Back to Journals » Journal of Inflammation Research » Volume 17
Exploring the Connections: Autophagy, Gut Microbiota, and Inflammatory Bowel Disease Pathogenesis
Authors Subramanian A, J A, T T , Kumarasamy V , Begum MY , Sekar M , Subramaniyan V , Wong LS , Al Fatease A
Received 24 July 2024
Accepted for publication 4 September 2024
Published 5 December 2024 Volume 2024:17 Pages 10453—10470
DOI https://doi.org/10.2147/JIR.S483958
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam Bachstetter
Arunkumar Subramanian,1 Afrarahamed J,1 Tamilanban T,1,2 Vinoth Kumarasamy,3 M Yasmin Begum,4 Mahendran Sekar,5 Vetriselvan Subramaniyan,6 Ling Shing Wong,7 Adel Al Fatease4
1Department of Pharmacology, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur, Tamilnadu, India; 2Faculty of Medicine, Bioscience and Nursing, MAHSA University, Selangor, Malaysia; 3Department of Parasitology & Medical Entomology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Cheras, Kuala Lumpur, Malaysia; 4Department of Pharmaceutics, College of Pharmacy, King Khalid University, Abha, Saudi Arabia; 5School of Pharmacy, Monash University Malaysia, Bandar Sunway, Subang Jaya, Selangor, Malaysia; 6Department of Medical Sciences, School of Medical and Life Sciences, Sunway University, Bandar Sunway, Selangor, Malaysia; 7Faculty of Health and Life Sciences, INTI International University, Nilai, Malaysia
Correspondence: Tamilanban T; Vinoth Kumarasamy, Email [email protected]; [email protected]
Abstract: Inflammatory Bowel Disease (IBD), which includes Crohn’s disease and ulcerative colitis, represents a complex and growing global health issue with a multifaceted origin. This review delves into the intricate relationship between gut microbiota, autophagy, and the development of IBD. The gut microbiota, a diverse community of microorganisms, plays a vital role in maintaining gut health, while imbalances in this microbial community, known as dysbiosis, are linked to IBD. Autophagy, a process by which cells recycle their components, is essential for gut homeostasis and the regulation of immune responses. When autophagy is impaired and dysbiosis occurs, they individually contribute to IBD, with their combined impact intensifying inflammation. The interconnectedness of gut microbiota, autophagy, and the host’s immune system is central to the onset of IBD. The review also examines how diet influences gut microbiota and its subsequent effects on IBD. It highlights the therapeutic potential of targeting the microbiota and modulating autophagic pathways as treatment strategies for IBD. Understanding these interactions could lead to personalized therapies within the rapidly advancing fields of microbiome research and immunology.
Keywords: inflammatory bowel disease, gut microbiota, autophagy, Crohn’s disease, ulcerative colitis
Graphical Abstract:

Introduction
Inflammatory Bowel Disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), refers to a group of chronic inflammatory conditions that impact the gastrointestinal tract.1 These conditions are marked by ongoing inflammation, abdominal pain, diarrhea, and a significant reduction in quality of life. The prevalence of IBD has been increasing globally in recent years, affecting millions of people and leading to substantial economic costs due to healthcare expenses, decreased productivity, and long-term treatment requirements.2 Despite considerable research efforts to understand IBD, its exact causes remain complex and not fully clarified. Recent scientific findings highlight the significant role of the gut microbiota and autophagy in the development and progression of IBD.3
The gut, often called the “second brain”, hosts a complex and ever-changing community of trillions of microorganisms, collectively known as the gut microbiota.4 This diverse collection includes bacteria, viruses, fungi, and other microbes, all of which play critical roles in digestion, nutrient absorption, and the regulation of the immune system.5 Maintaining a balanced and healthy microbiome is crucial for overall gut health.6 On the other hand, dysbiosis, which refers to an imbalance in the gut microbiota’s composition and function, has been strongly linked to the development and progression of inflammatory bowel disease (IBD).7
Parallel to the gut microbiota, autophagy, a conserved cellular process, is equally pivotal in sustaining intestinal homeostasis.8 Autophagy, derived from the Greek words “auto” (self) and “phagy” (eating), functions as an intracellular recycling mechanism, clearing out cellular waste and damaged organelles.9 It serves as the cell’s quality control system, ensuring the renewal of cellular components.10 This fundamental process also holds a critical role in innate and adaptive immune responses, particularly in the gut, where the mucosal lining is continuously exposed to a barrage of external threats.11
One key nexus between these three domains – the gut microbiota, autophagy, and IBD – is the intricate interaction between the host’s immune system and the gut microbiota.12 An essential function of autophagy is to preserve gut barrier integrity by facilitating the removal of intracellular pathogens and maintaining the mucus layer and tight junctions.8 When autophagy is compromised, as evidenced by certain genetic mutations such as ATG16 and IRGM, harmful intracellular pathogens may accumulate, triggering an overactive immune response and chronic inflammation.13
Adding on, the melanocortin receptors expressed on immune cells like macrophages, have been shown to reduce the production of pro-inflammatory cytokines such as TNF-α and IL-6, leading to a reduction in gut inflammation.14 The melanocortin system, comprising a series of receptors (MC1R to MC5R) and their endogenous ligands such as alpha-melanocyte stimulating hormone, plays a crucial role in inflammation, and immune responses. The dysregulation in the melanocortin system could lead to an inadequate anti-inflammatory response.15
Both dysfunctional autophagy and dysbiosis have been recognized as contributing factors in the development of inflammatory bowel disease (IBD). When these two conditions interact, their combined impact can be especially harmful.16 Autophagy plays a crucial role in regulating the composition and functionality of the gut microbiota, and this regulation significantly affects the microbiota itself.17
The development of inflammatory bowel disease (IBD) is often linked to impaired autophagy, which results in the buildup of pathogens and the loss of immune tolerance, ultimately causing persistent inflammation in the intestines.18 Additionally, individuals with IBD experience alterations in the composition and function of their gut microbiota, which further exacerbates the inflammatory response.19 Therefore, exploring the complex interactions among these factors is crucial to identifying new therapeutic approaches for IBD.20
This review paper aims to delve into the multifaceted interplay between gut microbiota, autophagy, and IBD. We will explore the role of the gut microbiota in IBD development, autophagy’s significance in maintaining gut health, the implications of autophagic dysfunction in IBD, the crosstalk between the gut microbiota and autophagy, and the resulting impact on chronic inflammation.21 Furthermore, we will discuss potential therapeutic avenues that target the gut microbiota or modulate autophagic pathways in the pursuit of alleviating IBD symptoms.22
In a world where IBD continues to afflict a growing number of individuals, comprehending the complex web of interactions between the gut microbiota, autophagy, and IBD represents a crucial step toward the development of more effective therapies and personalized treatment approaches.23 As the field of microbiome research and autophagy’s role in immunity continues to evolve, so does our understanding of IBD’s pathogenesis, bringing us closer to a future where patients can find relief from this debilitating condition.
