Back to Journals » Journal of Inflammation Research » Volume 17
Focal Staphylococcus Aureus Septic Arthritis Elicits Age and TLR2-Dependent Periarticular Bone Loss
Authors Schultz M , Hu Z , Deshmukh M, Henning P, Lerner UH, Mohammad M , Jin T
Received 6 September 2024
Accepted for publication 5 December 2024
Published 31 December 2024 Volume 2024:17 Pages 11901—11913
DOI https://doi.org/10.2147/JIR.S479718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Michelle Schultz,1,* Zhicheng Hu,1,2,* Meghshree Deshmukh,1 Petra Henning,3 Ulf H Lerner,3 Majd Mohammad,1 Tao Jin1,4
1Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; 2Center for Clinical Laboratories, the Affiliated Hospital of Guizhou Medical University, Guiyang, People’s Republic of China; 3Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Osteoporosis Centre and Centre for Bone and Arthritis Research at the Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; 4Department of Rheumatology, Sahlgrenska University Hospital, Gothenburg, Sweden
*These authors contributed equally to this work
Correspondence: Michelle Schultz, Department of Rheumatology & Inflammation, Institute of Medicine, The Sahlgrenska Academy at the University of Gothenburg, Guldhedsgatan 10A, Gothenburg, 41346, Sweden, Tel +46-31-3426475, Fax +46-31-823925, Email [email protected]
Introduction: Septic arthritis, primarily caused by Staphylococcus aureus (S. aureus), is a severe joint infection that leads to joint and bone damage. S. aureus lipoproteins (LPPs) bind to Toll-like Receptor 2 (TLR2), inducing arthritis and localized bone loss. Aging affects TLR2 immune response to pathogens. While intra-articular injections of S. aureus LPPs induces local bone resorption in mice, the influence of aging and TLR2 expression on bone mineral density (BMD) after S. aureus bacteremia remains unclear.
Methods: We analyzed distal femoral BMD in young and old TLR2 knock-out and wild-type mice following intravenous S. aureus infection. BMD was measured in both total and trabecular bone in old and young mice to determine age and TLR2-dependent responses to infection.
Results: In non-infected mice, BMD in both total and trabecular bone was mainly age-related and TLR2-independent. Following S. aureus bacteremia, young wild-type mice with TLR2 expression showed decreased combined cortical and trabecular BMD. This effect was absent in aged mice or TLR2 deficient mice. Focal septic arthritis, induced by S. aureus bacteremia, emerged as the primary cause to bone loss in the femur metaphysis. TLR2 appears to play a crucial role in focal septic arthritis-induced bone loss, as evidenced by in vitro findings demonstrating that staphylococcal LPPs, known TLR2 agonists, increase the Tnfsf11/Tnfrsf11b ratio in mouse pariosteal osteoblasts.
Conclusion: S. aureus bacteremia triggers local bone loss in murine arthritis, depending on both age and TLR2 expression.
Keywords: septic arthritis, Staphylococcus aureus, aging, TLR2, bone mineral density, mouse
Introduction
Septic arthritis, a severe joint infection primarily caused by Staphylococcus aureus (S. aureus), remains a medical emergency due to high mortality rates and potential for lasting joint damage.1–3 Studies indicate that bacterial components, rather than live bacteria, induce sustained inflammation.4 S. aureus-induced septic arthritis is linked to systemic bone mineral density (BMD) loss, a critical indicator for fracture risk and skeletal conditions such as osteoporosis.4–6
With age, immune system changes, including reduced T cell diversity and chronic inflammation, heighten susceptibility to infections and may impact bone metabolism.7 Toll-like Receptor 2 (TLR2), which recognizes bacterial lipoproteins (LPP) in the S. aureus cell wall, initiates a strong pro-inflammatory response through the TLR2 signaling pathway.8,9 Activation of this pathway recruits inflammatory cells, including macrophages and neutrophils, and triggers cytokine release (eg, TNF-a, IL-1b, IL-17), that intensify inflammation, resulting in joint damage and bone destruction.10,11 Aging may impair TLR2 responses, potentially increasing infection risk in older individuals; notably, advanced age is recognized as a significant risk factor for septic arthritis.12–15 Previous studies also suggest a link between septic arthritis and secondary osteoporosis.4
Our recent study shows that TLR2 deficiency and age impair immune responses to S. aureus bacteremia, with arthritis frequency and severity unaffected by TLR2 deficiency and age.16 TLR2 signaling, linked to joint inflammation and bone resorption through osteoclast activity, also appears crucial in S. aureus-related brain abscesses, where TLR2 inhibition has been shown to enhance anti-inflammatory treatment effectiveness.17–20 This supports the potential of TLR2 modulation as a therapeutic target in various S. aureus-related diseases.
