Back to Journals » International Journal of General Medicine » Volume 18
Expression of CXCR4 in the Primary Lesion of Recurrent Metastatic Breast Cancer and Its Association With Prognosis
Authors Huang D, Lin D, Liang S, Lin J
Received 12 December 2024
Accepted for publication 12 February 2025
Published 18 March 2025 Volume 2025:18 Pages 1543—1553
DOI https://doi.org/10.2147/IJGM.S511426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ching-Hsien Chen
DanChan Huang,1 DanXia Lin,2 SiXian Liang,2 Jing Lin1
1Department of Oncology, The First Affiliated Hospital of Shantou University Medical College, Shantou City, Guangdong Province, 515000, People’s Republic of China; 2Department of Breast Oncology, Cancer Hospital of Shantou University Medical College, Shantou City, Guangdong Province, 515000, People’s Republic of China
Correspondence: Jing Lin, Department of Oncology, The First Affiliated Hospital of Shantou University Medical College, No. 57, Changping Road, Longhu District, Shantou City, Guangdong Province, 515000, People’s Republic of China, Email [email protected]
Objective: This study examined CXCR4 expression in primary lesions of recurrent metastatic breast cancer patients, analyzing its association with clinicopathological features, chemotherapy efficacy, and prognosis.
Methods: Eighty-five early surgical specimens of advanced BCa were examined for CXCR4 expression using immunohistochemical staining. The relationships between CXCR4 expression and clinical pathological factors, such as tumor size, lymph node metastasis, tumor stage, and metastatic site, were statistically analyzed, along with their effect on the efficacy of platinum-based chemotherapy and prognosis in patients with advanced BCa.
Results: Significant associations were found between high CXCR4 levels in primary lesions of recurrent metastatic BCa and more frequent visceral metastases (p = 0.010), along with a higher rate of lymph node metastases (p = 0.022). Patients with advanced BCa showing high CXCR4 expression had lower efficacy with platinum-based chemotherapy (p = 0.002). Patients with high CXCR4 expression exhibited shorter disease-free survival (DFS) and overall survival (OS) compared to those with low expression, though the differences lacked statistical significance.
Conclusion: Patients with recurrent metastatic BCa with high expression of CXCR4 in primary lesions have poor efficacy with platinum-based chemotherapy, shorter DFS and OS, and poor prognosis. CXCR4 may be an important biomarker in metastatic BCa. It can be used not only as a predictor of metastasis and prognosis, but also as a therapeutic target and a tool to monitor treatment efficacy.
Keywords: breast cancer, CXCR4, chemotherapy efficacy, prognosis
Introduction
Breast cancer (BCa) is one of the most common malignant tumors in women, with metastasis being its primary cause of death.1,2 During the period 2015–2019, the incidence of BCa increased by 0.6–1% per year. The statistics show that BCa accounted for 32% of all newly diagnosed cases in 2024, as far as women are concerned. Between 2012–2019, the increase in incidence was even greater in women under 50 years of age. More importantly, BCa continues to lead the way in deaths.3 For patients with advanced BCa, chemotherapy is still a key treatment method, but sensitivity varies greatly.4 It is likely that chemotherapy will remain an important cancer treatment in the future, and better application and local administration may help to improve the efficacy of chemotherapeutic agents.5 The mechanism of chemoresistance in metastatic BCa is a complex process involving alterations in apoptotic pathways, cell cycle regulation, and tumor microenvironment. The emergence of these resistance mechanisms reduces the efficacy of chemotherapeutic agents and leads to tumor recurrence and metastasis, posing a great challenge to clinical treatment. Therefore, searching for biomarkers that indicate chemotherapy resistance and stratify patients to improve therapeutic efficacy is an urgent unmet clinical need.
