Back to Journals » Journal of Inflammation Research » Volume 17
Expression and Clinical Significance of Lymphocyte Subpopulations and Peripheral Inflammatory Markers in Glioma
Authors Zhou C, Xu L, Geng M, Hu S
Received 19 April 2024
Accepted for publication 28 October 2024
Published 22 November 2024 Volume 2024:17 Pages 9423—9451
DOI https://doi.org/10.2147/JIR.S474577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Chunxiao Zhou,* Lei Xu,* Mo Geng, Shaoshan Hu
Cancer Center, Department of Neurosurgery, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shaoshan Hu, Department of Neurosurgery, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang Province, 310003, People’s Republic of China, Tel +86 152 6816 6248, Email [email protected]
Purpose: Patients with glioma often fail to achieve satisfactory outcomes despite receiving surgery, radiotherapy, and chemotherapy. Photodynamic therapy (PDT) shows promise in addressing the limitations of traditional treatments. However, the immunological effects of PDT in glioma patients remain underexplored. This study aims to fill this gap by analyzing lymphocyte subpopulations and inflammatory markers in glioma patients undergoing PDT-assisted surgery.
Patients and Methods: To enhance our comprehension of the immunobiology of glioma within a clinical framework, we conducted a retrospective analysis of glioma patients from September 2019 to December 2023. Peripheral blood lymphocyte subpopulations (CD3+, CD19+, CD4+, CD8+, CD4+/CD8+) and hematological inflammatory factors were compared among 18 patients who underwent surgery with PDT, 10 patients treated with surgery alone, and healthy controls. Additionally, lymphocyte subpopulations from 48 healthy individuals and hematology inflammatory factors from 38 healthy controls were regarded as controls.
Results: PDT-assisted surgery resulted in significant alterations in lymphocyte subpopulations and inflammatory markers before and after treatment, particularly in CD4+ and CD8+ T cells. PDT-treated patients demonstrated a superior therapeutic response compared to surgery alone (P=0.035). Notably, primary glioma patients had more prolonged overall survival than recurrent glioma patients (P=0.039).
Conclusion: PDT-assisted surgery significantly affects lymphocyte subpopulations and inflammatory markers, enhancing immune response in glioma patients. These findings support the use of PDT as an effective adjuvant therapy. Monitoring lymphocyte subpopulations and inflammatory markers may be valuable for glioma prognosis and treatment optimization.
Keywords: glioma, lymphocyte subpopulations, inflammatory markers, photodynamic therapy
Introduction
Glioma is a type of tumor that originates from brain and spinal glial cells, accounting for 32% of all primary central nervous system malignant tumors and 80% of all malignant tumors.1–4 The lethality rate within the first year of diagnosis is close to 80%.5 According to the 2021 World Health Organization (WHO) “Fifth Central Nervous System Tumors Classification”, grade 1 and 2 gliomas are termed low-grade gliomas, while grades 3 and 4 are categorized as high-grade gliomas. Molecular diagnosis is included in tumor classification, and adult diffuse gliomas are divided into isocitrate dehydrogenase (IDH) wild-type and mutant (with or without 1p/19q-co-deletion) subtypes.6 Glioma carrying mutant IDH has different clinical characteristics from glioma carrying wild-type IDH, and IDH mutations may cause low-grade gliomas to progress to high-grade gliomas.7,8
Presently, glioma treatment involves a multimodal approach, comprising maximal surgical resection combined with postoperative chemoradiotherapy; however, it still exhibits a poor prognosis with a median survival of 12–18 months. Complete eradication of brain tumors is challenging, and even with radiologically complete resection, recurrence is common, posing a significant challenge to current treatment modalities. Photodynamic therapy (PDT) may emerge as a prominent anti-tumor treatment method in the future due to its advantages, such as high safety, precise targeting, low invasiveness, and minimal side effects.9 However, there are limited reports on the comprehensive comparison of different glioma subgroups, particularly regarding the impact of surgical resection supplemented by PDT on the immune system of glioma patients. Our study aims further to elucidate the changes in immunological indices after PDT and assess the safety and efficacy of PDT in treating glioma.
Currently, PDT has been extensively utilized in various types of tumors, including esophageal cancer, melanoma, and multidrug-resistant breast cancer.10–13 As an adjuvant treatment following malignant glioma resection, PDT has proven safe and effective, with reported survival extensions of 73% for patients with anaplastic astrocytoma and 75% for patients with glioblastoma multiforme.14–16 Most complications related to PDT are triggered by tumor resection rather than laser irradiation alone.17 The mechanism of PDT operates by employing designated red light wavelengths to irradiate and activate photosensitizers within tumor tissue, initiating the production of cytotoxic elements like reactive oxygen species, which ultimately results in the death of tumor cells.18 In addition to its direct cytotoxic effects, PDT exerts its antitumor effects primarily through modulation of the immune and inflammatory responses and microvascular destruction. PDT often induces a robust acute inflammatory response, thereby enhancing the immunogenicity of tumor cells and stimulating the body’s anti-tumor immune response.19,20 We found a positive correlation between PDT treatment and treatment efficacy in this study. Previous studies have shown that PDT improves median survival in high-grade gliomas and extends the 2-year survival rate of patients from 18% to 28%.21 An in vitro experiment demonstrated that 5-ALA PDT of GBM spheroids recruited and activated dendritic cells, while an in vivo experiment showed that G422 hGG mice exhibited increased immune cell infiltration and cytokine release following PDT.22,23
Despite its immune privilege, glioma can still induce extensive host immunosuppression.24,25 Effector immune cells, particularly T cells, are pivotal in antitumor responses, capable of crossing the blood-brain barrier and mediating antigen-specific reactions.26 However, glioblastoma multiforme (GBM) patients experience profound systemic and localized immunosuppression.27,28 Additionally, Nseyo et al found that urinary cytokines IL-1β and TNF-α were detectable in bladder cancer patients only after PDT with the highest fluence but not in the control group or the group treated with the lowest fluence.29 Fortunately, immune activation through PDT offers promising therapeutic potential for glioma patients. Many studies suggest that gliomas produce a range of inflammatory mediators and molecules known as damage-associated molecular patterns (DAMPs), which become translocated and upregulated on the cell membrane after PDT. This process activates host immune cells, including leukocytes that infiltrate the tumor site and produce proinflammatory factors and cytokines. Neutrophils rapidly migrate to the treatment site to clear