Gut Microbiota and Inflammatory Bowel Disease
Crohn’s disease and ulcerative colitis are the two main types of inflammatory bowel disease (IBD), a condition that impacts about 0.3% to 0.5% of people worldwide.1 IBD is marked by persistent inflammation in the digestive tract, and its precise origins are still not fully understood. Contributing factors may include immune system reactions, genetic factors, and the makeup of gut microbiota.3,24
Numerous studies have consistently highlighted significant differences in the composition of gut microbiota between individuals with IBD and those who are healthy,25,26 as illustrated in Figure 1, which shows the changes in gut microbiota in both healthy and diseased states. The human microbiota comprises a vast community of microorganisms, including bacteria, viruses, protozoa, and fungi, with bacteria being the most prevalent, reaching densities of 10^11 to 10^12 cells per millilitre.3,27,28 In healthy individuals, Firmicutes and Bacteroides dominate the gut flora, with over 99% of the bacteria belonging to species within the Bacteroides, Proteus, and Actinomycetes groups.29,30
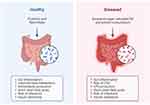 |
Figure 1 Gut Microbiota in Healthy and Diseased State. Created with Biorender.com. |
In the gastrointestinal system, over 1000 species of bacteria play pivotal roles in maintaining the host’s overall health, influencing aspects such as nutrition, immunity, metabolism, and protection against pathogens.31 These gut bacteria provide substantial energy to the intestinal epithelium, participate in the digestion of carbohydrates, and generate short-chain fatty acids (SCFAs) like butyric acid, propionic acid, and acetate.32 SCFAs influence the differentiation of T cells, particularly the induction of regulatory T cells, which are essential for maintaining immune tolerance. SCFAs can suppress the production of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-12, by inhibiting histone deacetylases and activating G-protein coupled receptors like GPR43 and GPR109A. Also, SCFAs influence the polarization of macrophages towards an anti-inflammatory M2 phenotype. This shift in macrophage function is associated with tissue repair and resolution of inflammation, contributing to gut homeostasis.33 Furthermore, beneficial gut bacteria can modulate the host’s immune response by regulating immune cells, while certain pathogenic bacteria can exacerbate intestinal damage by interacting with immune cells and releasing inflammatory cytokines through their metabolites.34
Beneficial Fungi such Hericium erinaceus (also known as Lion’s Mane) has gained attention for its potential therapeutic effects, including in IBD.35 Hericium erinaceus has shown to reduce the production of pro-inflammatory cytokines. Also, they inhibit the NF- κB signalling, a key regulator of inflammation in IBD.36 The pre-biotic effects of Hericium erinaceus promotes the growth of beneficial gut bacteria and reduce the levels of harmful bacteria, which is crucial in the exacerbation of IBD symptoms. Ex-vivo models of IBD have shown the anti-oxidant properties of the mushroom that protect gut tissues from oxidative stress.37,38 Further, Hericium erinaceus have reported to alleviate the stress and psychological factors39 that can play a significant role in the pathogenesis of IBD.
This passage examines the complex interactions between microbial populations, the immune system, metabolic products, and the intestinal lining in individuals with inflammatory bowel disease (IBD). It also investigates the potential of microbiota-based treatments as effective strategies for managing and treating IBD.40 The significance of these changes remains uncertain, but substantial alterations in the composition and function of the gut microbiota, potentially supporting disease states (referred to as dysbiosis), are commonly observed in individuals with IBD. A pivotal question still lingers: does dysbiosis play a role in causing or resulting from the inflammation and immune activation seen in IBD, or does it involve both processes? While the gut microbiota tends to exhibit relative stability over a person’s lifetime, various factors such as dietary modifications, environmental shifts, pathogenic infections, lifestyle choices, and the side effects of medications can disrupt this equilibrium. Consequently, the emergence of dysbiosis in IBD can impact the host’s responses, including immune and metabolic responses, aimed at restoring the balance in host-microbe interactions.
The host’s response includes factors like antimicrobial peptides (AMPs), reactive oxygen species, immune mediators, mucus, and various other changes that impact the composition and function of the gut microbiome, thereby reshaping the local gut ecosystems. Many studies have documented a decline in microbial diversity and a reduction in the abundance of less prevalent microbial groups, such as Gamma-proteobacteria, within the microbiota linked to inflammatory bowel disease (IBD).41,42
The gut microbiota, which is influenced in part by the host’s surroundings, plays a significant role in shaping intestinal health and disease states. In individuals with good health, these microorganisms serve crucial functions such as aiding in nutrient absorption, regulating the immune system, and maintaining the intestinal barrier.43 Disturbances in the gut microbiota, particularly in those with a genetic predisposition, can contribute to the onset of diseases. This concept is reinforced by the observation that genetically susceptible animals rarely develop colitis spontaneously when they are raised in a germ-free environment. For example, one study demonstrated that transferring gut microbiota associated with IBD to germ-free mice led to a reduction in Treg cells and a decrease in B-cell class switching in the colon. Recent research has also unveiled microbial-derived metabolites that directly influence the signaling of the host’s immune system.44
Nutrition plays a pivotal role in shaping the composition of our gut microbiota, and this influence extends to the management of IBD. The relationship between diet and IBD revolves around three key avenues.45 Firstly, the dietary choices we make can significantly impact the metabolic processes and overall composition of our intestinal microbiota. A diet rich in fibre, for instance, can promote the growth of beneficial bacteria, fostering a harmonious balance in the gut ecosystem. This, in turn, indirectly affects the immune system within our intestines, as a healthy microbiota is known to support a balanced immune response, which is crucial in IBD management.46
Secondly, certain food ingredients possess the ability to directly influence the integrity of the intestinal mucosal barrier. The mucosal barrier acts as the first line of defence in our gut, and when compromised, it can lead to increased permeability, potentially exacerbating IBD symptoms. Conversely, some dietary components may help to fortify this barrier, reducing the likelihood of harmful substances breaching it and triggering an immune response.47
Lastly, specific dietary components can directly impact the gut’s immune response. By consuming foods that favourably modulate the immune system, individuals with IBD can potentially reduce inflammation and alleviate symptoms. For example, omega-3 fatty acids found in certain fish have been associated with anti-inflammatory effects, which can be beneficial in managing IBD-related inflammation.48
The complex connection between nutrition and inflammatory bowel disease (IBD) highlights the critical role of careful dietary decisions in managing the condition. By choosing foods that encourage a healthy gut microbiome, strengthen the mucosal barrier, and positively affect the immune system, individuals can significantly contribute to minimizing the effects of IBD on their everyday life.49
Autophagy in Intestinal Homeostasis
Autophagy is a crucial cellular mechanism found in all eukaryotic organisms, playing a vital role in maintaining normal physiological functions. This process becomes particularly important in various situations, such as starvation, limited availability of growth factors, and increased energy demands.50 During these conditions, autophagy is activated to generate energy and support essential metabolic activities. Additionally, autophagy helps cells adapt to a wide range of stressors, including low oxygen levels (hypoxia), infections, the stress in the endoplasmic reticulum, tissue remodelling, the clearance of cellular waste, the removal of damaged organelles, tumour suppression, immune responses, and even in the regulation of cell death. Therefore, autophagy is not only crucial in situations of nutrient deprivation but also in managing diverse cellular stresses.51,52
At the most basic level, autophagy serves as a cleaning mechanism in all cells. This baseline autophagy is essential for preserving cellular homeostasis because it is in charge of regular cell turnover. Aggregates of polyubiquitinated proteins that arise with age, illness, and stress are partially eliminated via autophagy.53 It also plays a role in the formation of lymphocytes and antigen presentation, as well as the breakdown of invasive pathogens and the regulation of pro-inflammatory cytokines released by pathogens. Moreover, autophagy plays a critical role in bolstering antimicrobial defences, enhancing mucosal immune responses, and preserving the integrity of the intestinal barrier.54
Autophagy can cause cell death even though it is predominantly a survival process; this can happen independently of apoptosis or in conjunction with it. The various functions of autophagy in controlling inflammation and homeostasis are especially important when considering the intestinal mucosa. Because the intestinal mucosa is so close to the gut lumen, it is constantly in contact with food particles and gut bacteria.55 The mucosa’s resident immune cells and epithelial cells are essential for protecting against these antigens. Autophagy dysregulation has been linked to the emergence of multiple illnesses, such as IBD.
Inflammatory bowel disease (IBD) primarily includes two chronic conditions: ulcerative colitis and Crohn’s disease. Despite extensive research, the exact mechanisms driving IBD remain unclear.56 The disease’s onset and progression are influenced by multiple factors, including the host’s immune response, the composition of gut microbiota, genetic predispositions, and environmental influences. Genome-wide association studies (GWAS) have identified a link between genetic polymorphisms in autophagy-related genes and the development of IBD. Therefore, deepening our understanding of autophagy’s role in maintaining intestinal homeostasis and its contribution to the onset of inflammation is crucial for advancing new prevention and treatment strategies for inflammatory bowel diseases.57
ATG16L1 is essential for the formation of the autophagosome. It works in conjugation with other autophagy proteins like ATG5 and ATG12 to initiate the elongation of the autophagosome membrane, which engulfs cellular components for degradation. One of the most studied genetic variants of ATG16L1 is the T300A polymorphism, where threonine at position 300 is replaced by alanine. This variant is associated with an increased risk of CD.58 The T300a variant leads to a functional impairment in autophagy by making the ATG16L1 protein more susceptible to caspase-3-mediated cleavage during cellular stress.