The CXC chemokine receptor 4 (CXCR4) encodes a highly conserved G-protein-coupled receptor with 352 amino acids.6 CXCR4 has been found to be highly expressed in multiple types of cancers, including BCa,7 kidney cancer,8 ovarian cancer,9 prostate cancer,10 lung cancer,11 and colon cancer.12 CXC chemokine ligand 12 (CXCL12) binds specifically to the CXCR4 receptor. Studies in recent years have elucidated that the CXCL12/CXCR4 axis activates various signaling pathways and mediates the movement, chemotaxis, adhesion, secretion, angiogenesis, and proliferation of tumor cells.6,13–19 Additionally, CXCL12/CXCR4 axis activation has been identified to facilitate BCa cell malignant transformation and migration.20 More importantly, it has been established that CXCR4 plays a central role in tumor cell spread and metastatic development in more than 75% of cancers (including ovarian, lung, kidney, brain, etc).21 Similarly CXCL12 protein levels are highly expressed in organs known to be common sites of metastasis, including liver, bone marrow, and lung. This implies that tumor cells might employ chemokine-driven transport mechanisms typically utilized in organogenesis, angiogenesis and tissue regeneration. The CXCR4/CXCL12 axis also indirectly promotes tumor metastasis by mediating tumor cell invasion and proliferation as well as enhancing tumor-associated neoangiogenesis.6 One of the CXCR4/CXCL12 axis activators identified is POU1F1 transcription factor (Pit-1). Pit-1 overexpression enhances tumor growth and metastasis by enhancing CXCR4 expression and CXCL12 expression, thereby promoting angiogenic effects.22 Metastatic target tissues highly expressing CXCL12 are chemoattractants for primary tumor cells expressing high CXCR4, recruiting CXCR4-positive cancer cells to CXCL12-expressing organs,23 as shown by reduced metastasis to these tissues through CXCR4 blocking.22
In metastatic BCa mice, CXCR4 inhibitors reduce tumor cell metastasis and invasion to organ-specific sites by blocking CXCR4-CXCL12 interaction, offering a potential therapeutic approach to hinder tumor spread.13,24,25 Colon cancer cells are also more sensitive to chemotherapy drugs when treated with CXCR4 inhibitors both in vitro and in vivo.26,27 The FDA has approved AMD3100, commercially known as plerixafor, as a CXCR4 antagonist for autologous transplantation in Non-Hodgkin’s lymphoma or multiple myeloma patients. AMD3100 has demonstrated tremendous potential in the treatment of other diseases, such as leukemia and solid tumors.28 AMD3100 can promote chemosensitivity of ovarian cancer cells with high CXCR4 expression.29 In BCa, CXCR4 inhibitors are shown to eliminate trastuzumab resistance by blocking cell cycle progression and synergize with doxorubicin to inhibit tumor growth.30 This suggests that CXCR4 inhibitors not only directly inhibit tumor cell growth, but also enhance the efficacy of other chemotherapeutic agents by modulating the tumor microenvironment. Research has indicated CXCR4 inhibitors as promising agents to counteract multidrug resistance in acute myeloid leukemia (AML) and multiple myeloma (ALL),29 addressing a critical obstacle in treatment efficacy. In metastasis studies in BCa, self-assembly of a CXCR4 antagonist inhibits BCa multiorgan metastasis, improving drug solubility and enabling specific targeted therapy. Second, CXCR4 antagonists have also been shown to attenuate mobilization to sites of injury or inflammation.31 All of these previous studies suggest that CXCR4 has an important impact in cancer progression.
Even with progress in the characterization of other cancers and drug development, the function of CXCR4 in primary lesions of metastatic BCa patients remains unreported. This study aims to explore the correlation of CXCR4 expression levels in primary lesions of BC patients on platinum-based chemotherapy sensitivity and patient’s prognosis.
Materials and Methods
Patient Sample Collection
Metastatic BCa women (n = 85) were treated at the The First Affiliated Hospital of Shantou University Medical College from January 1996 to December 2008. Demographic and clinical information for each patient was obtained through a retrospective review of the hospital’s electronic patient records or the medical records department’s system. Inclusion criteria: (1) patients’ clinical data and follow-up data were complete; (2) all patients were treated for the first time with breast-conserving surgery or simple mastectomy and were confirmed as BCa by postoperative pathology; (3) patients had measurable target lesions clinically or radiographically before treatment; (4) Patients had early surgical tumor tissue paraffin blocks. Exclusion criteria: (1) history of other malignant tumors; (2) preoperative distant metastases; (3) comorbid endocrine-related diseases. The present study was approved by the Ethics Committee of The First Affiliated Hospital of Shantou University Medical College and written informed consent was provided by all patients prior to the study start.
Treatment Regimens and Determination of Efficacy
Platinum-based chemotherapy is widely used in the treatment of metastatic BCa. Platinum drugs belong to the cell cycle non-specific drugs, which mainly play an anti-tumor role by inhibiting the transcription and replication of DNA, thus promoting apoptosis of tumor cells. In addition, the choice of platinum drugs will be adjusted according to the specific conditions of patients. All patients received a standard platinum combination chemotherapy regimen: 75 mg/m2 of cisplatin or carboplatin on day 1 and either gemcitabine 1200 mg/m2 on days 1 and 8 or paclitaxel 175 mg/m2 on day 1, repeated every 3 weeks, with all patients treated for a minimum of 4 to 6 cycles or chemotherapy until progression. Platinum-based chemotherapy was terminated immediately when a patient receiving platinum-based chemotherapy experienced a sudden deterioration of the disease or when the patient was unable to tolerate the toxicity of the chemotherapy drug. The physician further created a personalized treatment plan based on the specifics of the patient’s disease progression.