photodamaged tumor cells, while dendritic cells (DCs) and macrophages facilitate migration and phagocytosis.30,31 Retrospective analysis indicates that severe and persistent reductions in CD4+ counts are common among newly diagnosed high-grade glioma (HGG) patients undergoing radiotherapy and temozolomide treatment, correlating with therapy and linked to early death from tumor progression.32 Further investigation reveals a significant downregulation of genes associated with T-cell activation in CD4+ and CD8+ T cells and an upregulation of suppressor genes in immunosuppressive regulatory T (Treg) cells in GBM patients.33 Numerous studies highlight the crucial role of neuroinflammatory responses in glioma development.34,35 Systemic inflammation increases neutrophils and a decrease in lymphocytes, leading to immune cell cytolytic activity suppression and immunosuppression. Lymphopenia reflects impaired cell-mediated immunity, while neutrophilia signifies systemic inflammation with suppressive anticancer activity and T-cell cytotoxicity.36 Platelet-induced angiogenesis and vascular endothelial growth factor secretion contribute to glioma progression in GBM patients, while platelet-derived growth factor contributes to oncogenic transformation and tumorigenesis.37 Platelets and neutrophils promote tumor cell survival, invasion, metastasis, and proliferation.38,39 Tumor-associated macrophages stimulate tumor development and angiogenesis by releasing TNF-α, vascular endothelial growth factor, and epidermal growth factor.40 Conversely, lymphocytes inhibit cancer cell proliferation and migration by inducing cytotoxic cell death.41,42 Reductions in lymphocyte levels weaken the host’s anticancer immunity, resulting in poor cancer prognosis. Additionally, these inflammatory cells secrete or induce inflammatory factors that critically impact symptoms and bodily functions, influencing s cancer patients’ quality of life and prognosis. Novel therapies targeting inflammatory cells and mediators offer promising avenues for advanced cancer treatment.43
Recently, several hematological indices, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), have emerged as potential novel biomarkers of baseline inflammatory processes,44–47 and may serve as excellent predictors for patients with glioma.48–50 Higher PLR and NLR, along with lower LMR, suggest a robust inflammatory response coupled with weakened immune defenses in cancer patients, correlating with diminished quality of life and treatment outcomes. Moreover, given their dynamic nature, NLR, PLR, and LMR promptly reflect host immunity status.51 Importantly, previous studies have indicated that these composite inflammation ratios may possess superior predictive ability compared to traditional inflammatory factors.52 Although lymphocyte subpopulations and hematological inflammatory markers are readily available as routine assays, their investigation in gliomas remains limited. Identifying patterns of expression changes in glioma patients could unveil prognostic factors and novel therapeutic avenues. Consequently, our study aims to elucidate the alterations in expression and clinical significance of hematological inflammatory markers and lymphocyte subpopulations in patients with different glioma types before and after treatment. Additionally, it seeks to assess their prognostic factors to improve guidance on glioma diagnosis and treatment, thus fostering new prognostic perspectives.
Materials and Methods
Patients and Controls
This study enrolled 28 glioma inpatients at Zhejiang Provincial People’s Hospital between September 2019 and December 2023. 18 patients underwent tumor resection followed by PDT using hematoporphyrin (dose 5 mg/kg), while the remaining 10 received only tumor resection. Glioma diagnoses were confirmed through routine and immunohistochemical staining of postoperative biopsies. Following diagnosis, patients underwent standard surgical, radiotherapeutic, and chemotherapeutic treatments as per glioma treatment guidelines. The administration of postoperative PDT was contingent upon clinical evaluations and institutional protocols. Subjects diagnosed with conditions or on medications affecting immune status, such as inflammatory disorders, were excluded. Control data were derived from lymphocyte subpopulation analyses of 48 healthy individuals and routine blood tests from 38 healthy individuals.
Diagnoses of glioma were established through computed tomography (CT), magnetic resonance imaging (MRI), and brain biopsies. Patient medical records were comprehensively reviewed to collect: (1) Demographic information: gender, age, marital status, occupation, and educational attainment; (2) Personal medical history: results from MRIs, CT scans, brain biopsies, tumor grading via blood tests, symptomatology, physical illness history, other cancer diagnoses, and medication use; (3) Family mental health history.
Photosensitizers and Laser Devices
The photosensitizer used was hematoporphyrin injection (Chongqing Mele Biopharmaceutical Co., Ltd)., with a molecular formula of C34H38N4O6 and a molecular weight of 598.70, excitable by visible red light at a wavelength of 630 nm. The treatment laser device (Guoyi Huake Medical Technology Group Co., Ltd.) emitted light at a wavelength of 635±3 nm, utilizing pulse output.
Treatment Strategy
Patients were stratified into three groups based on their treatment regimens: the PDT group, the non-PDT group, and the control group. The PDT group received an intravenous injection of hematoporphyrin 48h before the treatment. Treatment for the PDT group consisted of surgical resection followed by PDT and adjuvant chemotherapy and radiotherapy postoperatively. Hematoporphyrin was administered intravenously at a dosage of 5 mg/kg, followed by measures to protect the patient from sunlight exposure. PDT treatment commenced 48h following the infusion. Segmented irradiation was employed for lesions extending beyond the optical fiber’s reach. The standardized irradiation parameters included a wavelength of 630 nm, power of 1 W, energy density of 150 J/cm2, and an irradiation duration of 0.5h. Patients were advised to avoid direct sunlight or exposure to strong light sources for 1 month post-photosensitizer injection. The non-PDT group comprised patients who underwent surgical resection without subsequent PDT. Other treatments for the non-PDT group mirrored those of the PDT group.
Detection of Peripheral Blood Lymphocyte Subpopulations
Peripheral blood, heparinized to prevent coagulation, was collected for lymphocyte subpopulation analysis immediately before and 48h post-PDT. The following antibodies were utilized for flow cytometry: anti-CD3, anti-CD4, anti-CD8, and anti-CD19, all sourced from BD Biosciences, San Jose, CA, USA. Isotype controls lacking specific reactivity served as negative controls. The cell suspension was incubated at room temperature for 20 minutes. Following red blood cell lysis, the cells were washed and resuspended in 200 μL of phosphate-buffered saline (PBS). Subsequently, cell analysis was performed with a FACSCanto flow cytometer. CD3+ denotes total T cells, CD19+ represents total B cells, CD4+ identifies helper T cells, and CD8+ corresponds to cytotoxic T cells.