IRGM (Immunity related GTPase M) is another important gene involved in autophagy, particularly in the regulation of autophagosome formation and the control of intracellular pathogens. IRGM plays a role in the clearance of bacteria such as Mycobacterium tuberculosis and adherent-invasive Escherichia coli, both of which have been implicated in the pathology of CD. Genetic variants in the IRGM gene have been associated with an increased risk of IBD. A common single nucleotide polymorphism (SNPs), rs13361189, is associated with altered IRGM expression levels,59 which may lead to defective bacterial clearance and increased susceptibility to infection-driven inflammation in the gut.
It seeks to give readers a current grasp of autophagy mechanisms and how important they are for preserving intestinal homeostasis and controlling inflammation. It will explore how autophagy plays a role in the inflammatory response in both human and animal models. Additionally, it examines the recently discovered relationship between autophagy and the gut microbiota, emphasising the importance of this relationship about gut inflammation and its possible consequences for future treatment strategies.60
The three main intracellular pathways of autophagy - macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) are essential processes in eukaryotic cells. Although they all result in lysosomal degradation, they do so through somewhat different ways.60
The process of macroautophagy entails the creation of double membrane-bound structures known as autophagosomes, which contain organelles and cytoplasmic components. Eventually, these autophagosomes combine with lysosomes to undergo internal degradation.8 On the other hand, microautophagy plays a function in the preservation of organelles and membranes as well as cell survival under famine by directly engulfing and breaking down particular cellular targets within lysosomes. Whether microautophagy is a compensatory mechanism or happens concurrently with macroautophagy is still unknown.
The binding of the cytosolic chaperone HSC70 with a particular KFERQ-like pentapeptide motif on target proteins is essential for CMA. Target protein translocation into the lysosomal lumen is facilitated by the oligomerization of lysosome-associated membrane protein 2A (LAMP2A) as a result of this binding.61
Out of the three autophagy routes, macroautophagy has been the subject of the most research and has been connected to a wide range of physiological and pathological intestinal activities. For this review, we will mainly concentrate on macroautophagy and will call it autophagy from here on.62 Because autophagy mechanisms are highly conserved, research on them that started in yeast can also be applied to mammalian cells. The mammalian target of rapamycin complex 1 (mTORC1) and adenosine monophosphate-activated protein kinase (AMPK) are the primary regulators of autophagy.63
Whereas mTORC1 suppresses autophagy, AMPK promotes it. Growth factors and nutrient availability trigger mTORC1, which inhibits autophagy. On the other hand, AMPK is activated in response to famine, growth factor withdrawal, and ER stress, which inhibits mTORC1 and increases autophagy. The phosphorylation of Unc-51 like autophagy activating kinase (ULK1) at several locations is the cause of this conflicting regulation.63
Hypoxia-inducible factor 1 (HIF-1), p53, class I phosphatidylinositol-3-kinase (PI3K), death-associated protein kinase, and p53 are additional upstream regulators in the autophagy system. The production of autophagosomes, which are double-membrane vesicles containing bits of cytoplasm, is a defining feature of autophagy. At the phagophore assembly site (PAS), autophagosome development takes place in three stages: initiation, nucleation, and elongation. Though its precise source is unknown, the ER, Golgi, or plasma membrane are believed to be the sources of the separation membrane that forms the phagophore.64
Atg13, FIP200, Atg101, and ULK1 together form the ULK complex, which starts autophagosome formation at the PAS. Other proteins, such as beclin-1, Atg14, Vps15, and class III PI3K, are recruited by this complex during the nucleation stage and produce phosphatidylinositol-3-phosphate (PI3P) at the PAS. By giving downstream autophagy proteins a platform, PI3P enables the separation membrane to flex and swell.65
At the elongation stage, the Atg12–5 conjugate, which is activated by Atg7 and Atg10, forms a complex with Atg16L1. This complex binds to the isolation membrane and facilitates microtubule-associated protein 1’s lipidation to create LC3-II, which combines phosphatidylethanolamine (PE) and light chain 3 (LC3-I). LC3-II’s hydrophobic properties enable autophagosome closure and integration into the separation membrane which plays an integral part in the stages of Autophagy mechanism (depicted in Figure 2).66
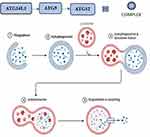 |
Figure 2 Stages of autophagy mechanism. Created with Biorender.com. |
The phosphorylated proteins and organelles are bound by adaptor proteins such as p62, which triggers selective autophagy. Additionally possible is non-selective autophagy. Used in conjunction with LC3-II, these adapters help locate and bring these components into the autophagosome. When two autophagosomes combine with a lysosome in the final stages of autophagy, an autolysosome is produced. Vesicle-associated membrane protein 8 (VAMP8), syntaxin 17, Rab7, and LAMP2 are among the proteins that mediate this fusion.67
When the inner membrane and autolysosome’s contents are broken down by hydrolases and released into the cytoplasm, the autophagy process is complete. It’s critical to keep in mind that recent studies have discovered alternative autophagic pathways, such as the Atg5/Atg7-independent pathway, suggesting that altered autophagy pathways and associated components may result from varying cell types or stress conditions. Table 1 displays the list of targets that can play a pivotal role in the Autophagy mechanism. To fully understand these differences, further research is needed, especially concerning specialised cell types like Paneth, Goblet, and enteroendocrine cells in the intestine.68
 |
Table 1 List of Target Involved in Autophagy in Intestinal Homeostasis |
The main function of the human intestinal system is to absorb nutrients from the food we eat. It is the largest digestive organ in the body. The gut’s inner lining and folds contain specialised cells termed absorptive enterocytes, which are primarily responsible for this absorption.69 But in the last ten years, an increasing amount of study has revealed that the stomach is an essential organ for the immune system. This is because potentially hazardous bacteria in the intestinal lumen continuously expose and challenge the gut.70
The intestinal mucosal barrier is a crucial component of the gut’s defence mechanism and has drawn a lot of interest lately. This barrier is essential for preserving the harmony of the gut microbiota and guaranteeing the harmonious coexistence of our bodies. We shall examine the elements, roles, and relationships of the intestinal mucosal barrier in the ensuing sections, with a focus on intestinal disorders.71
Research has shown that the intestinal mucosal barrier is composed of three primary layers. The outermost layer, known as the mucus layer, is made up of different proteins, carbohydrates, and lipids and coats the mucosal surface in a protective gel-like layer. Its main purpose is to keep the gut bacteria and the underlying epithelial cells from coming into direct touch.72
The intestinal mucosal barrier’s second layer consists of various epithelial cell types, including absorptive enterocytes, Paneth cells, goblet cells, microfold (M) cells, enteroendocrine cells, and tuft cells. Each of these cells plays a crucial role in maintaining gut health. Absorptive enterocytes are responsible for nutrient uptake, while Paneth cells contribute to antimicrobial defence. Goblet cells secrete mucus, aiding in protection and lubrication. Microfold cells facilitate antigen sampling, enteroendocrine cells release hormones that regulate digestion and gut function, and tuft cells are involved in immune responses and sensing. Together, these cells ensure proper nutrient absorption, mucosal protection, and overall gut health.73
The third layer, which is found in the submucosa, is made up of the immune system and inflammatory cells such as lymphocytes, neutrophils, and macrophages. Through actions like endocytosis, antigen presentation, and cytokine release, these cells can start self-defensive reactions that aid the body in warding off invasive invaders.74 However, an over-activation of self-defensive mechanisms can happen when the intestinal mucosal barrier is compromised and the balance of gut microbiota is upset. This may result in intestinal illnesses, such as inflammatory bowel disease, by causing problems such as oxidative stress, the production of inflammasomes, and other harmful reactions.75 Thus, maintaining the proper balance of gut microbiota and managing overactive self-defensive inflammatory and immunological responses become essential therapy approaches for inflammatory bowel disease.76
Recent years have seen a major increase in the amount of attention focused on autophagy, a vital metabolic process that breaks down and recycles long-lived or misfolded proteins and organelles under stressful situations. It has long been known that autophagy plays a role in the aetiology and development of several diseases, including IBD.77 Research on IBD has shown that autophagy is essential for controlling the onset and course of the disease by modulating the immune system and inflammation. According to recent research, autophagy targeting shows promise as an IBD treatment approach. Our goal in doing this review is to present a current analysis of the research on autophagy and IBD, illuminating the function of autophagy in the management of this illness.78
Ulcerative colitis (UC) is a persistent chronic illness affecting the entire length of the colon and rectum. Although its exact cause remains unclear, UC is associated with immune system irregularities, environmental influences, disruptions in gut microbiota, external infections, and specific genetic mutations. Typically, UC starts in the rectum and progresses to the distal colon, potentially leading to widespread inflammation throughout the large intestine. This progression can result in severe complications such as toxic megacolon, malnutrition, and even colorectal cancer.79 Recent studies have extensively explored the role of autophagy in UC. It has been found that Activating Transcription Factor 4 (ATF4), a critical protein involved in autophagy, is significantly reduced in the inflamed intestinal mucosa of patients with active UC compared to normal mucosa. These findings suggest that a reduction in autophagy may contribute to the development of UC.80