The longest dimensions of the lesions were measured according to the RECIST 1.1, and the efficacy of the target lesions was clinically assessed.32 Complete response (CR) is defined as the disappearance of the target lesion after platinum-based chemotherapy, and partial response (PRe) is defined as at least a 30% reduction in the combined diameter of the target lesion. Patient response rate to platinum-based therapy was calculated using the sum of CR and PRe. Progression of disease (PD) is defined as an increase of at least 20% in the sum of the diameters of the target lesions. Stable disease (SD) is defined as neither sufficient shrinkage to qualify for CR or PRe nor sufficient increase to qualify for PD. PD and SD reflect the patient’s insensitivity to platinum-based chemotherapy. The nonresponse rate is calculated by adding SD and PD.33,34
Methods
General Information
Clinical data were collected, including age at diagnosis, menstrual status, family history, tumor size, lymph node metastasis, tumor staging, histopathological type, metastasis sites and times, death times, estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2), platinum-based chemotherapy regimens, and therapeutic efficacy.
Immunohistochemistry and Scoring Criteria
The tumor tissue paraffin blocks were preserved and provided by the Department of Pathology, Shantou University Medical College. CXCR4 was analyzed using immunohistochemistry (IHC) on fresh tissue blocks (≥ 50 tumor cells; reviewed by pathologist Wei Xiaolong). IHC was performed using rabbit CXCR4 monoclonal antibody AB124824 (Abcam Inc. Cambridge, UK). The slides were heated in a citrate buffer (pH 6.0) using a microwave for 18 minutes, starting with 5 minutes on high heat and then 13 minutes on low heat. Endogenous peroxidase was blocked using 3% H2O2 solution. To block non-specific protein binding, normal goat serum was applied at room temperature for 20 minutes. Tissues were incubated overnight in AB124824 with 1:200 dilution. The reaction was visualized using an antibody (GK500510A, GTVisionTM I) and DAB chromogen.
CXCR4 positivity was determined by evaluating the intensity of cell staining and the percentage of cells stained. The degree of staining was based on 0 points for no staining, 1 point for light yellow, 2 points for yellow, and 3 points for brown-yellow. Scores were assigned according to the percentage of positive cells: 0 for 5% or less, 1 for 6–25%, 2 for 26–50%, 3 for 51–75%, and 4 for 76% or more. Determination based on the product of the two integrals above: a product of 0 indicated negative (-), ≥1 indicated positive. Specifically, scores of 1–4 indicated weakly positive (+), scores of 5–8 indicated positive (++), and scores of ≥9 indicated strongly positive (+++). Negative (-) and weakly positive (+) were considered low expression, whereas positive (++) and strongly positive (+++) were considered high expression.35 The above results were reviewed independently by two senior pathologists in a double-blind fashion to minimize errors.
Follow-up Program and Determination Criteria
Patients start to follow up after surgery, every 3 months in the first 2 years after surgery; every 6 months after 2 years; after 5 years, every year. Recurrence is defined as the reappearance of tumor lesions in the breast, chest wall, or regional lymph nodes of the patient by imaging. Metastasis is defined as bone metastasis, lung metastasis, liver metastasis, and brain metastasis by imaging. Follow-up was conducted by outpatient clinic, telephone inquiry to the patients themselves or their family members. Among the 98 patients, the shortest follow-up time was 15 years and the longest was 18 years. The follow-up period ended on August 31, 2023. Disease-free survival (DFS) was defined as the interval between BCa diagnosis and metastasis. Based on the time between diagnosis and death or last follow-up/contact, overall survival (OS) was calculated. OS1 is the length of time since metastasis was diagnosed to death or the last follow-up.
Statistical Analysis
All statistical analysis and presentation of all data were performed using Python. Count data were analyzed using the Chi-squared test and the Fisher’s exact test. By using the Kaplan-Meier method, the median overall survival was estimated. The Log rank test was used to evaluate differences between survival groups. Respective hazard ratios were obtained by Cox regression. Independent variables with statistically significant (P < 0.05) differences on one-way analysis of variance were included in multifactor Cox regression analysis to screen for independent predictors (P < 0.05). Potential confounding variables were adjusted. Hazard ratios (HR) were calculated. All statistical results with p<0.05 were considered statistically significant.
Results
Immunohistochemistry Findings
The expression levels of CXCR4 in primary lesions were detected using IHC. The IHC results of BCa primary lesions showed that CXCR4 was predominantly expressed in the cytoplasm. Out of 85 patients, 41 (48.24%) patients showed positive (++) and strongly positive (++++) IHC results for CXCR4 (Figure 1C and D), indicating high CXCR4 expression, while 44 (51.76%) patients showed negative (-) and weakly positive (+) IHC results (Figure 1A and B), indicating low CXCR4 expression.