Measurement of Blood Cell Count Composite Inflammation Ratios
Blood cell counts were obtained from each participant upon admission, either before PDT treatment or 48h before surgical resection. An automated analyzer (XE-2100, Sysmex, Kobe, Japan) was utilized to analyze the cell counts, which were subsequently used to calculate composite inflammation ratios, namely NLR, PLR, and LMR. The neutrophil-to-lymphocyte ratio (NLR) was determined by dividing the neutrophil count by the lymphocyte count. The platelet-to-lymphocyte ratio (PLR) was computed by dividing the platelet count by the lymphocyte count. The lymphocyte-to-monocyte ratio (LMR) was calculated as the ratio of the lymphocyte count to the monocyte count.
Efficacy Evaluation
Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were evaluated based on data from the 2022 WHO guidelines. Study efficacy evaluation: Responses of CR and PR were categorized as effective treatments, whereas SD and PD were considered ineffective treatments. Outcomes of CR and PR were deemed indicative of effective treatment, while SD and PD were classified as indicative of ineffective treatment.
Survival Analysis
Overall Survival (OS) was defined as the duration from surgical resection until death or the last follow-up. The Kaplan-Meier method was utilized to estimate survival functions, while the Log rank test was employed to evaluate differences in survival outcomes.
Statistical Analysis
Statistical analyses were conducted using SPSS Statistics 26.0 and GraphPad Prism 8.0. Continuous variables were reported as mean ± standard deviation (SD) and median with interquartile range (IQR), whereas categorical variables were presented as counts and percentages (n, %). The Kruskal–Wallis H-test was applied for inter-group comparisons, while the Bonferroni correction was utilized for pairwise comparisons. The non-parametric tests (Wilcoxon signed-rank test and Wilcoxon test) were used to compare paired data. The Pearson chi-square test was employed to compare categorical variables. All p-values were two-sided, with values <0.05 deemed statistically significant and <0.01 considered highly significant.
Results
Demographic and Clinical Characteristics of Glioma Patients
From December 2019 to December 2023, 28 glioma patients were enrolled. Table 1 presents the patients’ demographics and clinical characteristics, such as age, gender, onset of disease, clinical stage, IDH1/2 mutation, and treatment regimen. 18 patients underwent PDT, while 10 received only surgical treatment. All patients, irrespective of their group, were administered systemic therapy. Among the 14 glioma patients with IDH1/2 mutations, 6 received PDT and 8 did not; among the 14 patients without IDH1/2 mutations, 12 received PDT and 2 did not. The median patient age was 54 years. No statistically significant differences were observed in gender or age between the groups (P > 0.05), as detailed in Table 1.
 |
Table 1 The General Information of Patients with Glioma in This Study |
Lymphocyte Subpopulation Analysis
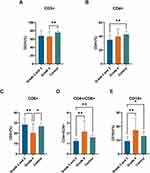
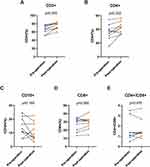
Significant differences in CD3+, CD19+, CD4+, CD8+, and CD4+/CD8+ ratios were observed between the control group and patients with grade 2 and 3 and grade 4 glioma upon enrollment (P < 0.05) (Table 2). Pairwise analyses revealed lower levels of CD3+ in grade 4 glioma patients and CD4+ in grade 2 and 3 glioma patients compared to healthy controls (Figure 1A and B). Grade 4 glioma patients exhibited reduced CD8+ levels relative to grade 2 and 3 glioma patients and healthy controls (Figure 1C). Additionally, CD4+/CD8+ ratios and CD19+ levels in grade 2 and 3 glioma patients were reduced relative to healthy controls (Figure 1D and E). Subsequent analysis focused on the changes in peripheral blood immune cells in grade 2 and 3 and grade 4 glioma patients pre- and post-PDT treatment, as depicted in Tables 3 and Table 4. The systemic immune cell count trends in grade 2 and 3 and grade 4 patients varied before and after PDT treatment. Specifically, no statistical difference was observed in immune cell counts in stage II–III glioma patients pre- and post-PDT treatment (Figure 2A–C). Post-PDT treatment, grade 4 glioma patients demonstrated a significant reduction in CD3+ cells, CD4+ cells, and the CD4+T/CD8+ ratio compared to pre-treatment levels (Figure 2D–F). In addition, when IDH was included in the subgroups, as shown in Table 5 and Table 6, we found that lymphocyte subpopulation did not change significantly regardless of whether grade 2 and 3 glioma patients had IDH1/2 mutations, which may be because lymphocyte subpopulation is not as sensitive to the immune status of glioma patients as hematological inflammatory factors.
 |
Table 2 Comparative Analysis of Lymphocyte Subpopulations Among Grade 2 and 3 and Grade 4 Glioma Patients versus Healthy Controls at Enrollment |
 |
Table 3 Changes in Lymphocyte Subpopulations Levels 48h Before and After PDT Treatment in Grade 2 and 3 Glioma Patients (n=6) [%; Median (Range)] |
 |
Table 4 Changes in Lymphocyte Subpopulations Levels 48h Before and After PDT Treatment in Grade 4 Glioma Patients (n=12) [%; Median (Range)] |
 |
Table 5 Comparative Analysis of Lymphocyte Subpopulations Between IDH1/2 Mutation- and + Glioma Patients in Grade 2 and 3 (n=11) [%; Median (Range)] |
 |
Table 6 Comparative Analysis of Lymphocyte Subpopulations Between IDH1/2 Mutation- and + Glioma Patients in Grade 4 (n=17) [%; Median (Range)] |
Next, we focus on the immune profiles of primary and recurrent gliomas. Analysis revealed statistically significant differences in levels of CD3+, CD19+, CD4+, CD8+, and the CD4+/CD8+ ratio among the primary glioma group, recurrent glioma group, and healthy controls (P < 0.05) (Table 7). Pairwise comparisons indicated that levels of CD3+ and CD4+ in recurrent glioma patients were lower than in healthy controls (Figure 3A and B). Concurrently, CD8+ levels were higher in recurrent glioma patients than in primary glioma patients, without significant differences from healthy controls (Figure 3C). Notably, levels of the CD4+/CD8+ and CD19+ ratio in recurrent glioma patients were lower compared to primary glioma patients yet showed no significant difference from healthy controls (Figure 3D and E). The subsequent analysis examined lymphocyte subpopulation shifts in primary and recurrent glioma patients pre- and post-PDT treatment, detailed in Table 8 and Table 9. Pre- and post-PDT treatment, systemic immune cell counts in primary and recurrent glioma patients demonstrated varying patterns. Specifically, no statistical difference was observed in immune cell counts in primary glioma patients pre- and post-PDT treatment (Figure 4A and B). After PDT treatment, levels of CD4+ and the CD4+/CD8+ ratio in recurrent glioma patients were significantly decreased compared to pre-PDT treatment levels (Figure 4C and D). After incorporating IDH1/2 mutations into this group, we found that lymphocyte subpopulations did not differ significantly between the IDH1/2 mutation group and the non-mutated group in patients with primary and recurrent gliomas (Table 10 and Table 11).