Recent research indicates that activating the intestinal nuclear receptor vitamin D receptor (VDR) might reduce intestinal inflammation by promoting autophagy and inhibiting inflammasome activation. Studies have also revealed that patients with ulcerative colitis (UC) exhibit low levels of VDR expression and diminished vitamin D/VDR signalling. Additionally, it has been observed that human intestinal epithelial cells from individuals with active UC lack mTOR-dependent autophagy flux.81 These observations suggest a link between UC and baseline deficiencies in autophagy. Furthermore, during episodes of intestinal inflammation, certain bacteria, such as adherent-invasive Escherichia coli, are capable of adhering to intestinal epithelial cells and evading autophagic elimination by being engulfed by macrophages.82
A type of localised or regional enteritis, CD is linked to pathogenetic variables such as weakened immunity, stress in the environment, heredity, and specific gene alterations. Its aetiology is unknown. CD frequently begins in the area of perineal blindness and progresses throughout the colon and distal ileum, sometimes accompanied by extraintestinal problems. Genetic variables seem to have a stronger influence on CD than they do on UC.83
In a recent European twin study, the proband concordance rates for monozygotic twins were 6.3% for UC and 58.3% for CD. Notably, among dizygotic twins, the concordance for CD dropped to 3.9% with no discernible effect on UC.84 Mutations in important autophagy-related genes are strongly correlated with CD pathophysiology, as revealed by genome-wide association studies (GWAS) in 2007. Among these were important autophagy-related proteins, such as ATG16L1, and immunity-related GTPase M (IRGM).85
Studies have shown that differences in genes related to autophagy, including IRGM, ULK-1, and XBP-1, impact the ability of macrophages to manage the proliferation of adherent-invasive E. coli, which is linked to Crohn’s Disease (CD). Specifically, patients with Crohn’s Disease carrying the IRGM mutation exhibited significantly better survival rates of adherent-invasive E. coli compared to both healthy controls and patients with ulcerative colitis (UC) (p = 0.05). Conversely, individuals with CD-associated polymorphisms in ULK-1 and XBP-1 experienced lower survival rates of these bacteria (p = 0.033 and p = 0.026, respectively).86,87
ATG16L1 single nucleotide polymorphisms (SNPs) such as rs2241880 and rs6754677 have also been found in CD patients and have been connected to the beginning of the disease. It has been determined that NOD2 is essential for bringing ATG16L1 to the cell membrane, which triggers autophagy (shown in Figure 3).88 Disturbances in the intestinal microbiota’s balance and overindulgence in gut inflammatory responses have been linked to mutations in the nucleotide-binding oligomerisation domain-containing protein 2 (NOD2) in CD patients.89 Beyond NOD2, a review examined the connection between the mutation or deletion of several autophagy-related genes and the pathogenesis of inflammatory bowel disease (IBD), namely CD. Examples include leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2), ATG5, transcription factor 4 (TCF4), and ATG5 in Paneth cells.90
 |
Figure 3 Detailed Autophagy Pathway (adapted from KEGG database).91 ATG16 is a key gene associated with autophagy, specifically involved in autophagosome formation. Single nucleotide polymorphisms (SNPs), specifically rs2241880 and rs6754677 in the ATG16 gene, have been identified in patients with Crohn’s disease. These genetic variations are linked to the onset of CD, indicating a genetic predisposition to altered autophagic processes. The presence of mutations, particularly in the interaction between ATG16 and other autophagy-related genes, can hinder the efficient clearance of CD-associated bacteria from epithelial cells. |
The study showed that mutations in genes related to autophagy can prevent CD-associated bacteria from being removed from epithelial cells. This is especially the case when these mutations interact with ATG16L1, which prevents autophagosome formation and the autophagy-dependent removal of intracellular bacteria. In conclusion, new research has shed light on the function of autophagy in the onset and advancement of inflammatory bowel disease.92
Intersection of Gut Microbiota, Autophagy, and IBD
The human stomach’s commensal microbiota is a distinct ecosystem that has coevolved in symbiosis with humans. This microbial community is primarily composed of bacteria, but there are additional colonies of fungi, viruses, archaea, and protozoa.93 The host gives its microbial residents sustenance and a favourable environment. In exchange, the gut microbiota plays a multitude of roles in nutrition metabolism, immune system modulation, and preventing colonisation by enteric pathogens, all of which promote the host’s overall homeostasis. For example, the synthesis of some vitamins, such as K and B-group vitamins, and the fermentation of carbohydrates and dietary fibres to generate short-chain fatty acids (SCFAs), depend on the gut microbiota.
The organs receive energy from these fermentation byproducts, which also control several cellular functions such as inflammation, mucus production, and intestinal barrier integrity.94,95 Gut bacteria and the products they generate play a function in the physiological regulation of the gut mucosa and organs that are far from it through their interactions with the host.8,96,97 Thus, to maintain the host’s health throughout life, the mutualistic relationship between the gut microbiota and the host needs to be maintained.98
Intestinal homeostasis is critically dependent on the gut flora. A healthy and varied microbial population supports both pathogen defence and the immune system’s optimal operation. IBD onset and progression have been linked to dysbiosis, an imbalance in the gut microbiota’s composition. When beneficial bacteria are reduced and dangerous bacteria proliferate, dysbiosis can result, which can inflame the gut and cause an aberrant immunological response.24
Dysbiosis can also result from immune system dysfunction and abnormalities in host metabolism, which can alter the symbiotic equilibrium between the gut microbiota and the host, in addition to environmental stressors.99,100 Within the cell, a multistep intracellular process known as macroautophagy/autophagy, which eats some of the cytoplasm, is started by the formation of a phagophore, a membranous cup-shaped structure. The autophagosome is a closed, double-membrane vacuole that forms when the phagophore lengthens and eventually closes. Its maturation is completed when it fuses with lysosomes.101–103 Autophagy was first defined as a lysosomal catabolic process that takes place during a famine and breaks down and recycles cytoplasmic macromolecules to assist the cell in regaining its energy balance and biosynthesizing necessary components.104
The function of intestinal stem cells and secretory cells is regulated by autophagy.9 Among other things, autophagy is necessary for the survival and growth of neurons in the central nervous system.105 The crucial role that autophagy plays in preserving homeostasis and, consequently, health status is supported by the observed embryonic or neonatal lethality of mice lacking for the majority of autophagy-related core genes, as well as the link between autophagy defects and a variety of diseases and disorders.106,107
In the context of IBD, the reciprocal effects of gut microbiota and autophagy form a dynamic interplay that greatly determines disease development. One essential mechanism for preserving cellular homeostasis is autophagy, a cellular activity.108 It entails the breakdown and recycling of intracellular pathogens and damaged organelles, among other cellular constituents. Within the intestinal environment, autophagy is essential for getting rid of invasive microbes and helps control the immune system.109
Conversely, the variety and structure of the gut microbiota directly affect autophagic activity. The best autophagy is linked to a broad and well-balanced microbial community, which supports gut health maintenance and pathogen removal.108 This delicate equilibrium can be upset by dysbiosis, which is characterised by an imbalance in the composition of gut microbes. Different changes in the microbiota under dysbiotic circumstances might either stimulate or hinder autophagy.107
Because of the complex relationship between autophagy and gut microbiota, dysregulated autophagy may exacerbate the pathophysiology of IBD. Intestinal cells’ capacity to efficiently eliminate intracellular pathogens is reduced when autophagy is compromised, which may contribute to the persistence of dangerous microbes. This persistence fosters an environment that is favourable to chronic inflammation, along with impaired clearance of cellular debris.110–112
On the other hand, autophagic mechanisms may be impacted by dysbiosis-induced changes in the makeup of the gut microbiota. Autophagy may be influenced by the presence of pathogenic bacteria or the reduction of beneficial ones, which could further tilt the scales in favour of a pro-inflammatory state in the gut. This reciprocal link highlights how intricate the interaction between autophagy and the gut flora is when it comes to IBD.113–115
Understanding the reciprocal effects between gut microbiota and autophagy is essential for grasping the molecular mechanisms that drive the progression of inflammatory bowel disease (IBD). Investigating how disruptions in gut microbiota composition or autophagy can exacerbate inflammation provides valuable insights for developing potential treatment strategies. By delving into the intricate interactions between autophagy and gut microbiota, new therapeutic methods for managing and treating IBD could emerge.116–118
Interrelation Between IBD and Urinary System
The interrelation between IBD and the urinary system is a reflection of the interconnectedness of the gut and urinary tract, often referred to as the gut-urinary axis. This relationship is complex and involves several key mechanisms.119 Both the gut and the urinary tract share common immune responses. In IBD, systemic inflammation can lead to immune dysregulation resulting in conditions such as urinary tract infections. Also, patients with IBD may experience secondary urinary symptoms including dysuria, frequency, and urgency, as a result of pelvic inflammation or irritation of the bladder.120 Chronic inflammation in IBD can lead to conditions like nephrolithiasis and other renal complications.121 Understanding this interrelation is important for managing IBD, as addressing urinary symptoms and complications can significantly improve the quality of life for patients.