Correlation Between CXCR4 Expression and Clinical Characteristics of BCa Patients
All patients were categorized into a CXCR4 low expression group (n = 44) and a CXCR4 (n = 41) high expression group based on their IHC test results. The clinicopathologic characteristics of all subjects are shown in Table 1. There was no significant difference in age (p = 0.824), menstrual status (p = 0.510), family history (p = 0.423), tumor tissue type (p = 0.423), ER expression (p = 0.506), PR expression (p = 0.388), or HER2 expression levels (p = 0.385) between the two groups, as shown in Table 1. Of interest, patients in the CXCR4 high expression group had a greater number of metastatic foci (p = 0.010), more lymph node metastases (p = 0.022), and higher tumor staging (p = 0.042) than those in the CXCR4 low expression group. The expression of CXCR4 in the tumor microenvironment can affect the interaction between tumor cells and surrounding stromal cells, promoting tumor angiogenesis and metastasis. CXCR4 is usually highly expressed in metastatic tissues,36 and prominent CXCR4 expression characterizes all major histological forms of invasive BCa.37 These are all evidence supporting the correlation of CXCR4 with metastasis and tumor staging.
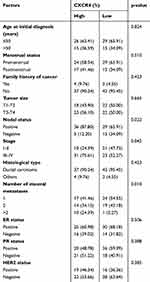 |
Table 1 Relationship of CXCR4 Expression in the Primary Lesion and Breast Cancer Clinical Characteristics. Statistics Was Performed Using Chi-Square Test |
Correlation of CXCR4 Expression Level in Primary Lesions on the Platinum Chemotherapy Efficacy in BCa Patients
Four patients were excluded due to their chemotherapy efficacy after relapse and metastasis not being able to be evaluated (Table 2). BCa patients with low CXCR4 expression had a 65.85% response rate (CR and PRe) to platinum-based chemotherapy after metastasis. It showed that BCa patients with low expression of CXCR4 had good efficacy after platinum-based chemotherapy. In contrast, the response rate of high expression patients was 30.00% and non-response rate was as high as 70%. There was a significant difference between the two groups in the efficacy of platinum-based chemotherapy (P = 0.002), indicating that patients with high expression of CXCR4 had poorer efficacy of platinum-based chemotherapy.
 |
Table 2 A Significant Difference (p=0.002) Suggests Lower Efficacy of Platinum Chemotherapy in High CXCR4 Expression Patients by Using the Chi-Square Test |
Association of CXCR4 Expression With BCa Disease-Free Survival and Overall Survival
The Kaplan-Meier analysis was carried out to compare BCa patient groups with low and high CXCR4 expression. The median follow-up was 137 months in the CXCR4 low expression group and 142 months in the CXCR4 high expression group. Among the 84 BCa patients, 75 cases had recurrence and metastasis, and the DFS rate was 89.29%. There were 41 cases in the CXCR4 low expression group and 34 cases in the CXCR4 high expression group (Figure 2A). Among all cases, 82 cases died, and the OS rate was 87.62%. Among them, 42 cases were in the CXCR4 low expression group and 40 cases were in the CXCR4 high expression group (Figure 2B). The median DFS and OS of patients in the CXCR4 low expression group were 37.0 and 57.0 months, respectively. The median DFS and OS of patients in the CXCR4 high expression group were 34.0 and 52.0 months, respectively. The difference between the two groups in DFS (p = 0.808) and OS (p = 0.115) was not statistically significant by Log Rank test. We also performed an additional analysis focusing on specific OS metrics (OS1). From the time metastasis was detected until the patient’s death or last follow-up, 80 cases were noted, with 40 cases each in the CXCR4 low and high expression groups. Patients with lower CXCR4 expression had a longer median OS1 of 28.0 months, compared to 17.0 months in the CXCR4 high expression group, although not statistically significant (p=0.053) (Figure 2C). For patients with high CXCR4 expression, closer monitoring and more aggressive treatment strategies may be required in long-term management due to the greater invasive and metastatic capacity of tumor cells. These patients may be more susceptible to disease recurrence and metastasis and therefore may require more frequent imaging and more intensive treatment regimens. Patients with low CXCR4 expression may have a better prognosis and may be treated with relatively conservative therapeutic strategies in long-term management, but monitoring should not be ignored.