 |
Table 7 Comparative Analysis of Lymphocyte Subpopulations in Primary versus Recurrent Glioma Patients and Healthy Controls at Enrollment |
 |
Table 8 Changes in Lymphocyte Subpopulations Levels 48h Before and After PDT Treatment in Primary Glioma Patients (n=8) [%, M(Range)] |
 |
Table 9 Changes in Lymphocyte Subpopulations Levels 48h Before and After PDT Treatment in Recurrent Glioma Patients (n=10) [%, M(Range)] |
 |
Table 10 Comparative Analysis of Lymphocyte Subpopulations Between IDH1/2 Mutation- and + Primary Glioma Patients (n=10) [%; Median (Range)] |
 |
Table 11 Comparative Analysis of Lymphocyte Subpopulations Between IDH1/2 Mutation- and + Recurrent Glioma Patients (n=18) [%; Median (Range)] |
Following the evaluation of PDT responses in glioma across various groups, we analyzed peripheral blood lymphocyte subpopulations in patients who had undergone surgical resection but not PDT. Other markers showed no significant changes (refer to Table 12 and Figure 5). We hypothesize that adjuvant PDT may enhance immune regulation following surgical resection in glioma patients, potentially leading to a significant accumulation of immune cells at the tumor site.
 |
Table 12 Changes in Lymphocyte Subpopulations Levels 48h Before and After Surgical Resection in Glioma Patients (n=10) [%, M(Range)] |
Hematological Inflammatory Marker Analysis
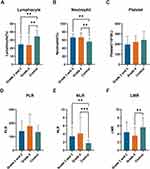
Statistically significant differences were observed in lymphocyte classification, neutrophil percentage, NLR, and LMR levels between the control group and patients with grade 2 and 3 and grade 4 glioma at enrollment (P<0.05) (Table 13). However, no significant differences were found in platelet levels and PLR, as detailed in Table 13 and Figure 6C and D. A study on advanced non-small cell lung cancer (NSCLC) showed that the variation of OS and PFS among NSCLC subtypes was considerable. The increase of PLR in advanced NSCLC patients was not associated with OS and PFS, which explained that PLR was not as sensitive in grade 4 glioma.53 Pairwise comparisons revealed that lymphocyte counts in stage II–IIIgrade 2 and 3 and stage IVgrade 4 glioma patients were significantly lower. In contrast, neutrophil percentage and NLR were considerably higher than those in healthy controls (Figure 6A, B, and E). Additionally, LMR levels were lower in grade 4 glioma patients than in the control group (Figure 6F). Notably, NLR and PLR were higher, and LMR lower, in grade 4 than in grade 2 and 3, although these differences were not statistically significant (Figure 6E and F). Subsequently, we analyzed hematological inflammatory markers before and after PDT treatment in these glioma patients, as presented in Table 14 and Table 15. The results indicated that, except for the nonsignificant differences in platelet levels in patients with grade 2 and 3 glioma before and after PDT treatment, the remaining hematological inflammatory biomarkers showed statistically significant changes (P<0.05). Specifically, lymphocyte classification and LMR exhibited a decreasing trend in patients with grade 2 and 3 and grade 4 glioma following PDT treatment (Figure 7A and F), with a significant decrease in platelet count observed in grade 4 patients (Figure 7C). The decrease in platelet counts for grade 2 and 3, however, did not reach statistical significance. It should be noted that in patients with grade 2 and 3 and grade 4, neutrophil percentage, NLR, and PLR exhibited a significant upward trend following. We found an interesting thing that when IDH was included in the subgroups, as shown in Table 16 and Table 17, we found that grade 2 and 3 glioma patients had no significant changes in inflammatory markers regardless of whether they had IDH1/2 mutations (Table 16 and Figure 8). However, for the grade 4 glioma group, we found that patients with IDH1/2 mutations had lower lymphocyte percentage than those without mutations (Figure 8A), while neutrophil percentage and NLR were higher than those without mutations (Figure 8B and C).
 |
Table 13 Comparative Analysis of Hematological Inflammatory Markers in Grade 2 and 3 and Grade 4 Glioma Patients versus Healthy Controls at Enrollment |
 |
Table 14 Changes in Hematological Inflammatory Markers 48h Before and After PDT in Grade 2 and 3 Glioma Patients (n=6) [%; Median (Range)] |
 |
Table 15 Changes in Hematological Inflammatory Markers 48h Before and After PDT in Grade 4 Glioma Patients (n=12) [%, M(Range)] |
 |
Table 16 Comparative Analysis of Hematological Inflammatory Markers Between IDH1/2 Mutation- and + Glioma Patients in Grade 2 and 3 (n=10) [%; Median (Range)] |
 |
Table 17 Comparative Analysis of Hematological Inflammatory Markers Between IDH1/2 Mutation- and + Glioma Patients in Grade 4 (n=17) [%; Median (Range)] |
Next, we turned our attention to primary and recurrent gliomas. The results revealed statistically significant differences in lymphocyte percentage, neutrophil percentage, NLR, and LMR levels between primary and recurrent glioma groups and healthy controls at enrollment (P<0.05) (Table 18). However, no significant differences were found in platelet levels and PLR, as illustrated in Table 18 and Figure 9C and D. Pairwise comparisons indicated that lymphocyte levels in both primary and recurrent glioma patients were significantly lower than in healthy controls (Figure 9A). In contrast, neutrophil levels and NLR were higher (Figure 9B and E). In addition, the LMR levels in patients with recurrent glioma were lower than those in the control group and lower than those in patients with primary glioma; however, the latter difference was not statistically significant (Figure 9F). Subsequently, we examined the alterations in routine blood and hematological inflammatory markers in patients with primary and recurrent glioma before and after PDT treatment, as detailed in Table 19 and Table 20. Specifically, lymphocyte counts and LMR decreased in primary and recurrent glioma patients after PDT treatment (Figure 10A and F), while neutrophil percentage and NLR significantly increased (Figure 10B and D). Platelet levels and PLR increased in patients with primary glioma 48h post-PDT treatment; however, no significant changes were observed in patients with recurrent glioma (Figure 10C and E). In this study, our data showed that there was no statistical difference in routine blood and hematological inflammatory markers between patients with and without IDH1/2 mutations, whether in primary or recurrent gliomas, as shown in Tables 21 and 22. After assessing the response of PDT across various glioma groups, we investigated alterations in routine blood and hematological inflammatory markers in patients undergoing surgical resection without subsequent PDT. The analysis revealed statistically significant alterations in lymphocyte percentage, neutrophil percentage, and platelet counts, as well as in NLR, PLR, and LMRpre- and post-surgery (P<0.05) (Table 23). Specifically, increases were observed in neutrophil percentage, NLR, and PLR compared to preoperative measurements (Figure 11B, D, and E). At the same time, decreases were noted in lymphocytes, platelets, and LMR (Figure 11A, C and F). The observed changes in hematological biomarkers suggest they may serve as sensitive indicators of systemic inflammatory responses in these patients.