Therapeutic Implications and Future Perspectives
Defective autophagy, as this review discusses, may significantly influence the development of IBD by upsetting intestinal homeostasis, altering the composition of the gut microbiota, reducing the removal of intracellular microorganisms and increasing intestinal irritation. Therefore, attempts have been undertaken to create autophagy-based IBD therapies.122
Therefore, methods for directly modulating autophagy without causing harm should be discovered to create an effective medication for the treatment of IBD. It’s interesting to note that several substances included in food, such as curcumin and vitamin D, have been demonstrated to promote autophagy.123,124 IBD pathophysiology is significantly influenced by vitamin D insufficiency, and recent research has demonstrated that vitamin D3 therapy of IECs increases autophagy activation.125
This implied that supplementing with vitamin D may help IBD patients with their shortage and raise their autophagy levels, both of which would be advantageous. Similarly, it has been shown that glutamine limits stress-induced cellular death by modulating the MTOR and MAPK/p38 pathways, which increases autophagy in IECs both in basal and stress-induced conditions.126 Gene therapy is another possible treatment approach for IBD that may help but not cure it. More than one miRNA has been claimed to prevent the expression of genes linked with autophagy, hence suppressing autophagy.127
Currently, anti-inflammatory agents (such as mesalamine, olsalazine), anti-TNF agents (such as Infliximab, Adalimumab, Golimumab), anti-integrin agents (such as Vedolizumab, Natalizumab), Anti-IL-12/23 agents (such as Ustekinumab, Risankizumab), JAK inhibitors (such as Tofacitinib, Filgotinib, Upadacitinib) are widely used for the management of IBD.128 Emerging therapies such as Fecal Microbiota Transplantation, Specific probiotics, Stem cell therapy are being explored to reduce inflammation and promote gut healing as strategic options for treating IBD.129–131 Ongoing clinical trials are evaluating the efficacy and safety of drugs including Tofacitinib, Tilpisertib fosmecarbil for the treatment of IBD.132
More effective and individualized therapeutic strategies for patients with inflammatory bowel disease may be developed by taking into account the intricate relationships among gut bacteria, autophagy, and the immune system.133,134
Treatments Based on Microbiota
Potential treatment approaches include faecal microbiota transplantation (FMT), probiotics, and prebiotics that alter the gut microbiota. Restoring a balanced population of helpful bacteria may be able to reduce the dysbiosis linked to inflammatory bowel disease.135
Interventions in Nutrition
Diets that are high in fibre and designed to support a balanced gut microbiota may be taken into consideration. Dietary changes may have an impact on the course of IBD because nutrition is a major factor in determining the makeup of the gut flora.136
Modulation of Autophagy
Creating methods to improve autophagy function could be a goal for treatment. This can entail finding tiny compounds or medications that trigger autophagy pathways to preserve cellular homeostasis and reduce inflammatory reactions.137,138
Genetic Methods and Precision Medicine
Given that autophagy-related gene polymorphisms in IBD have a genetic component, tailored treatment strategies based on unique genetic profiles may be investigated. One potential application of precision medicine is to target particular genetic alterations linked to autophagy malfunction.139
Immunomodulatory Medication
Treatments intended to control the immune system, particularly those affecting pathways connected to autophagy, may be studied. Interventions that improve intracellular pathogen clearance and stop the hyperactive immune response associated with IBD may fall under this category.140
Reducing Inflammation
Given the function of autophagy in the regulation of inflammation, it may be advantageous to develop medications that precisely target the inflammatory pathways linked to IBD. The creation of medications that improve autophagy-mediated inflammasome suppression may be necessary to achieve it.141
Vitamin D Administration
Given the connection between autophagy and vitamin D/VDR signalling, vitamin D supplementation may be taken into consideration for individuals with IBD. By stimulating vitamin D receptors, intestinal inflammation may be decreased and autophagy may be regulated.142
Environmental and Lifestyle Interventions
Encouragement of gut-healthy lifestyle choices, like consistent exercise and stress reduction, may support other treatment approaches. The management of IBD may benefit from addressing environmental factors that influence the gut flora.143
Clinical Studies and Investigations
Encouraging and taking part in clinical trials centred on autophagy modulation, microbiota-based therapeutics, and other cutting-edge techniques can yield important information for upcoming therapy possibilities. Finding novel therapeutic targets and improving current approaches require ongoing study.144
Conclusion
To sum up, the intricate and diverse interaction among gut microbiota, autophagy, and IBD holds great significance for comprehending the aetiology and possible treatment approaches. The gut microbiota, which is frequently referred to as the “second brain”, is essential for preserving gut health in general, and dysbiosis has been directly linked to the development and course of IBD. Maintaining intestinal homeostasis requires the conserved cellular process known as autophagy, which also serves as a cellular recycling mechanism. Dysfunctional autophagy has been linked to the pathophysiology of IBD.
As a result, ongoing clinical trials and research centred on modulating autophagy, microbiota-based therapies, and innovative approaches are essential for creating more effective medications and personalized treatments for inflammatory bowel disease (IBD). A deeper understanding of how gut microbiota and autophagy interact with IBD is moving us closer to a future where patients no longer have to suffer from the severe impacts of this complex and challenging condition.