Our study expands previous research by investigating the effect of TLR2 and age on femoral BMD in S. aureus infections.16 We found that young, TLR2-expressing mice experienced significant total BMD loss after infection, a pattern not observed in older or TLR2-deficient mice, suggesting an age and TLR2-dependent mechanism in bone loss. We identify focal septic arthritis as a contributor to bone loss in the murine femur metaphysis. In non-infected models, age-related BMD reductions occurred independently of TLR2, indicating intrinsic age effects on bone density. Additionally, we investigated staphylococcal lipoproteins in osteoclastogenesis, suggesting that TLR2 recognition of bacterial components not only initiates joint inflammation but also promotes bone resorption, likely via osteoclast activation. These findings suggest a novel link between immune responses and bone health in S. aureus infections, offering promising directions for future therapeutic strategies.
Materials and Methods
Ethics Statement
In Sweden, ethical approval for mouse studies is evaluated and granted by the local Ethics Committee for Animal Research. For the current study, ethical approval was provided by the Ethics Committee of Animal Research of Gothenburg, under permit number 5.8.18–02443/2021. All the experiments were conducted in accordance with the guidelines and recommendations of the Swedish Board of Agriculture.
Mice
Female C57BL/6 wild-type (WT) mice and TLR2−/− B6.129-Tlr2tm1 Kir/J (TLR2−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). All mouse strains were housed and bred without backcrossing until they reached a designated age for experiments under controlled laboratory conditions at the animal facility of the Department of Rheumatology and Inflammation Research in Gothenburg, Sweden. The selected age groups for WT mice and TLR2−/− mice were comprised of young mice (13–28 weeks) and old mice (73–89 weeks). Female NMRI mice, aged 8 weeks, were used for the study of S. aureus lipoprotein-induced arthritis. The mice were obtained from Envigo (Venray, The Netherlands).
Preparation of Bacterial Strains
S. aureus Newman WT,21 SA113, and SA113Δlgt22 strains were prepared as described.23 The S. aureus Newman strain is well-established as an arthritogenic strain, notable for its lack of antibiotic resistance. Briefly, the bacterial strains were stored at −20°C. For experimental use, S. aureus Newman strain was thawed, washed with sterile phosphate-buffered saline (PBS), and adjusted to a concentration of 1.5×106 colony-forming units (CFUs) for intravenous injection. Because of the potential for impaired immune response and increased mortality in aged and TLR2-deficient mice, the bacterial concentration was kept lower than the typical arthritogenic dose of 4.0–6.0×106 CFUs to accommodate these vulnerable groups.23 For heat-killed bacteria, S. aureus strains (SA113 and SA113Δlgt) were heat-killed at 95°C for 45 min.
Experimental Protocols for S. Aureus Bacteremia Mouse Model
The mouse bacteremia model was established as described.16 Intravenous injections of 200 µL of S. aureus suspension were inoculated via the tail vein. The mice were monitored daily for 10 days post-infection. The clinical outcomes of S. aureus bacteremia in mice in this study, including mortality rates and septic arthritis frequency, have been described elsewhere.16 Surviving mice were euthanized on day 10, followed by the collection of knee samples. Data were pooled from two independent experiments (n=44 and n=16 knee samples).
Induction of S. Aureus Lipoprotein-Induced Arthritis
NMRI mice were intra-articularly injected in the knee joint with either 20 µL of PBS that contained purified S. aureus lipoprotein (LPP) (4 µg/knee) or 20 µL of PBS as controls. Clinical severity was evaluated by measuring the differences in knee joint diameters with a caliper. On day 3 after injection, the knee joints were collected for further tartrate-resistant acid phosphatase (TRAP) staining.
Histopathologic Examination
The joints were subjected to decalcification, followed by paraffin embedding and microtome sectioning. The resulting tissue sections were stained with TRAP. The staining was performed using a customized TRAP buffer that consisted of 0.2 M acetate buffer, 0.3 M sodium tartrate, 10 mg/mL naphthol AS-MX phosphate, 0.1% Triton X-100, and 0.3 mg/mL Fast Red Violet LB (Sigma-Aldrich). Following deparaffinization and incubation with acetate buffer, the tissue samples were incubated in the TRAP buffer overnight and subsequently counterstained with Fast Green.