 |
Table 18 Comparative Analysis of Hematological Inflammatory Markers in Primary versus Recurrent Glioma Patients and Healthy Controls at Enrollment |
 |
Table 19 Changes in Hematological Inflammatory Markers Levels 48h Before and After PDT in Primary Glioma Patients (n=8) [%, M(Range)] |
 |
Table 20 Changes in Hematological Inflammatory Markers Before and After PDT in Recurrent Glioma Patients (n=10) [%, M(Range)] |
 |
Table 21 Comparative Analysis of Hematological Inflammatory Markers Between IDH1/2 Mutation- and + Primary Glioma Patients (n=10) [%; Median (Range)] |
 |
Table 22 Comparative Analysis of Hematological Inflammatory Markers Between IDH1/2 Mutation- and + Recurrent Glioma Patients (n=18) [%; Median (Range)] |
 |
Table 23 Changes in Hematological Inflammatory Markers Before and After Surgical Resection in Glioma Patients (n=10) [%, M(Range)] |
Efficacy Evaluation
The efficacy evaluation and potential influencing factors for glioma treatment are shown in Table 24. Following surgery, patients underwent standard chemoradiotherapy and were monitored through clinical and radiological assessments every three months until death. The treatment proved effective for 18 patients (CR+PR, 64.3%), while 18 patients experienced disease progression (SD+PD, 35.7%). As of the last follow-up, 1 patient had died, and 5 had shown disease progression. Of the 18 patients treated with PDT, 8 achieved complete remission (CR), 7 achieved partial response (PR), none maintained stable disease (SD), and 3 exhibited progressive disease (PD). We investigated factors influencing treatment efficacy in glioma patients and found PDT to be a significant determinant (p=0.035, Table 24). A positive correlation exists between PDT treatment and treatment efficacy.
 |
Table 24 Comparison of Various Efficacy Types |
Clinical Prognostic Determinants of Glioma
Survival analysis was conducted across the entire cohort. Univariate survival analysis using the Kaplan-Meier method indicated that the disease onset was a significant prognostic factor (p=0.039, Figure 12). The median follow-up duration for all glioma patients was 19 months. The OS curve for the patients is depicted in Figure 13. The patients’ 34-month OS rate was 70.9%. Kaplan-Meier analysis of OS for both the primary and recurrent glioma groups is presented in Figure 12. Given that some patients still receive treatment and brief follow-up durations, the overall median OS has not yet been established. As other related OS parameters in the univariate analysis did not meet the pre-established significance threshold, multivariate analysis was not undertaken, as indicated in Table 25.
 |
Table 25 Prognostic Factor Analysis |
 |
Figure 12 Kaplan-Meier curve of overall survival of patients with glioma in primary group and recurrent group. |
 |
Figure 13 Kaplan-Meier curves of overall survival for all glioma patients. |
Discussion
The limited efficacy of immunotherapy in treating gliomas underscores the urgent need to enhance our understanding of immunity in central nervous system diseases. This study demonstrates that, compared to controls, patients in the grade 2 and 3 and grade 4 groups and those in the primary and recurrent groups exhibit decreased lymphocyte percentage and increased neutrophil percentage, indicative of systemic inflammation in glioma. Additionally, the CD3+, CD4+, and CD8+ lymphocyte subsets were also reduced in these groups compared to controls. These findings suggest that the glioma patients in this study generally exhibit compromised immune function. In preclinical models of glioma, CD4+ T cell function represents a critical protective mechanism in antitumor immunity, and a reduced frequency of antitumor CD4+ T cells in high-grade glioma (HGG) patients correlates with poor OS.54 Furthermore, CD4+/CD8+ ratio in the blood serves as an indicator of systemic immune activation.55 This study included 10 cases of primary and 18 cases of recurrent glioma. The results indicated that patients with recurrent glioma exhibited a more intense immunosuppressive status than those with primary glioma, as evidenced by significant decreases in CD4+/CD8+, CD19+, and lymphocyte percentage. Some studies suggest increased CD4+ T cells during short-term relapses of recurrent glioma.54 However, this study found no significant changes in CD4+ T cells among patients with primary and recurrent gliomas, potentially attributable to longer relapse periods. The proportion of CD8+ T cells was significantly higher in the recurrent group compared to the primary group. The tumor microenvironment (TME) of gliomas plays a pivotal role in driving immune suppression. Immunosuppressive cells within the glioma microenvironment, such as myeloid-derived suppressor cells and tumor-associated macrophages, further complicate the role of CD8+ T cells.56 These immune cells secrete immunosuppressive cytokines, including IL-10 and TGF-β, which inhibit the proliferation and cytotoxic function of CD8+ T cells. Therefore, the observed increase in CD8+ T cells in recurrent gliomas may reflect a compensatory response rather than effective antitumor activity, as these cells are likely suppressed by the surrounding TME. While our study demonstrates a higher proportion of CD8+ T cells in recurrent glioma patients, this increase does not necessarily translate to improved immune function. Instead, these CD8+ T cells may become exhausted or inhibited by the immunosuppressive components of the TME.57 Our findings are consistent with those of Brennan,58 supporting the notion that CD8+ T cells are crucial for glioma progression.59 Patients with II–III stage glioma generally exhibit good health, normal organ function, and adequate nutritional status. In contrast, IV stage patients often experience partial immune cell depletion due to distant metastases, organ dysfunction, malnutrition, and cachexia. In this study, CD8+T cell levels were lower in grade 4 compared to stages II–III, reflecting a degree of immune suppression in grade 4 glioma patients. NLR, PLR, and LMR are recognized as novel inflammatory biomarkers, with increases in NLR and PLR and decreases in LMR associated with poorer glioma prognosis.49,50 Consistent with these findings, patients in grade 4 exhibited higher NLR and PLR values than those in stages II–III. In contrast, LMR values were lower in grade 4 and recurrent cases than in primary cases, suggesting that preoperative NLR and PLR may serve as useful indicators for predicting glioma grading. IDH mutation is very meaningful in glioma. Compared with wild-type glioma, glioma with IDH1/2 mutation shows a more indolent course and good prognosis; however, these tumors usually relapse. We