Abbreviations
IBD, Inflammatory Bowel Disease; CD, Crohn’s Disease; UC, Ulcerative Colitis; SCFAs, Short Chain Fatty Acids; AMP, Antimicrobial Peptides; GWAS, Genome-Wide Association Studies; CMA, Chaperone-Mediated Autophagy; HSC70, Heat shock cognate 71-kDa protein; LAMP2A Lysosome-associated membrane protein 2A; mTORC1, mammalian target of rapamycin complex 1; AMPK, adenosine monophosphate-activated protein kinase; ATG16L1, Autophagy-related Gene 16-Like 1; IRGM, Immunity related GTPase M; ULK1, Unc-51 like autophagy activating kinase; HIF-1, Hypoxia-inducible factor 1; PI3K, Phosphatidylinositol-3-kinase; PAS, Phagophore assembly site; ATG, Autophagy-related Protein; PI3P, Phosphatidylinositol-3-phosphate; PE, Phosphatidylethanolamine; LC3-I, Light chain 3; VAMP8, Vesicle-associated membrane protein 8; ATF4, Activating transcription factor 4; VDR, Vitamin D receptor; IRGM, immunity-related GTPase M; XBP-1, X box-binding protein 1 spliced-1; LRRK2, leucine-rich repeat serine/threonine-protein kinase 2; TCF4, Transcription Factor 4; FMT, Faecal Microbiota Transplantation.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through large group Research Project under grant number (RGP2/24/45). We also express our sincere gratitude to the Dean, SRM College of Pharmacy for the support and motivation provided by her throughout the study. All the authors of this manuscript are thankful to their respective Departments/Universities for successful completion of this study. Graphical Abstract was created with BioRender.com.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
2. Dharni K, Singh A, Sharma S, et al. Trends of inflammatory bowel disease from the global burden of disease study (1990–2019). Indian J Gastroenterol. 2024;43(1):188–198. doi:10.1007/s12664-023-01430-z
3. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi:10.1136/gutjnl-2015-309990
4. Sasso JM, Ammar RM, Tenchov R, et al. Gut microbiome–brain alliance: a landscape view into mental and gastrointestinal health and disorders. ACS Chem Neurosci. 2023;14(10):1717–1763. doi:10.1021/acschemneuro.3c00127
5. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi:10.1016/j.cell.2014.03.011
6. Quaglio AEV, Grillo TG, Oliveira De ECS, Stasi Di LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28(30):4053–4060. doi:10.3748/wjg.v28.i30.4053
7. Foerster EG, Mukherjee T, Cabral-Fernandes L, Rocha JDB, Girardin SE, Philpott DJ. How autophagy controls the intestinal epithelial barrier. Autophagy. 2022;18(1):86–103. doi:10.1080/15548627.2021.1909406
8. Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11–42. doi:10.1016/j.cell.2018.09.048
9. Deretic V, Levine B. Autophagy balances inflammation in innate immunity. Autophagy. 2018;14(2):243–251. doi:10.1080/15548627.2017.1402992
10. Keller MD, Torres VJ, Cadwell K. Autophagy and microbial pathogenesis. Cell Death Differ. 2020;27(3):872–886. doi:10.1038/s41418-019-0481-8
11. Zhang M, Sun K, Wu Y, Yang Y, Tso P, Wu Z. Interactions between intestinal microbiota and host immune response in inflammatory bowel disease. Front Immunol. 2017;8:8. doi:10.3389/fimmu.2017.00942
12. Kee BP, Ng JG, Ng CC, Hilmi I, Goh KL, Chua KH. Genetic polymorphisms of ATG16L1 and IRGM genes in Malaysian patients with Crohn’s disease. J Dig Dis. 2020;21(1):29–37. doi:10.1111/1751-2980.12829
13. Rinne P, Taylor AW, Montero-Melendez T. Editorial: melanocortins and melanocortin receptors in the regulation of inflammation: mechanisms and novel therapeutic strategies. Front Immunol. 2023;14. doi:10.3389/fimmu.2023.1226886
14. Gravina AG, Pellegrino R, Durante T, et al. The melanocortin system in inflammatory bowel diseases: insights into its mechanisms and therapeutic potentials. Cells. 2023;12(14):1889. doi:10.3390/cells12141889
15. Iida T, Onodera K, Nakase H. Role of autophagy in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2017;23(11):1944. doi:10.3748/wjg.v23.i11.1944
16. Busnelli M, Manzini S, Chiesa G. The gut microbiota affects host pathophysiology as an endocrine organ: a focus on cardiovascular disease. Nutrients. 2019;12(1):79. doi:10.3390/nu12010079
17. Andhavarapu S, Mubariz F, Arvas M, Bever C, Makar TK. Interplay between ER stress and autophagy: a possible mechanism in multiple sclerosis pathology. Exp Mol Pathol. 2019;108:183–190. doi:10.1016/j.yexmp.2019.04.016
18. Khan I, Ullah N, Zha L, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): cause or Consequence? IBD treatment targeting the gut microbiome. Pathogens. 2019;8(3):126. doi:10.3390/pathogens8030126
19. Cai Z, Wang S, Li J. Treatment of Inflammatory Bowel disease: a comprehensive review. Front Med Lausanne. 2021;8. doi:10.3389/fmed.2021.765474
20. Dowdell AS, Colgan SP. Metabolic host–microbiota interactions in autophagy and the pathogenesis of inflammatory bowel disease (IBD). Pharmaceuticals. 2021;14(8):708. doi:10.3390/ph14080708
21. Celiberto LS, Graef FA, Healey GR, et al. Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology. 2018;155(1):36–52. doi:10.1111/imm.12939
22. Hold GL. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20(5):1192. doi:10.3748/wjg.v20.i5.1192
23. Schaubeck M, Clavel T, Calasan J, et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65(2):225–237. doi:10.1136/gutjnl-2015-309333
24. Mukhopadhya I, Hansen R, Meharg C, et al. The fungal microbiota of de-novo paediatric inflammatory bowel disease. Microbes Infect. 2015;17(4):304–310. doi:10.1016/j.micinf.2014.12.001
25. Lopetuso LR, Ianiro G, Scaldaferri F, Cammarota G, Gasbarrini A. Gut virome and inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(7):1708–1712. doi:10.1097/MIB.0000000000000807
26. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi:10.1053/j.gastro.2014.02.009
27. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi:10.1038/s41586-019-1237-9
28. Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017;179:204–222. doi:10.1016/j.trsl.2016.08.002
29. Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28(S4):9–17. doi:10.1111/jgh.12294
30. Shin Y, Han S, Kwon J, et al. Roles of short-chain fatty acids in inflammatory bowel disease. Nutrients. 2023;15(20):4466. doi:10.3390/nu15204466
31. Allen-Vercoe E, Coburn B. A microbiota-derived metabolite augments cancer immunotherapy responses in mice. Cancer Cell. 2020;38(4):452–453. doi:10.1016/j.ccell.2020.09.005
32. Gravina AG, Pellegrino R, Auletta S, et al. Hericium erinaceus, a medicinal fungus with a centuries-old history: evidence in gastrointestinal diseases. World J Gastroenterol. 2023;29(20):3048–3065. doi:10.3748/wjg.v29.i20.3048
33. Diling C, Xin Y, Chaoqun Z, et al. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget. 2017;8(49):85838–85857. doi:10.18632/oncotarget.20689
34. Ren Y, Geng Y, Du Y, et al. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J Nutr Biochem. 2018;57:67–76. doi:10.1016/j.jnutbio.2018.03.005
35. Gravina AG, Pellegrino R, Palladino G, et al. Hericium erinaceus, in combination with natural flavonoid/alkaloid and B3/B8 vitamins, can improve inflammatory burden in Inflammatory bowel diseases tissue: an ex vivo study. Front Immunol. 2023;14:1215329. doi:10.3389/fimmu.2023.1215329
36. Priori EC, Ratto D, De Luca F, et al. Hericium erinaceus extract exerts beneficial effects on gut-neuroinflammaging-cognitive axis in elderly mice. Biology. 2023;13(1). doi:10.3390/biology13010018
37. Stappenbeck TS, Virgin HW. Accounting for reciprocal host–microbiome interactions in experimental science. Nature. 2016;534(7606):191–199. doi:10.1038/nature18285
38. Torres J, Hu J, Seki A, et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut. 2020;69(1):42–51. doi:10.1136/gutjnl-2018-317855
39. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi:10.1016/j.chom.2014.02.005
40. Qiu P, Ishimoto T, Fu L, Zhang J, Zhang Z, Liu Y. The gut microbiota in inflammatory bowel disease. Front Cell Infect Microbiol. 2022;12. doi:10.3389/fcimb.2022.733992
41. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1–10. doi:10.1007/s12328-017-0813-5
42. Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37(1):47–55. doi:10.1007/s00281-014-0454-4
43. Wang ZK. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol. 2014;20(40):14805. doi:10.3748/wjg.v20.i40.14805