Micro-Computed Tomography
Septic arthritis was identified by computed tomography (CT)-detected bone erosions for two reasons: 1) all CT-confirmed erosions in our model were consistently linked to septic arthritis, as supported by prior data;24 2) clinical assessment of deeper joints (eg, knees, hips, shoulders) is challenging, and CT imaging provides greater reliability for detecting septic arthritis in our animal model. Following termination of the experiment, the mouse knee joints were fixed in 4% formaldehyde. Before scanning, the joints were transferred to PBS and scanned using a SkyScan1176 micro-computed tomography scanner (micro-CT, Bruker, Antwerp, Belgium). Scanning parameters included a voltage of 55 kV, current of 455μA, voxel size of 9μm, aluminum filter of 0.2 mm, and exposure time of 755ms. The scanning angular rotation was set to 180° and the X-ray projections were captured at 0.42° intervals. CTvox software (version 2.7.0; Bruker) was used for image reconstruction and morphometric analysis. To obtain bone mineral density measurements of the distal femur metaphysis in the mouse knee joints, the CT-Analyser software (version 1.14.1.1; Bruker) was used. BMD and bone morphometric analyses were based on a 0.86 mm section of the femoral bone, corresponding to 100 micro-CT image slices, obtained 50 slices away from the reference point. The growth plate was selected as the reference point for this experiment. Total BMD measurements refer to measurements of the combined trabecular and cortical bone, whereas trabecular measurements refer only to the trabecular bone within this region. Erosion scoring of the knee joints was blindly executed individually by M.S., Z.H., and M.M., involving the assessment of reconstructed 3-dimensional images. The scoring system ranged from 0 to 3, with higher scores indicating a greater extent of erosion, as described elsewhere.24,25
Calvarial Osteoblast Cultures and Gene Expression Analyses
Calvarial periosteal cells were isolated from 3–5 days old C57BL/6N mice by sequential collagenase treatment, as previously described.26,27 Isolated cells were cultured in complete α-MEM medium (cat no 22561–021, Gibco, Thermo Fisher Scientific) supplemented with 10% heat-inactivated fetal bovine serum (FBS; cat no F7524, Sigma), 2 mM GlutaMAX (cat no 35050–038, Gibco), 50 µg/mL gentamicin (cat no 15750–037, Gibco), 100 U/mL penicillin, and 100 µg/mL streptomycin (cat no 15140–148, Gibco) for 3–5 days prior to the start of the experiment. At the start of the experiments, the cells were re-seeded in 48-well plates at a density of 20.000 cell/cm2 and cultured in the presence or absence of Pam2Cys (EMC, Tübingen, Germany, 20 ng/mL), Pam3Cys (EMC, Tübingen, Germany, 20 ng/mL), heat-killed SA113WT (3x107 cfu/mL), or SA113Δlgt (3x107 cfu/mL) for 24h. Cells were harvested for gene expression analysis by lysis in RLT buffer (Qiagen), followed by RNA purification using an RNAeasy Micro kit (cat. no 74004, Qiagen). Single-strand cDNA was synthesized using a High-Capacity cDNA Reverse Transcription kit (cat. no. 4374967, Applied Biosystems). Quantitative real-time PCR (qPCR) analyses were performed using predesigned Taqman Assays and Taqman Fast Advance Master Mix and the StepOnePlus Real-Time PCR system (Applied Biosystems). The following predesigned real-time PCR assays were used for gene expression analysis: Tnfsf11 (Rankl; Mm00441908_m1), Tnfrsf11b (Opg; Mm00435452_m1), Tnfa (Mm00443258_m1), House-keeping gene 18S (cat no 4310893E) was used as an endogenous control. Data were calculated as fold changes relative to the mean of the vehicle (Veh) group. Cell culture stimulation experiments were performed twice, and similar results were obtained.