further subdivided grade 2 and 3 glioma patients according to whether they had IDH1/2 mutation or not. We found that there was no significant change in inflammatory factors in grade 2 and 3 glioma patients regardless of whether they had IDH1/2 mutation or not. However, for grade 4 glioma, the lymphocyte classification of patients with IDH1/2 mutation was lower than that of patients without mutation, while the neutrophil classification and NLR were higher than those of patients without mutation, which just confirmed that IDH mutation can cause immunosuppression and promote tumor progression.60 Glioma is characterized by high recurrence rates and limited efficacy following traditional treatments. PDT may address some of these shortcomings. This study focuses on the changes in immune function before and after PDT. Surprisingly, PDT treatment led to a statistically significant reduction in platelets, CD3+, CD4+, and CD4+/CD8+ in grade 4 patients. This may be due to enhanced local immunity following surgical resection and PDT, which attracts numerous circulating immune cells to the tumor site. However, the bone marrow’s production of new immune cells may not keep pace, leading to decreased blood cell counts. In contrast, no significant changes were observed in lymphocyte subpopulations (CD3+, CD19+, CD4+, CD8+, and CD4+/CD8+) in grade 2 and 3 patients after PDT. Similarly, in more immunosuppressed recurrent glioma patients, percentages of CD4+, CD4+/CD8+, and lymphocytes further decreased after surgical resection with adjuvant PDT. This is likely because limited bone marrow output leads to the recruitment of immune cells to tumor sites, enhancing local immunity and exerting anti-tumor effects. Similarly, in recurrent glioma patients with more immunosuppression, levels of CD4+, CD4+/CD8+, and lymphocytes further decreased after surgical resection with adjuvant PDT. This is likely because limited bone marrow output leads to the recruitment of immune cells to tumor sites, enhancing local immunity and exerting anti-tumor effects. PDT did not significantly alter lymphocyte subsets (CD3+, CD19+, CD4+, CD8+, and CD4+/CD8+) in patients with primary glioma. PDT did not considerably alter lymphocyte subsets (CD3+, CD19+, CD4+, CD8+, and CD4+/CD8+) in primary glioma patients. These findings suggest that grade 4 and recurrent glioma immune function respond more favorably to PDT. Following photooxidative damage induced by PDT, neutrophils are the first to reach the tumor site, directing monocytes/macrophages to invade the irradiated tumor margins to remove damaged and dead cells.61,62 Consistent with these findings, an increase in neutrophils was observed post-PDT across all groups, including stages II–III and IV, as well as primary and recurrent cases. Regarding inflammatory markers, both subgroups displayed similar trends post-PDT: increases in NLR and PLR, and a decrease in LMR. Although some studies have demonstrated that PDT possesses an immunosuppressive effect, this effect is only partial. PDT indeed promotes the induction of tumor-specific immunity. Antigen-specific T cell-mediated responses are vital for tumor ablation and play a significant role in achieving tumor eradication through PDT.63,64 Some researchers contend that PDT preferentially targets rapidly dividing cells, and in conditions like oral lichen planus, it may cause abnormal T cell death.65
Relative to other cancers, few clinical studies have explored immunological changes in glioma following PDT. This study involved 28 glioma patients, of whom 18 underwent PDT irradiation post-maximal tumor resection, while 10 received only the surgical intervention. Efficacy analysis revealed that treatment outcomes varied significantly between groups; patients receiving PDT demonstrated superior outcomes to those who did not (P=0.035); specifically, the effective rate of patients receiving PDT treatment was 83.3%, while the effective rate of patients not receiving PDT treatment was 40.0%. These results indicate significant benefits of combining surgery with PDT. Moreover, a detailed analysis of prognostic factors showed that OS for the primary group was superior to that of the recurrent group (P=0.039). Specifically, the median OS for the primary group was 34 months, whereas the median survival for the recurrence group has not yet been reached, a promising finding. Despite the small sample size, our study suggests a potentially beneficial effect of PDT on the recovery of patients’ immune systems relative to conventional treatments. However, accumulating clinical data suggest that PDT’s effectiveness may not be uniform across all malignant glioma cases, highlighting the importance of patient selection to maximize benefits.
This study’s limitations must be acknowledged. As a single-center retrospective study, it is subject to selection bias, and its findings require external validation. Furthermore, the short follow-up period and absence of certain outcome measures may lead to overestimating the benefits of combining surgery with PDT. Most critically, the limited number of participants restricted our capacity for robust statistical analysis, particularly for subgroup comparisons. Further multicenter, prospective randomized trials are necessary to evaluate PDT’s impact on the glioma immune microenvironment and its synergistic effects with other therapies.
Conclusion
In summary, monitoring changes in lymphocyte subpopulations and peripheral blood inflammatory markers offers a valuable approach to assessing disease severity and progression in glioma patients. These biomarkers may be important tools for auxiliary diagnosis, evaluating therapeutic efficacy, and guiding prognostic interventions. Additionally, this study highlights that PDT, when used as an adjunct to surgical resection, has a more significant effect on the immune function of patients with recurrent and grade 4 gliomas. These findings provide new insights into the immunomodulatory potential of PDT, suggesting its role in enhancing treatment outcomes in advanced glioma cases.
Ethics Approval and Informed Consent
Informed consent was secured from all participants. This research received approval from the Ethics Committee of Zhejiang Provincial People’s Hospital and adhered to the ethical principles outlined in the Declaration of Helsinki.
Author Contributions
Chunxiao Zhou and Lei Xu contributed to the work equally and should be regarded as co-first authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Zhejiang Provincial People’s Hospital Talent Introduction Project (No. C-2021-QDJJ03-01), Project of Medicine and Health Science and Technology of Zhejiang Province (No. 2024KY015).