COX Model Analysis of Prognostic Factors for Advanced BCa
CXCR4 expression and other variables were analyzed in univariate and multivariate analysis. Univariate analysis revealed that family history (p = 0.008), tumor size (p = 0.030), tumor staging (p = 0.011), DFS time (p = 0.000), and CXCR4 expression (p = 0.024) were predictive indicators for OS (Table 3). Further multifactorial COX regression analyses were performed, and after adjusting for all factors with significance in the univariate analyses, independent influences affecting OS in patients with BCa were identified. Multivariate analysis showed that family history (p = 0.026), tumor size (p = 0.020), and DFS time (p = 0.002) were important prognostic factors for OS after adjusting for multiple potential confounding variables (age, menstrual status, node status, pathological type, etc). (Table 3).
 |
Table 3 CXCR4 Expression Predicts OS According to Univariate Analysis (p=0.024), but Not in Multivariate Analysis |
Discussion
The study shed light on how CXCR4 expression influences disease progression and treatment outcomes in 85 BCa patients. CXCR4 is found in various subcellular localizations. High expression of CXCR4 in the nucleus is associated with a better prognosis in lung cancer,38 and high expression of CXCR4 in the cell membrane is significantly associated with a decrease in DFS.39 Cytoplasmic high expression of CXCR4 in the cytoplasm is a poor prognostic factor for lung cancer.40 Therefore, CXCR4 expression in different subcellular localizations may lead to different biological behaviors and may have clinical applications. Our analysis showed that CXCR4 was predominantly expressed in the cytoplasm, with nearly identical distribution of expression among patients; 48.24% of primary lesions showed high levels and 51.76% showed low levels as determined by IHC. We also identified a significant association between high CXCR4 expression and increased rates of visceral and lymph node metastasis (p = 0.010 and p = 0.022, respectively). This suggests that in patients with BCa, the association between high cytoplasmic CXCR4 expression and poor prognosis was even more pronounced. Moreover, our research revealed that patients with high CXCR4 expression showed low efficacy of platinum-based chemotherapy (p=0.002). This indicates that assessing CXCR4 expression levels could play a key role in guiding therapeutic strategies, emphasizing the need for alternative treatments for BCa patients with high CXCR4 expression. The study by Liu et al focuses on the progression of multiple cancer types with copper death-associated gene expression and highlights the potential application in immunotherapy.41 While another study analyzes copper cell death genes as possible candidate biomarkers for cancer diagnosis, prognosis, and treatment through pan-cancer genomics, and deeply analyzes the potential association with common cancer pathways.42,43 Although the present study validated CXCR4 as an independent influencer of metastasis and prognosis in BCa, the specific role of CXCR4 in a variety of cancer diseases and its associated cancer pathways have yet to be explored in depth.
In this study, we found a significant difference in the efficacy of platinum-based chemotherapy between the two groups (p = 0.002), with a 64.71% increase in the median OS1 in patients with lower CXCR4 expression, suggesting that patients with high CXCR4 expression have a poorer outcome after platinum-based chemotherapy. High CXCR4 expression is considered a marker of poor prognosis in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). In addition, preclinical models of ALL and AML suggest that inhibition of CXCR4 enhances the efficacy of chemotherapy. A study demonstrates that CXCR4 antagonists significantly inhibit acquired resistance to gefitinib in lung adenocarcinoma cell lines harboring EGFR mutations.44 CXCR4 antagonists remodel the tumor microenvironment, favoring the entry of T effectors and decreasing regulatory T cells in order to enhance the efficacy of anti-programmed death therapies.45 Overall, it is currently believed that CXCR4 antagonists may improve the prognosis of cancer patients by enhancing the therapeutic efficacy of conventional therapies or immunotherapies. Although some CXCR4 antagonists have completed clinical trials,46 their specific application in BCa needs to be further explored.
Our analysis did not find a direct correlation between CXCR4 and DFS, OS, or OS1. However, patients with lower CXCR4 expression had a longer median OS1 (28.0 months) compared to those with higher expression (17.0 months), though this difference was not statistically significant (p = 0.053). This trend warrants further investigation as it might indicate a potential impact of CXCR4 expression on the long-term outcomes of BCa patients. Univariate analysis confirmed that CXCR4 expression was a predictive factor for OS (p = 0.024), thereby reinforcing its role as a prognostic marker. However, this correlation was not evident in the multivariate analysis, suggesting that the prognostic value of CXCR4 might be influenced by other factors not considered in this study. This discrepancy underscores the complexity of cancer biology and the interplay of various factors in disease progression and treatment response.