44. Babickova J. Pathological and therapeutic interactions between bacteriophages, microbes and the host in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11321. doi:10.3748/wjg.v21.i40.11321
45. Eom T, Kim YS, Choi CH, Sadowsky MJ, Unno T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J Microbiol. 2018;56(3):189–198. doi:10.1007/s12275-018-8049-8
46. Stecher B, Conway T, Cohen P. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol Spectr. 2015;3(3). doi:10.1128/microbiolspec.MBP-0008-2014
47. Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16(8):461–472. doi:10.1038/nrm4024
48. Hu J, Nie Y, Yan X. The role of autophagy in the gut pathogens clearance and evasion. Curr Protein Pept Sci. 2015;16(7):632–645. doi:10.2174/1389203716666150630134205
49. Martin PK, Cadwell K. Regulation of interferon signaling in response to gut microbes by autophagy. Gut Microbes. 2020;11(1):126–134. doi:10.1080/19490976.2019.1614395
50. Martin PK, Marchiando A, Xu R, et al. Autophagy proteins suppress protective type I interferon signalling in response to the murine gut microbiota. Nat Microbiol. 2018;3(10):1131–1141. doi:10.1038/s41564-018-0229-0
51. Pant A, Yao X, Lavedrine A, et al. Interactions of autophagy and the immune system in health and diseases. Autophagy Reports. 2022;1(1):438–515. doi:10.1080/27694127.2022.2119743
52. Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14(2):207–215. doi:10.1080/15548627.2017.1378838
53. Hurley JH, Young LN. Mechanisms of Autophagy Initiation. Annu Rev Biochem. 2017;86(1):225–244. doi:10.1146/annurev-biochem-061516-044820
54. Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Rev Immunol. 2016;16(11):661–675. doi:10.1038/nri.2016.100
55. Hampe J, Franke A, Rosenstiel P, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39(2):207–211. doi:10.1038/ng1954
56. Ajayi TA, Innes CL, Grimm SA, et al. Crohn’s disease IRGM risk alleles are associated with altered gene expression in human tissues. Am J Physiol Gastrointest Liver Physiol. 2019;316(1):G95–G105. doi:10.1152/ajpgi.00196.2018
57. Chen SL, Li CM, Li W, et al. How autophagy, a potential therapeutic target, regulates intestinal inflammation. Front Immunol. 2023:14. doi:10.3389/fimmu.2023.1087677
58. Rufini S, Ciccacci C, Di fusco D, et al. Autophagy and inflammatory bowel disease: association between variants of the autophagy-related IRGM gene and susceptibility to Crohn’s disease. Digestive Liver Dis. 2015;47(9):744–750. doi:10.1016/j.dld.2015.05.012
59. Kim S, Eun H, Jo EK. Roles of autophagy-related genes in the pathogenesis of inflammatory bowel disease. Cells. 2019;8(1):77. doi:10.3390/cells8010077
60. Parkes M. Evidence from genetics for a role of autophagy and innate immunity in IBD pathogenesis. Dig Dis. 2012;30(4):330–333. doi:10.1159/000338119
61. Nguyen HTT, Lapaquette P, Bringer MA, Darfeuille-Michaud A. Autophagy and Crohn’s Disease. J Innate Immun. 2013;5(5):434–443. doi:10.1159/000345129
62. Blumberg R, Cho J, Lewis J, Wu G. Inflammatory bowel disease: an update on the fundamental biology and clinical management. Gastroenterology. 2011;140(6):1701–1703. doi:10.1053/j.gastro.2011.03.013
63. Murdoch TB, Xu W, Stempak JM, et al. Pattern recognition receptor and autophagy gene variants are associated with development of antimicrobial antibodies in Crohn’s disease. Inflamm Bowel Dis. 2012;18(9):1743–1748. doi:10.1002/ibd.22884
64. Cooney R, Baker J, Brain O, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–97. doi:10.1038/nm.2069
65. Plantinga TS, Crisan TO, Oosting M, et al. Crohn’s disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Gut. 2011;60(9):1229–1235. doi:10.1136/gut.2010.228908
66. Hu W, Chan H, Lu L, et al. Autophagy in intracellular bacterial infection. Semin Cell Dev Biol. 2020;101:41–50. doi:10.1016/j.semcdb.2019.07.014
67. Hooper KM, Barlow PG, Henderson P, Stevens C. Interactions between autophagy and the unfolded protein response: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(4):661–671. doi:10.1093/ibd/izy380
68. Gardet A, Xavier RJ. Common alleles that influence autophagy and the risk for inflammatory bowel disease. Curr Opin Immunol. 2012;24(5):522–529. doi:10.1016/j.coi.2012.08.001
69. Kaser A, Blumberg RS. Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease. Gastroenterology. 2011;140(6):1738–1747.e2. doi:10.1053/j.gastro.2011.02.048
70. Scharl M, Rogler G. Inflammatory bowel disease: dysfunction of autophagy? Dig Dis. 2012;30(Suppl. 3):12–19. doi:10.1159/000342588
71. Wang SL, Shao BZ, Zhao SB, et al. Impact of paneth cell autophagy on inflammatory bowel disease. Front Immunol. 2018:9. doi:10.3389/fimmu.2018.00693
72. El-Khider F, McDonald C. Links of autophagy dysfunction to inflammatory bowel disease onset. Dig Dis. 2016;34(1–2):27–34. doi:10.1159/000442921
73. Iida T, Yokoyama Y, Wagatsuma K, Hirayama D, Nakase H. Impact of autophagy of innate immune cells on inflammatory bowel disease. Cells. 2018;8(1):7. doi:10.3390/cells8010007
74. Suh HW, Kim JK, Kim TS, Jo EK. New insights into vitamin D and autophagy in inflammatory bowel diseases. Curr Med Chem. 2017;24(9):898–910. doi:10.2174/0929867323666161202151856
75. Jones SA, Mills KHG, Harris J. Autophagy and inflammatory diseases. Immunol Cell Biol. 2013;91(3):250–258. doi:10.1038/icb.2012.82
76. Hosomi S, Kaser A, Blumberg RS. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 2015;31(1):81–88. doi:10.1097/MOG.0000000000000144
77. Huang S, Li F, Jin D. Research updates: the mechanism of autophagy in inflammatory bowel disease. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022;38(6):559–564.
78. Fiocchi C. Current perspectives in inflammatory bowel disease: stress response and autophagy, host–microbe mutualism, immune duality and plasticity, and early versus late disease. Curr Opin Gastroenterol. 2010;26(4):299–301. doi:10.1097/MOG.0b013e32833c114d
79. Lassen KG, Xavier RJ. Genetic control of autophagy underlies pathogenesis of inflammatory bowel disease. Mucosal Immunol. 2017;10(3):589–597. doi:10.1038/mi.2017.18
80. Lu XC, Tao Y, Wu C, et al. Association between variants of the autophagy related gene – IRGM and susceptibility to crohn’s disease and ulcerative colitis: a meta-analysis. PLoS One. 2013;8(11):e80602. doi:10.1371/journal.pone.0080602
81. Palomino-Morales RJ, Oliver J, Gómez-García M, et al. Association of ATG16L1 and IRGM genes polymorphisms with inflammatory bowel disease: a meta-analysis approach. Genes Immun. 2009;10(4):356–364. doi:10.1038/gene.2009.25
82. Teimoori-Toolabi L, Samadpoor S, Mehrtash A, Ghadir M, Vahedi H. Among autophagy genes, ATG16L1 but not IRGM is associated with Crohn’s disease in Iranians. Gene. 2018;675:176–184. doi:10.1016/j.gene.2018.06.074
83. Brain O, Cooney R, Simmons A, Jewell D. Functional consequences of mutations in the autophagy genes in the pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2012;18(4):778–781. doi:10.1002/ibd.21832
84. Grant SF, Baldassano RN, Hakonarson H. Classification of genetic profiles of Crohn’s disease: a focus on the ATG16L1 gene. Expert Rev Mol Diagn. 2008;8(2):199–207. doi:10.1586/14737159.8.2.199
85. Hooper KM, Barlow PG, Stevens C, Henderson P. Inflammatory bowel disease drugs: a focus on autophagy. J Crohn's Colitis. 2017;11(1):118–127. doi:10.1093/ecco-jcc/jjw127
86. Alula KM, Theiss AL. Autophagy in crohn’s disease: converging on dysfunctional innate immunity. Cells. 2023;12(13):1779. doi:10.3390/cells12131779
87. Kabi A, Nickerson KP, Homer CR, McDonald C. Digesting the genetics of inflammatory bowel disease: insights from studies of autophagy risk genes. Inflamm Bowel Dis. 2012;18(4):782–792. doi:10.1002/ibd.21868
88. Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi:10.1093/nar/28.1.27
89. Jin M, Zhang Y. Autophagy and Inflammatory Diseases. Int J Cell Biol. 2020;391–400. doi:10.1007/978-981-15-4272-5_26
90. Matijašić M, Meštrović T, Čipčić Paljetak H, Perić M, Barešić A, Verbanac D. Gut microbiota beyond bacteria—mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int J Mol Sci. 2020;21(8):2668. doi:10.3390/ijms21082668
91. Cury DB, Moss AC, Schor N. Nephrolithiasis in patients with inflammatory bowel disease in the community. Int J Nephrol Renovasc Dis. 2013;6:139–142. doi:10.2147/IJNRD.S45466