Disclosure
The authors report no competing interests in this work.
References
1. Louis DN, Perry A, Reifenberger G. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1
2. Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28(7):1457–1472. doi:10.1093/annonc/mdx106
3. Mamelak AN, Jacoby DB. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin Drug Delivery. 2007;4(2):175–186. doi:10.1517/17425247.4.2.175
4. Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Gene. 2012;205(12):613–621. doi:10.1016/j.cancergen.2012.10.009
5. Hakyemez B, Erdogan C, Yildirim N, Parlak MJTB. Glioblastoma multiforme with atypical diffusion-weighted MR findings. The British Journal of Radiology. 2005;78(935):989–992. doi:10.1259/bjr/12830378
6. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi:10.1093/neuonc/noab106
7. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. New Engl J Med. 2009;360(8):765–773. doi:10.1056/NEJMoa0808710
8. Miller JJ, Loebel F, Juratli TA, et al. Accelerated progression of IDH mutant glioma after first recurrence. Neuro Oncol. 2019;21(5):669–677. doi:10.1093/neuonc/noz016
9. Kaleta-Richter M, Kawczyk-Krupka A, Aebisher D, Bartusik-Aebisher D, Czuba Z, Cieślar G. The capability and potential of new forms of personalized colon cancer treatment: immunotherapy and Photodynamic Therapy. Photodiagn Photodyn Ther. 2019;25:253–258. doi:10.1016/j.pdpdt.2019.01.004
10. Phua SZF, Xue C, Lim WQ, et al. Light-responsive prodrug-based supramolecular nanosystems for site-specific combination therapy of cancer. Chem Mater. 2019;31(9):3349–3358.
11. Wu H, Minamide T, Yano TJDE. Role of photodynamic therapy in the treatment of esophageal cancer. Digestive Endoscopy: Official Journal of the Japan Gastroenterological Endoscopy Society. 2019;31(5):508–516. doi:10.1111/den.13353
12. Nackiewicz J, Kliber-Jasik M, Skonieczna MJJP. A novel pro-apoptotic role of zinc octacarboxyphthalocyanine in melanoma me45 cancer cell’s photodynamic therapy (PDT). Journal of Photochemistry and Photobiology. B, Biology. 2019;190:146–153. doi:10.1016/j.jphotobiol.2018.12.002
13. Aniogo EC, Plackal Adimuriyil George B, Abrahamse HJC. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell International. 2019;19(1):91. doi:10.1186/s12935-019-0815-0
14. Lamberti MJ, Morales Vasconsuelo AB, Ferrara MG, Rumie Vittar NBJP. Photobiology, Recapitulation of hypoxic tumor–stroma microenvironment to study photodynamic therapy implications. Photochemistry and Photobiology. 2020;96(4):897–905. doi:10.1111/php.13220
15. Cramer SW, Chen CC. Photodynamic Therapy for the Treatment of Glioblastoma. Front Surg. 2019;6:81. doi:10.3389/fsurg.2019.00081
16. Stylli SS, Kaye AH, MacGregor L, Howes M, Rajendra P. Photodynamic therapy of high grade glioma - long term survival. J Clin Neurosci. 2005;12(4):389–398. doi:10.1016/j.jocn.2005.01.006
17. Muragaki Y, Akimoto J, Maruyama T, et al. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J Neurosurg. 2013;119(4):845–852. doi:10.3171/2013.7.JNS13415
18. Fitzgerald F. Photodynamic Therapy (PDT). Nova Science Publishers, Incorporated; 2017.
19. Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62(6):1604–1608.
20. Hirschberg H, Berg K, Peng Q. Photodynamic therapy mediated immune therapy of brain tumors. Neuroimmunol Neuroinflamm. 2018;5(7):27. doi:10.20517/2347-8659.2018.31
21. Pichlmeier U, Bink A, Schackert G, Stummer WJN. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro-Oncology. 2008;10(6):1025–1034. doi:10.1215/15228517-2008-052
22. Etminan N, Peters C, Lakbir D, et al. Heat-shock protein 70-dependent dendritic cell activation by 5-aminolevulinic acid-mediated photodynamic treatment of human glioblastoma spheroids in vitro. Br J Cancer. 2011;105(7):961–969. doi:10.1038/bjc.2011.327
23. Li F, Cheng Y, Lu J, Hu R, Wan Q, Feng H. Photodynamic therapy boosts anti-glioma immunity in mice: a dependence on the activities of T cells and complement C3. J Cell Biochem. 2011;112(10):3035–3043. doi:10.1002/jcb.23228
24. Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim MJJo IR. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. J Immunol Res. 2011;2011:1.
25. Engelhardt S, Patkar S, Ogunshola OJB. Cell-specific blood–brain barrier regulation in health and disease: a focus on hypoxia. British Journal of Pharmacology. 2014;171(5):1210–1230. doi:10.1111/bph.12489
26. Johanns TM, Bowman-Kirigin JA, Liu C, Dunn GPJN. Targeting neoantigens in glioblastoma: an overview of cancer immunogenomics and translational implications. Neurosurgery. 2017;64(CN_suppl_1):165–176. doi:10.1093/neuros/nyx321
27. Dunn GP, Okada HJN. Principles of immunology and its nuances in the central nervous system. Neuro-Oncology. 2015;17(suppl_7):vii3–vii8. doi:10.1093/neuonc/nov175