The limitations of this study are mainly in the following aspects. This study is a single-center retrospective study, accompanied by confounding factors and the possibility of selection bias. In addition, the long time of onset and the small number of included cases of the patients enrolled in this study had an impact on the reliability and stability of the results. The BCa treatment method and effect may have an impact on the prognosis, but further investigation is needed because of the challenges in obtaining data from the treatment schedules of the enrolled patients and the period over which diabetes developed. In the future, we will validate this in a larger cohort, which will help determine whether the current findings have broad clinical applicability. Second, BCa is a complex disease that often involves abnormalities in multiple signaling pathways. Therefore, future validation studies in larger multicenter cohorts are needed and to explore the application of combination therapies targeting CXCR4 for BCa treatment in combination with other related drugs to generate synergistic effects and overcome drug resistance. This will not only help to improve treatment efficacy, but also help to promote the development of personalized medicine in BCa. In addition, the specific mechanism of CXCR4 needs to be further explored and studied in BCa metastatic animal models.
In conclusion, our findings emphasized the significant role of CXCR4 expression in metastasis and treatment response in BCa patients. In the BCa microenvironment, CXCR4 expression levels may be altered. The current study showed that the identification of novel depleted T-cell CD8+ markers provided new insights into understanding the state of immune cells in the tumor environment, with genetically characterized molecular subtypes and immune scoring systems capable of accurately predicting immune infiltration, survival, and response to immune checkpoint blockers. This implies that drugs or immunomodulatory therapies can be designed by specifically targeting features of the immune microenvironment, which is critical for the development of new strategies for immunotherapy against BCa, and provides new ideas for future probes targeting the interaction of CXCR4 with the immune microenvironment. This study helps to understand CXCR4 as a biomarker, provides direction for more precise patient stratification and personalized treatment strategies, and accurately predicts a patient’s response to specific treatments, thus selecting the most appropriate treatment option for the patient.
Data Available
Data is available from the corresponding author on request.
Ethics Statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 helsinki Declaration and its later amendments or comparable ethical standards. All subjects were approved by The First Affiliated Hospital of Shantou University Medical College (No.B-2024-109).
Acknowledgments
We would like to express our heartfelt gratitude to Professor Su Min and Professor Tian Dongping from the Department of Pathology at Shantou University Medical College for their help and support in immunohistochemical experiments and pathological diagnosis. We are deeply thankful to Professor Zhang Guojun and Professor Du Caiwen for their guidance on experimental design. Our sincere thanks also go to Dr. Huang Dongmin for handling and statistical analysis of the data.
Funding
The clinical application research of hospice care closed-loop management in improving the quality of life of terminal cancer patients based on the four level linkage of Internet plus medical consortium (STKJ2024061).
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Chen W, Hoffmann AD, Liu H, Liu X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precision Oncology. 2018;2(1):4. doi:10.1038/s41698-018-0047-0
2. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nature Reviews Disease Primers. 2019;5:66. doi:10.1038/s41572-019-0111-2
3. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
4. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi:10.1001/jama.2018.19323
5. Sonkin D, Thomas A, Teicher BA. Cancer treatments: past, present, and future. Cancer Genet. 2024;286-287:18–24. doi:10.1016/j.cancergen.2024.06.002
6. Shi Y, Riese DJ, Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Frontiers in Pharmacology. 2020;11:574667. doi:10.3389/fphar.2020.574667
7. Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. nature. 2001;410(6824):50–56. doi:10.1038/35065016
8. Pan J, Mestas J, Burdick MD, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Molecular Cancer. 2006;5(1):1–14. doi:10.1186/1476-4598-5-56
9. Y-p J, X-h W, Shi B, W-x W, G-r Y. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecologic Oncology. 2006;103(1):226–233. doi:10.1016/j.ygyno.2006.02.036
10. Hirata H, Hinoda Y, Kikuno N, et al. CXCL12 G801A polymorphism is a risk factor for sporadic prostate cancer susceptibility. Clinical Cancer Research. 2007;13(17):5056–5062. doi:10.1158/1078-0432.CCR-07-0859
11. Gangadhar T, Nandi S, Salgia R. The role of chemokine receptor CXCR4 in lung cancer. Cancer Biology & Therapy. 2010;9(6):409–416. doi:10.4161/cbt.9.6.11233
12. Lv S, Yang Y, Kwon S, et al. The association of CXCR 4 expression with prognosis and clinicopathological indicators in colorectal carcinoma patients: a meta‐analysis. Histopathology. 2014;64(5):701–712. doi:10.1111/his.12321
13. Yang Y, Li J, Lei W, et al. CXCL12-CXCR4/CXCR7 axis in cancer: from mechanisms to clinical applications. International Journal of Biological Sciences. 2023;19(11):3341. doi:10.7150/ijbs.82317
14. Goïta AA, Guenot D. Colorectal cancer: the contribution of CXCL12 and its receptors CXCR4 and CXCR7. Cancers. 2022;14(7):1810. doi:10.3390/cancers14071810
15. Mir MA, Naik AQ, Mzuh S, Zafar T. CXCL12–CXCR4 Axis in Cancer Metastasis. In: Cytokine and Chemokine Networks in Cancer. Springer; 2023:191–217.