92. Blaak EE, Canfora EE, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11(5):411–455. doi:10.3920/BM2020.0057
93. LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. doi:10.1016/j.copbio.2012.08.005
94. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22(10):1079–1089. doi:10.1038/nm.4185
95. Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–255. doi:10.1038/s41579-020-00460-0
96. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi:10.1056/NEJMra1600266
97. Al Nabhani Z, Lepage P, Mauny P, et al. Nod2 deficiency leads to a specific and transmissible mucosa-associated microbial dysbiosis which is independent of the mucosal barrier defect. J Crohn's Colitis. 2016;10(12):1428–1436. doi:10.1093/ecco-jcc/jjw095
98. Yang M, Liu Y, Xie H, et al. Gut microbiota composition and structure of the Ob/Ob and Db/Db Mice. Int J Endocrinol. 2019;2019:1–9. doi:10.1155/2019/1394097
99. Galluzzi L, Baehrecke EH, Ballabio A, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36(13):1811–1836. doi:10.15252/embj.201796697
100. Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15(7):713–720. doi:10.1038/ncb2788
101. Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22(11):733–750. doi:10.1038/s41580-021-00392-4
102. Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. doi:10.1038/cr.2013.169
103. Nikoletopoulou V, Papandreou ME, Tavernarakis N. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ. 2015;22(3):398–407. doi:10.1038/cdd.2014.204
104. Kuma A, Komatsu M, Mizushima N. Autophagy-monitoring and autophagy-deficient mice. Autophagy. 2017;13(10):1619–1628. doi:10.1080/15548627.2017.1343770
105. Lavoie S, Conway KL, Lassen KG, et al. The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife. 2019;8. doi:10.7554/eLife.39982
106. Tsuboi K, Nishitani M, Takakura A, Imai Y, Komatsu M, Kawashima H. Autophagy protects against colitis by the maintenance of normal gut microflora and secretion of mucus. J Biol Chem. 2015;290(33):20511–20526. doi:10.1074/jbc.M114.632257
107. Yang L, Liu C, Zhao W, et al. Impaired autophagy in intestinal epithelial cells alters gut microbiota and host immune responses. Appl Environ Microbiol. 2018;84(18). doi:10.1128/AEM.00880-18
108. Bretin A, Lucas C, Larabi A, et al. AIEC infection triggers modification of gut microbiota composition in genetically predisposed mice, contributing to intestinal inflammation. Sci Rep. 2018;8(1):12301. doi:10.1038/s41598-018-30055-y
109. Bretin A, Carrière J, Dalmasso G, et al. Activation of the EIF2AK4-EIF2A/eIF2α-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection. Autophagy. 2016;12(5):770–783. doi:10.1080/15548627.2016.1156823
110. Palm NW, de Zoete MR, Cullen TW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–1010. doi:10.1016/j.cell.2014.08.006
111. Zhang H, Zheng L, McGovern DPB, et al. Myeloid ATG16L1 facilitates host–bacteria interactions in maintaining intestinal homeostasis. J Immunol. 2017;198(5):2133–2146. doi:10.4049/jimmunol.1601293
112. Cheng S, Ma X, Geng S, et al. Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates gut barrier injury. mSystems. 2018;3(5). doi:10.1128/mSystems.00137-18
113. Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. doi:10.1016/j.cmet.2011.02.018
114. Silwal P, Kim YS, Basu J, Jo EK. The roles of microRNAs in regulation of autophagy during bacterial infection. Semin Cell Dev Biol. 2020;101:51–58. doi:10.1016/j.semcdb.2019.07.011
115. Zheng L, Wei F, Li G. The crosstalk between bacteria and host autophagy: host defense or bacteria offense. J Microbiol. 2022;60(5):451–460. doi:10.1007/s12275-022-2009-z
116. Casanova JE. Bacterial autophagy: offense and defense at the host–pathogen interface. Cell Mol Gastroenterol Hepatol. 2017;4(2):237–243. doi:10.1016/j.jcmgh.2017.05.002
117. Evenepoel P, Poesen R, Meijers B. The gut-kidney axis. Pediatr Nephrol. 2017;32(11):2005–2014. doi:10.1007/s00467-016-3527-x
118. Romano L, Pellegrino R, Arcaniolo D, et al. Lower urinary tract symptoms in patients with inflammatory bowel diseases: a cross-sectional observational study. Dig Liver Dis. 2024;56(4):628–634. doi:10.1016/j.dld.2023.10.010
119. Aronson JK, Ferner RE. Biomarkers—A General Review. Curr Protoc Pharmacol. 2017;76(1). doi:10.1002/cpph.19
120. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of crohn’s disease 2016: part 1 Diagnosis and Medical Management. J Crohn's Colitis. 2017;11(1):3–25. doi:10.1093/ecco-jcc/jjw168
121. Melmed GY, Elashoff R, Chen GC, et al. Predicting a change in diagnosis from ulcerative colitis to crohn’s disease: a nested, case-control study. Clin Gastroenterol Hepatol. 2007;5(5):602–608. doi:10.1016/j.cgh.2007.02.015
122. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338. doi:10.1038/ajg.2015.233
123. Allen PB, Bonovas S, Danese S, Peyrin-Biroulet L. Evolving primary and secondary endpoints in randomized controlled trials leading to approval of biologics and small molecules in IBD: an historical perspective. Expert Opin Biol Ther. 2020;20(2):151–161. doi:10.1080/14712598.2020.1702020
124. Jiang Y, Jarr K, Layton C, et al. Therapeutic implications of diet in inflammatory bowel disease and related immune-mediated inflammatory diseases. Nutrients. 2021;13(3):890. doi:10.3390/nu13030890
125. Bretto E, Ribaldone DG, Caviglia GP, Saracco GM, Bugianesi E, Frara S. Inflammatory bowel disease: emerging therapies and future treatment strategies. Biomedicines. 2023;11(8):2249. doi:10.3390/biomedicines11082249
126. Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.651415
127. Higashiyama M, Hokari R. New and emerging treatments for inflammatory bowel disease. Digestion. 2023;104(1):74–81. doi:10.1159/000527422
128. Hazel K, O’Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020;11:2040622319899297. doi:10.1177/2040622319899297
129. AlAmeel T, AlMutairdi A, Al-Bawardy B. Emerging therapies for ulcerative colitis: updates from recent clinical trials. Clin Exp Gastroenterol. 2023;16:147–167. doi:10.2147/CEG.S375969
130. Subramanian A, Jahabardeen A, Thamaraikani T, Vellapandian C. More on the interplay between gut microbiota, autophagy, and inflammatory bowel disease is needed. World J Gastroenterol. 2024;30(27):3356–3360. doi:10.3748/wjg.v30.i27.3356
131. Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology. 2021;161(4):1118–1132. doi:10.1053/j.gastro.2021.07.042
132. Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145(1):16–27. doi:10.1016/j.jaci.2019.11.003
133. Boyapati RK, Rossi AG, Satsangi J, Ho GT. Gut mucosal DAMPs in IBD: from mechanisms to therapeutic implications. Mucosal Immunol. 2016;9(3):567–582. doi:10.1038/mi.2016.14
134. Mawdsley JE. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481–1491. doi:10.1136/gut.2005.064261
135. Subramanian A, Tamilanban T, Alsayari A, et al. Trilateral association of autophagy, mTOR and Alzheimer’s disease: potential pathway in the development for Alzheimer’s disease therapy. Front Pharmacol. 2022:13. doi:10.3389/fphar.2022.1094351
136. Khatri V, Kalyanasundaram R. Therapeutic implications of inflammasome in inflammatory bowel disease. THE FASEB Journal. 2021;35(5). doi:10.1096/fj.202002622R
137. Matondo A, Kim SS. Targeted-mitochondria antioxidants therapeutic implications in inflammatory bowel disease. J Drug Target. 2018;26(1):1–8. doi:10.1080/1061186X.2017.1339196
138. Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21(3):147–159. doi:10.1111/1751-2980.12849
139. Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14(5):269–278. doi:10.1038/nrgastro.2016.208
140. Elhag DA, Kumar M, Saadaoui M, et al. Inflammatory bowel disease treatments and predictive biomarkers of therapeutic response. Int J Mol Sci. 2022;23(13):6966. doi:10.3390/ijms23136966
141. Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 2020;17(20):7618. doi:10.3390/ijerph17207618
142. Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi:10.3390/microorganisms7010014
143. Oligschlaeger Y, Yadati T, Houben T, Condello Oliván CM, Shiri-Sverdlov R. Inflammatory bowel disease: a stressed “gut/feeling. Cells. 2019;8(7):659. doi:10.3390/cells8070659
144. Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol. 2016;7. doi:10.3389/fmicb.2016.00455
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