28. Parney IF. Basic concepts in glioma immunology. Adv Exp Med Biol. 2012;746:42–52.
29. Nseyo UO, Whalen RK, Duncan MR, Berman B, Lundahl SLJU. Urinary cytokines following photodynamic therapy for bladder cancer a preliminary report. Urology. 1990;36(2):167–171. doi:10.1016/0090-4295(90)80220-h
30. Radogna F, Diederich M. Stress-induced cellular responses in immunogenic cell death: implications for cancer immunotherapy. Biochemical Pharmacology. 2018;153:12–23. doi:10.1016/j.bcp.2018.02.006
31. Castano AP, Mroz P, Hamblin MRJNRC. Photodynamic therapy and anti-tumour immunity. Nature Reviews. Cancer. 2006;6(7):535–545. doi:10.1038/nrc1894
32. Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi:10.1158/1078-0432.CCR-11-0774
33. Learn CA, Fecci PE, Schmittling RJ, et al. Profiling of CD4+, CD8+, and CD4+CD25+CD45RO+FoxP3+ T cells in patients with malignant glioma reveals differential expression of the immunologic transcriptome compared with T cells from healthy volunteers. Clin Cancer Res. 2006;12(24):7306–7315. doi:10.1158/1078-0432.CCR-06-1727
34. Michelson N, Rincon-Torroella J, Quiñones-Hinojosa A, Greenfield JP. Exploring the role of inflammation in the malignant transformation of low-grade gliomas. J Neuroimmunol. 2016;297:132–140. doi:10.1016/j.jneuroim.2016.05.019
35. Sen E. Targeting inflammation-induced transcription factor activation: an open frontier for glioma therapy. Drug Discovery Today. 2011;16(23–24):1044–1051. doi:10.1016/j.drudis.2011.09.003
36. Gong YT, Zhang LJ, Liu YC, et al. Neutrophils as potential therapeutic targets for breast cancer. Pharmacol Res. 2023;198:106996. doi:10.1016/j.phrs.2023.106996
37. Di Vito C, Navone SE, Marfia G, et al. Platelets from glioblastoma patients promote angiogenesis of tumor endothelial cells and exhibit increased VEGF content and release. Platelets. 2017;28(6):585–594. doi:10.1080/09537104.2016.1247208
38. Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front Immunol. 2020;11:1749. doi:10.3389/fimmu.2020.01749
39. Hajizadeh F, Aghebati Maleki L, Alexander M, et al. Tumor-associated neutrophils as new players in immunosuppressive process of the tumor microenvironment in breast cancer. Life Sci. 2021;264:118699. doi:10.1016/j.lfs.2020.118699
40. Teng JJ, Zhang J, Zhang TY, Zhang S, Li BS. Prognostic value of peripheral blood lymphocyte-to-monocyte ratio in patients with solid tumors: a meta-analysis. Onco Targets Ther. 2016;9:37–47. doi:10.2147/OTT.S94458
41. Fulda S. Promises and Challenges of Smac Mimetics as Cancer Therapeutics. Clin Cancer Res. 2015;21(22):5030–5036. doi:10.1158/1078-0432.CCR-15-0365
42. Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Current Opini Immunol. 2007;19(3):339–347. doi:10.1016/j.coi.2007.04.007
43. Crusz SM, Balkwill FRJN. Inflammation and cancer: advances and new agents. Nature Reviews. Clinical Oncology. 2015;12(10):584–596. doi:10.1038/nrclinonc.2015.105
44. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Translational Lung Cancer Res. 2019;8(6):886–894. doi:10.21037/tlcr.2019.11.16
45. Yasumatsu R, Wakasaki T, Hashimoto K, et al. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck. 2019;41(8):2610–2618. doi:10.1002/hed.25737
46. Matsuda A, Yamada T, Matsumoto S, et al. Prognostic Role of the Platelet-to-Lymphocyte Ratio for Patients With Metastatic Colorectal Cancer Treated With Aflibercept. In vivo. 2020;34(5):2667–2673.
47. Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi:10.1038/s41568-020-0285-7
48. Koudriavtseva T, Villani V, Lorenzano S, et al. Neutrophil-to-lymphocyte ratio, Factor VIII and Antithrombin III: inflammatory-clotting biomarkers in glioma. EXCLI J. 2021;20:1152–1169. doi:10.17179/excli2021-3831
49. Wang J, Xiao W, Chen W, Hu Y. Prognostic significance of preoperative neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with glioma. EXCLI J. 2018;17:505–512. doi:10.17179/excli2017-978
50. Wang Y, Xu C, Zhang Z. Prognostic value of pretreatment lymphocyte-to-monocyte ratio in patients with glioma: a meta-analysis. BMC Med. 2023;21(1):486. doi:10.1186/s12916-023-03199-6
51. Guo J, Fang J, Huang X, et al. Prognostic role of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in prostate cancer: a meta-analysis of results from multivariate analysis. International Journal of Surgery (London, England). 2018;60:216–223. doi:10.1016/j.ijsu.2018.11.020
52. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflamm. 2021;18(1):51. doi:10.1186/s12974-021-02090-6
53. Qiang G, Liang C, Xiao F, et al. Prognostic significance of platelet-to-lymphocyte ratio in non-small-cell lung cancer: a meta-analysis. OncoTargets and Therapy. 2016;9:869–876. doi:10.2147/OTT.S96804
54. Chen D, Varanasi SK, Hara T, et al. CTLA-4 blockade induces a microglia-Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma. Immunity. 2023;56(9):2086–2104.e2088. doi:10.1016/j.immuni.2023.07.015
55. Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br J Cancer. 2007;96(12):1839–1848. doi:10.1038/sj.bjc.6603792
56. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska BJL. Immune microenvironment of gliomas. Laboratory Investigation; a Journal of Technical Methods and Pathology. 2017;97(5):498–518. doi:10.1038/labinvest.2017.19
57. Magri S, Musca B, Bonaudo C, et al. Sustained accumulation of blood-derived macrophages in the immune microenvironment of patients with recurrent glioblastoma after therapy. Cancers. 2021;13(24):6178. doi:10.3390/cancers13246178
58. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi:10.1016/j.cell.2013.09.034
59. Guo X, Pan Y, Xiong M, et al. Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat Commun. 2020;11(1):2177. doi:10.1038/s41467-020-15770-3
60. Massara M, Persico P, Bonavita O, et al. Neutrophils in Gliomas. Front Immunol. 2017;8:1349. doi:10.3389/fimmu.2017.01349
61. Leong S, Chan AH, Levy JG, Hunt DW. Transcutaneous photodynamic therapy alters the development of an adoptively transferred form of murine experimental autoimmune encephalomyelitis. Photochem Photobiol. 1996;64(5):751–757. doi:10.1111/j.1751-1097.1996.tb01830.x
62. Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br J Cancer. 1995;71(3):549–555. doi:10.1038/bjc.1995.108
63. Korbelik M, Cecic I, Merchant S, Sun J. Acute phase response induction by cancer treatment with photodynamic therapy. Int J Cancer. 2008;122(6):1411–1417. doi:10.1002/ijc.23248
64. Gollnick SO, Evans SS, Baumann H, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88(11):1772–1779. doi:10.1038/sj.bjc.6600864
65. Kvaal SI, Angell-Petersen E, Warloe T. Photodynamic treatment of oral lichen planus. Oral Surg Oral Medi Oral Pathol Oral Radiol. 2013;115(1):62–70. doi:10.1016/j.oooo.2012.08.448
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.