data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry. 2023;28(12):5319–5327. doi:10.1038/s41380-023-02138-4
2. Boyer L, Falissard B, Nuss P, et al. Real-world effectiveness of long-acting injectable antipsychotic treatments in a nationwide cohort of 12,373 patients with schizophrenia-spectrum disorders. Mol Psychiatry. 2023;28(9):3709–3716. doi:10.1038/s41380-023-02175-z
3. Chong HY, Teoh SL, Wu DB, et al. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–373. doi:10.2147/NDT.S96649
4. Geriani D, Savithry KS, Shivakumar S, Kanchan T. Burden of care on caregivers of schizophrenia patients: a correlation to personality and coping. J Clin Diagn Res. 2015;9(3):VC01–VC04. doi:10.7860/JCDR/2015/11342.5654
5. Wei Y, Peng Y, Li Y, et al. Caregivers’ burden and schizophrenia patients’ quality of life: sequential mediating effects of expressed emotion and perceived expressed emotion. Front Psychiatry. 2022;13:961691. doi:10.3389/fpsyt.2022.961691
6. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59. doi:10.1007/s40263-020-00779-5
7. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2021;154(4 Suppl):1–63. PMID: 9090368. doi:10.1176/ajp.154.4.1
8. MacEwan JP, Forma FM, Shafrin J, et al. Patterns of adherence to oral atypical antipsychotics among patients diagnosed with schizophrenia. J Manag Care Spec Pharm. 2016;22(11):1349–1361. doi:10.18553/jmcp.2016.22.11.1349
9. Caseiro O, Perez-Iglesias R, Mata I, et al. Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J Psychiatr Res. 2012;46(8):1099–1105. doi:10.1016/j.jpsychires.2012.05.001
10. Jorgensen KT, Bog M, Kabra M, et al. Predicting time to relapse in patients with schizophrenia according to patients’ relapse history: a historical cohort study using real-world data in Sweden. BMC Psychiatry. 2021;21(1):634. doi:10.1186/s12888-021-03634-z
11. Weiser M, Davis JM, Brown CH, et al. Differences in antipsychotic treatment discontinuation among veterans with schizophrenia in the U.S. Department of Veterans Affairs. Am J Psychiatry. 2021;178(10):932–940. doi:10.1176/appi.ajp.2020.20111657
12. Lin D, Thompson-Leduc P, Ghelerter I, et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(5):469–481. doi:10.1007/s40263-021-00815-y
13. Janzen D, Bolton JM, Leong C, Kuo IF, Alessi-Severini S. Second-generation long-acting injectable antipsychotics and the risk of treatment failure in a population-based cohort. Front Pharmacol. 2022;13:879224. doi:10.3389/fphar.2022.879224
14. Milz R, Benson C, Knight K, et al. The effect of longer dosing intervals for long-acting injectable antipsychotics on outcomes in schizophrenia. Neuropsychiatr Dis Treat. 2023;19:531–545. doi:10.2147/NDT.S395383
15. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. doi:10.4088/JCP.15032su1
16. Brasso C, Bellino S, Bozzatello P, et al. Second generation long-acting injectable antipsychotics in schizophrenia: the patient’s subjective quality of life, well-being, and satisfaction. J Clin Med. 2023;12(22):6985. doi:10.3390/jcm12226985
17. Kane JM, Mychaskiw MA, Lim S, et al. Treatment journey from diagnosis to the successful implementation of a long-acting injectable antipsychotic agent in young adults with schizophrenia. J Clin Psychiatry. 2023;84(3):22m14544. doi:10.4088/JCP.22m14544
18. Pilon D, Joshi K, Tandon N, et al. Treatment patterns in Medicaid patients with schizophrenia initiated on a first- or second-generation long-acting injectable versus oral antipsychotic. Patient Prefer Adherence. 2017;11:619–629. doi:10.2147/PPA.S127623
19. Pappa S, Mason K. Partial compliance with long-acting paliperidone palmitate and impact on hospitalization: a 6-year mirror-image study. Ther Adv Psychopharmacol. 2020;10:2045125320924789. doi:10.1177/2045125320924789
20. Mason K, Barnett J, Pappa S. Effectiveness of 2-year treatment with aripiprazole long-acting injectable and comparison with paliperidone palmitate. Ther Adv Psychopharmacol. 2021;11:20451253211029490. doi:10.1177/20451253211029490
21. Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–134. doi:10.1080/13696998.2017.1379412
22. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1–3):176–181. doi:10.1016/j.schres.2011.04.030
23. Achtyes E, Simmons A, Skabeev A, et al. Patient preferences concerning the efficacy and side-effect profile of schizophrenia medications: a survey of patients living with schizophrenia. BMC Psychiatry. 2018;18(1):292. doi:10.1186/s12888-018-1856-y
24. Pappa S, Barnett J, Gomme S, et al. Shared and supported decision making in medication in a mental health setting: how far have we come? Community Ment Health J. 2021;57(8):1566–1578. doi:10.1007/s10597-021-00780-2
25. Blackwood C, Sanga P, Nuamah I, et al. Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the patient-reported medication preference questionnaire. Patient Prefer Adherence. 2020;14:1093–1102. doi:10.2147/PPA.S251812
26. Lencer R, Garcia-Portilla MP, Bergmans P, et al. Impact on carer burden when stable patients with schizophrenia transitioned from 1-monthly to 3-monthly paliperidone palmitate. Compr Psychiatry. 2021;107:152233. doi:10.1016/j.comppsych.2021.152233