16. Daniel SK, Seo YD, Pillarisetty VG, editors. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. In: Seminars in Cancer Biology. Elsevier; 2020.
17. Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell‐derived chemokines in host immune response. Cancer Science. 2007;98(11):1652–1658. doi:10.1111/j.1349-7006.2007.00606.x
18. Ho TK, Shiwen X, Abraham D, Tsui J, Baker D. Stromal-cell-derived factor-1 (SDF-1)/CXCL12 as potential target of therapeutic angiogenesis in critical leg ischaemia. Cardiology Research and Practice. 2012;2012. doi:10.1155/2012/143209
19. Barbolina MV, Kim M, Liu Y, et al. Microenvironmental regulation of chemokine (CXC-motif) receptor 4 in ovarian carcinoma. Molecular Cancer Research. 2010;8(5):653–664. doi:10.1158/1541-7786.MCR-09-0463
20. Ben-Batalla I, Seoane S, Garcia-Caballero T, et al. Deregulation of the Pit-1 transcription factor in human breast cancer cells promotes tumor growth and metastasis. The Journal of Clinical Investigation. 2010;120(12):4289–4302. doi:10.1172/JCI42015
21. Mortezaee K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: a critical mediator of metastasis. Life Sci. 2020;249:117534. doi:10.1016/j.lfs.2020.117534
22. Martinez-Ordoñez A, Seoane S, Cabezas P, et al. Breast cancer metastasis to liver and lung is facilitated by Pit-1-CXCL12-CXCR4 axis. Oncogene. 2018;37(11):1430–1444. doi:10.1038/s41388-017-0036-8
23. Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17(8):2074–2080. doi:10.1158/1078-0432.CCR-10-2636
24. Tulotta C, Stefanescu C, Chen Q, Torraca V, Meijer A, Snaar-Jagalska B. CXCR4 signaling regulates metastatic onset by controlling neutrophil motility and response to malignant cells. Scientific Reports. 2019;9(1):2399. doi:10.1038/s41598-019-38643-2
25. Zhang J, Liu C, Mo X, Shi H, Li S. Mechanisms by which CXCR4/CXCL12 cause metastatic behavior in pancreatic cancer. Oncology Letters. 2018;15(2):1771–1776. doi:10.3892/ol.2017.7512
26. Song J-S, Chang -C-C, Wu C-H, et al. A highly selective and potent CXCR4 antagonist for hepatocellular carcinoma treatment. Proceedings of the National Academy of Sciences. 2021;118(13):e2015433118. doi:10.1073/pnas.2015433118
27. Nengroo MA, Maheshwari S, Singh A, et al. CXCR4 intracellular protein promotes drug resistance and tumorigenic potential by inversely regulating the expression of death receptor 5. Cell Death & Disease. 2021;12(5):464. doi:10.1038/s41419-021-03730-8
28. Lei HW, Huang BR, Cai J, et al. CXCR4 antagonist AMD3100 enhances therapeutic efficacy of transcatheter arterial chemoembolization in rats with hepatocellular carcinoma. The Kaohsiung Journal of Medical Sciences. 2022;38(8):781–789. doi:10.1002/kjm2.12540
29. Salomonnson E, Stacer AC, Ehrlich A, Luker KE, Luker GD. Imaging CXCL12-CXCR4 signaling in ovarian cancer therapy. PLoS One. 2013;8(1):e51500. doi:10.1371/journal.pone.0051500
30. Saha T, Lukong KE. Breast cancer stem-like cells in drug resistance: a review of mechanisms and novel therapeutic strategies to overcome drug resistance. Front Oncol. 2022;12:856974. doi:10.3389/fonc.2022.856974
31. Hu C, Yong X, Li C, et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J Surg Res. 2013;183(1):427–434. doi:10.1016/j.jss.2013.01.019
32. Schwartz LH, Litière S, De Vries E, et al. RECIST 1.1—Update and clarification: from the RECIST committee. European Journal of Cancer. 2016;62:132–137. doi:10.1016/j.ejca.2016.03.081
33. Bahri S, Chen J-H, Mehta RS, et al. Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Annals of Surgical Oncology. 2009;16:1619–1628. doi:10.1245/s10434-009-0441-5
34. Hayward J, Rubens R, Carbone P, Heuson J-C, Kumaoka S, Segaloff A. Assessment of response to therapy in advanced breast cancer. A project of the programme on clinical oncology of the international union against cancer, Geneva, Switzerland. European Journal of Cancer. 1978;14(11):1291–1292. doi:10.1016/0014-2964(78)90238-4