Measurement of Cytokine/Chemokine Levels in Mouse Blood
The blood levels of IL6 and keratinocyte-derived chemokine (KC) from four groups of mice infected with S. aureus were quantified using DuoSet ELISA Kits (R&D Systems Europe, Abingdon, UK) according to the manufacturer’s instructions.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (version 9.2.0, GraphPad Software, La Jolla, CA, United States). Comparisons between experimental groups were performed using the Mann–Whitney U-test, Spearman’s rank correlation test, Kruskal–Wallis test followed by Dunn’s multiple comparison test, and ordinary one-way ANOVA followed by Šídák’s or Dunnett´s multiple comparison test. The results are presented as mean ± standard error of the mean. Significance levels are presented as follows: *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
Results
Age-Related Bone Loss in Total and Trabecular Metaphyseal Bone in Healthy Mice is TLR2 Independent
To study the effects of increased age and TLR2 on BMD, femoral metaphyseal BMD was analyzed in WT and TLR2−/− mice aged between 13–16 weeks (young) and 75–82 weeks (old) under non-infected conditions. To mitigate the potential impact of genetic drift on BMD, statistical comparisons were performed exclusively between old and young mice of the same strain. Significantly lower values for total and trabecular BMD were observed in both WT and TLR2−/− mice in the older age groups than in their younger counterparts (Figure 1A and B). These data suggest that advanced age significantly contributes to a reduction in the total femoral metaphyseal BMD in mice. Similarly, an increase in age was observed to significantly reduce trabecular bone volume fraction and trabecular number in the older groups compared to the younger groups (Figure 1C and D). Trabecular thickness was significantly reduced in TLR2−/−/old mice compared to TLR2−/−/young mice, whereas no significant differences were observed between the two WT age groups (Figure 1E). Trabecular separation was significantly increased in WT/old and TLR2−/−/old mice compared to that in their younger counterparts (Figure 1F). Figure 1G shows representative micro-CT images of the different groups.
 |
Figure 1 Age-related bone loss in total and trabecular metaphyseal bone in healthy mice is TLR2 independent. Knee samples from healthy female C57BL/6 wild-type (WT) mice and Toll-like receptor (TLR) 2 deficient (TLR2−/−) mice, aged 13–16 weeks (young) or 75–82 weeks (old)- including WT/young (n=10 knee joints), WT/old (n=10 knee joints), TLR2−/−/young (n=12 knee joints) and TLR2−/−/old (n=8 knee joints), were collected for bone analysis. Measurements of metaphyseal (A) total- and (B) trabecular bone mineral density (BMD, g/cm3). Graphs showing quantitative evaluations of metaphyseal (C) trabecular bone volume fraction, (D) trabecular number, (E) trabecular thickness, and (F) trabecular separation in the respective group of mice. (G) Representative 2D micro-computed tomography images of femoral metaphysis of young and old WT and TLR2−/− mice. Statistical evaluations were performed using the Mann–Whitney U-test between young and old mice of the same genotype (A–F). The data are presented as a scatterplot and expressed as the means with standard error of the mean. **p<0.01, ***p<0.001 and ****p<0.0001; ns= not significant. |
Only Wild Type Young Mice Displayed Bone Loss After Septic Arthritis Induced by S. Aureus Bacteremia
Intra-articular injections of S. aureus lipoproteins (LPP) have been shown to induce BMD loss18 as well as mediate arthritogenic and bone destructive effects.17,28 To further investigate the relationship between septic arthritis resulting from S. aureus bacteremia and its impact on BMD, including the factors of aging and TLR2, an examination was conducted using WT and TLR2−/− mice of young (13–28 weeks) and old (73–89 weeks) age. Aged WT mice exhibited significantly higher mortality compared to their younger counterparts, while young TLR2−/− mice showed a tendency toward higher mortality than young WT mice. Among all groups, aged TLR2−/− mice demonstrated the highest mortality rate (Supplementary Figure 1). The blood levels of IL6 and KC did not differ significantly among those four groups of mice 10 days post infection (Supplementary Figure 2). The mice were intravenously inoculated with the S. aureus Newman WT strain, followed by micro-CT evaluation of femoral BMD at 10 days post-infection. BMD measurements of infected mice were compared with those of healthy WT and TLR2−/− mice in young (13–16 weeks) and old (75–82 weeks) age groups. Total BMD measurements were lower in WT Young/Infected mice than in WT Young/Healthy mice (Figure 2A). This effect was not observed in case of TLR2 deficient mice (Figure 2B), suggesting that TLR2 contributes to bone loss after infection of WT mice. No significant difference in total BMD was observed between WT Old/Healthy and WT Old/Infected or TLR2−/− Old/Healthy and TLR2−/− Old/Infected mice (Figure 2A and B), suggesting that both TLR2 and age contribute to the total BMD reduction due to septic arthritis. We further analyzed the trabecular BMD changes after septic arthritis. The differences observed in total BMD between WT Young/Infected and WT Young/Healthy mice were not observed in the metaphyseal trabecular bone. Surprisingly, trabecular BMD was higher in TLR2−/− Old/Infected mice than in TLR2−/− Old/Healthy mice (Figure 2D).