27. Nasrallah HA, Weiden PJ, Walling DP, et al. Aripiprazole lauroxil 2-month formulation with 1-day initiation in patients hospitalized for an acute exacerbation of schizophrenia: exploratory efficacy and patient-reported outcomes in the randomized controlled ALPINE study. BMC Psychiatry. 2021;21(1):492. doi:10.1186/s12888-021-03420-x
28. Pungor K, Sanchez P, Pappa S, et al. The Patient, Investigator, Nurse, Carer Questionnaire (PINC-Q): a cross-sectional, retrospective, non-interventional study exploring the impact of less frequent medication administration with paliperidone palmitate 3-monthly as maintenance treatment for schizophrenia. BMC Psychiatry. 2021;21(1):300. doi:10.1186/s12888-021-03305-z
29. Rise MB, Stølan LO, Erdner A, et al. Patients’ perspectives on three-monthly administration of antipsychotic treatment with paliperidone palmitate - a qualitative interview study. Nord J Psychiatry. 2021;75(4):257–265. doi:10.1080/08039488.2020.1841289
30. Barnett J, Pappa S. Switching from monthly to three-monthly long-acting injectable paliperidone: a survey on subjective satisfaction and safety. Patient Prefer Adherence. 2023;17:1603–1610. doi:10.2147/PPA.S410028
31. Robinson DG, Suett M, Wilhelm A, et al. Patient and healthcare professional preferences for characteristics of long-acting injectable antipsychotic agents for the treatment of schizophrenia. Adv Ther. 2023;40(5):2249–2264. doi:10.1007/s12325-023-02455-8
32. Kim H, Sefcik JS, Bradway C. Characteristics of qualitative descriptive studies: a systematic review. Res Nurs Health. 2017;40(1):23–42. doi:10.1002/nur.21768
33. Maxwell JA. Understanding and validity in qualitative research. Harv Educ Rev. 1992;62(3):279–300. doi:10.17763/haer.62.3.8323320856251826
34. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:114523. doi:10.1016/j.socscimed.2021.114523
35. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi:10.1093/intqhc/mzm042
36. O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–1251. doi:10.1097/ACM.0000000000000388
37. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82. doi:10.1177/1525822X05279903
38. Kane JM, McEvoy JP, Correll CU, Llorca PM. Controversies surrounding the use of long-acting injectable antipsychotic medications for the treatment of patients with schizophrenia. CNS Drugs. 2021;35(11):1189–1205. doi:10.1007/s40263-021-00861-6
39. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25–40. doi:10.2147/CPAA.S32735
40. Harlin M, Yildirim M, Such P, et al. A randomized, open-label, multiple-dose, parallel-arm, pivotal study to evaluate the safety, tolerability, and pharmacokinetics of aripiprazole 2-month long-acting injectable in adults with schizophrenia or bipolar I disorder. CNS Drugs. 2023;37(4):337–350. doi:10.1007/s40263-023-00996-8
41. Coppola D, Liu Y, Gopal S, et al. A one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophrenia. BMC Psychiatry. 2012;12:26. doi:10.1186/1471-244X-12-26
42. Bosanac P, Castle DJ. Why are long-acting injectable antipsychotics still underused? BJPsych Advances. 2015;21(2):98–105. doi:10.1192/apt.bp.114.013565
43. Wang Y, Harlin M, Larsen F, et al. Population pharmacokinetics and dosing simulations for aripiprazole 2-month ready-to-use long-acting injectable in adult patients with schizophrenia or bipolar I disorder. Clin Pharmacol Drug Dev. 2024;13(6):631–643. doi:10.1002/cpdd.1397
44. Citrome L, Such P, Yildirim M, et al. Safety and efficacy of aripiprazole 2-month ready-to-use 960 mg: secondary analysis of outcomes in adult patients with schizophrenia in a randomized, open-label, parallel-arm, pivotal study. J Clin Psychiatry. 2023;84(5):23m14873. doi:10.4088/JCP.23m14873
45. Wang D, Schneider-Thoma J, Siafis S, et al. Efficacy, acceptability and side-effects of oral versus long-acting- injectables antipsychotics: systematic review and network meta-analysis. Eur Neuropsychopharmacol. 2024;83:11–18. doi:10.1016/j.euroneuro.2024.03.003
46. Beitinger R, Kissling W, Hamann J. Trends and perspectives of shared decision-making in schizophrenia and related disorders. Curr Opin Psychiatry. 2014;27(3):222–229. doi:10.1097/YCO.0000000000000057
47. Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res. 2003;61(1):75–88. doi:10.1016/S0920-9964(02)00316-X
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Preferred Conversation Topics with Respect to Treatment Decisions Among Individuals with Type 2 Diabetes
Tichler A, Hertroijs DFL, Ruwaard D, Brouwers MCGJ, Hiligsmann M, de Jong JD, Elissen AMJ
Patient Preference and Adherence 2023, 17:719-729
Published Date: 17 March 2023
Patients’ Preferences for Attributes of Oral vs Injectable Androgen Deprivation Therapy in Prostate Cancer: A Discrete Choice Experiment

Collins SP, Hong A, Hauber B, Flanders SC, Will O, Maculaitis MC, Gatoulis SC, Chakoian M, Thorley J
Patient Preference and Adherence 2025, 19:1397-1409
Published Date: 9 May 2025