35. Hao L, Zhang C, Qiu Y, et al. Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human breast cancer. Cancer Letters. 2007;253(1):34–42. doi:10.1016/j.canlet.2007.01.005
36. Sacanna E, Ibrahim T, Gaudio M, et al. The role of CXCR4 in the prediction of bone metastases from breast cancer: a pilot study. Oncology. 2011;80(3–4):225–231. doi:10.1159/000327585
37. Sobolik T, Su YJ, Wells S, Ayers GD, Cook RS, Richmond A. CXCR4 drives the metastatic phenotype in breast cancer through induction of CXCR2 and activation of MEK and PI3K pathways. mol Biol Cell. 2014;25(5):566–582. doi:10.1091/mbc.e13-07-0360
38. Spano JP, Andre F, Morat L, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15(4):613–617. doi:10.1093/annonc/mdh136
39. Wagner PL, Hyjek E, Vazquez MF, et al. CXCL12 and CXCR4 in adenocarcinoma of the lung: association with metastasis and survival. J Thorac Cardiovasc Surg. 2009;137(3):615–621. doi:10.1016/j.jtcvs.2008.07.039
40. Wang M, Chen GY, Song HT, Hong X, Yang ZY, Sui GJ. Significance of CXCR4, phosphorylated STAT3 and VEGF-A expression in resected non-small cell lung cancer. Exp Ther Med. 2011;2(3):517–522. doi:10.3892/etm.2011.235
41. Liu H. Expression and potential immune involvement of cuproptosis in kidney renal clear cell carcinoma. Cancer Genet. 2023;274-275:21–25. doi:10.1016/j.cancergen.2023.03.002
42. Liu H. Pan-cancer profiles of the cuproptosis gene set. Am J Cancer Res. 2022;12(8):4074–4081.
43. Liu H, Tang T. Pan-cancer genetic analysis of cuproptosis and copper metabolism-related gene set. Front Oncol. 2022;12:952290. doi:10.3389/fonc.2022.952290
44. Zhu Q, Zhang Z, Lu C, et al. Gefitinib promotes CXCR4-dependent epithelial to mesenchymal transition via TGF-beta1 signaling pathway in lung cancer cells harboring EGFR mutation. Clin Transl Oncol. 2020;22(8):1355–1363. doi:10.1007/s12094-019-02266-w
45. D’Alterio C, Buoncervello M, Ierano C, et al. Targeting CXCR4 potentiates anti-PD-1 efficacy modifying the tumor microenvironment and inhibiting neoplastic PD-1. J Exp Clin Cancer Res. 2019;38(1):432. doi:10.1186/s13046-019-1420-8
46. Tahirovic YA, Pelly S, Jecs E, et al. Small molecule and peptide-based CXCR4 modulators as therapeutic agents. A patent review for the period from 2010 to 2018. Expert Opin Ther Pat. 2020;30(2):87–101. doi:10.1080/13543776.2020.1707186
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Novel lncRNA Panel for Risk Stratification and Immune Landscape in Breast Cancer Patients

Li C, Wang X, Chen T, Li W, Yang Q
International Journal of General Medicine 2022, 15:5253-5272
Published Date: 27 May 2022
Establishment and Validation of a Model for Disease-Free Survival Rate Prediction Using the Combination of microRNA-381 and Clinical Indicators in Patients with Breast Cancer
Shen J, Wang M, Li F, Yan H, Wang R, Zhou J
Breast Cancer: Targets and Therapy 2022, 14:375-389
Published Date: 30 November 2022
Chromobox Family Proteins as Putative Biomarkers for Breast Cancer Management: A Preliminary Study Based on Bioinformatics Analysis and qRT-PCR Validation
Tian H, Zhao T, Li Y, Sun N, Ma D, Shi Q, Zhang G, Chen Q, Zhang K, Chen C, Zhang Y, Qi X
Breast Cancer: Targets and Therapy 2022, 14:515-535
Published Date: 30 December 2022
Prognostic Significance of Preoperative Lactate Dehydrogenase to Albumin Ratio in Breast Cancer: A Retrospective Study
He J, Tong L, Wu P, Wu Y, Shi W, Chen L
International Journal of General Medicine 2023, 16:507-514
Published Date: 8 February 2023
The Prognostic Role of HuR Varies Between Different Subtypes of Breast Cancer Patients: Data Mining and Retrospective Analysis
Liao Y, Liao Y, Li J, Li Y, Fan Y
Breast Cancer: Targets and Therapy 2023, 15:135-146
Published Date: 11 February 2023