 |
Figure 2 Only wild type young mice displayed bone loss after septic arthritis induced by S. aureus bacteremia. C57BL/6 wild-type (WT) and Toll-like receptor (TLR) 2 deficient (TLR2−/−) mice aged 13–28 weeks (young), or 73–89 weeks (old)- including WT Young/Infected (n=16 knee joints), WT Old/Infected (n=10 knee joints), TLR2−/− Young/Infected (n=18 knee joints) and TLR2−/− Old/Infected (n=16 knee joints), were intravenously inoculated with Staphylococcus aureus Newman WT strain at a dose of 1.5×106 colony-forming units per mouse. Knee samples were collected for bone analysis on day 10 after infection. Knee samples collected for bone analysis from healthy female C57BL/6 WT mice and TLR2−/− mice, aged 13–16 weeks (young) or 75–82 weeks (old)- including WT Young/Healthy (n=10 knee joints), WT Old/Healthy (n=10 knee joints), TLR2−/− Young/Healthy (n=12 knee joints) and TLR2−/− Old/Healthy (n=8 knee joints), were collected for bone analysis. Measurement of total bone mineral density (BMD, g/cm3) in healthy and infected (A) WT and (B) TLR2−/− mice. Trabecular BMD measurements of healthy and infected (C) WT and (D) TLR2−/− mice. Statistical evaluations were performed using Kruskal–Wallis test with Dunn’s posttest (A) or Ordinary one-way ANOVA with Šídák’s posttest (B–D) between the healthy and infected mice of the same age. The data were pooled from two independent experiments and are presented as a scatterplot with the data expressed as the means with standard error of the mean. *p<0.05 and **p<0.01; ns= non significant. |
Focal Septic Arthritis is the Main Cause of Bone Loss in Femur Metaphysis After Septic Arthritis Induced by S. Aureus Bacteremia
To investigate the impact of focal septic arthritis induced by S. aureus bacteremia on bone density, the knee joints of infected WT and TLR2−/− mice were categorized based on the presence or absence of erosion. The division of joints was determined by analyzing the reconstructed 3D micro-CT images and assigning severity scores ranging from 0 to 3, with a cutoff point of 1 indicating erosion. Subsequently, BMD measurements were compared between the groups. Significant differences between the total and trabecular BMD were observed only between the eroded and non-eroded WT/young joints (Figure 3A and B). Our results imply that focal septic arthritis induces local bone loss in the femur metaphysis of young mice expressing TLR2. Representative 2D and 3D micro-CT images of the corresponding groups are shown in Figure 3C. Additionally, we examined the correlation between BMD and bone erosion scores in various mouse subgroups. Significant negative correlations were observed between young and old wild-type mice as well as in young TLR2 deficient mice. Interestingly, these correlations were absent in old TLR2 deficient mice (Table 1). This strengthens the conclusion that both aging and TLR2 play pivotal roles in the reduction of trabecular BMD associated with focal septic arthritis following S. aureus bacteremia.
 |
Table 1 TLR2 is the Major Determinant for the Correlation Between Focal Septic Arthritis and Bone Resorption in S. Aureus Bacteremia |
Staphylococcal Lipoproteins Enhance Tnfsf11/Tnfrsf11b Ratio in Mouse Periosteal Osteoblasts
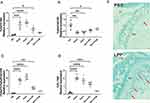
We further utilized widely recognized TLR2 agonists, synthetic lipopeptides (Pam2Cys and Pam3Cys), heat-killed S. aureus SA113 expressing lipoproteins, and S. aureus lacking lipoproteins (SA113Δlgt) to examine the potential stimulatory impact of staphylococcal lipoproteins on osteoclastogenesis. This was achieved by evaluating Tnfsf11/Tnfrsf11b ratio in mouse calvarial periosteal osteoblasts. The RANKL/RANK signaling pathway stimulates osteoclastogenesis, whereas osteoprotegerin (OPG) protects against excessive bone resorption by binding to RANKL. The RANKL/OPG ratio reflects the potential to induce osteoclast differentiation.29 We investigated whether staphylococcal lipoproteins induce RANKL production and alter the RANKL/OPG ratio in mouse parietal osteoblasts. Staphylococcal lipopeptide stimulation led to increased expression of the osteoclastogenic cytokine gene Tnfsf11 and proinflammatory cytokine Tnfα mRNA (Figure 4A and D), while downregulating Tnfrsf11b mRNA (Figure 4B). This resulted in a significant increase in RANKL/OPG ratio (Figure 4C). Deletion of Lpp diacylglyceryl transferase (lgt) resulted in a lack of prelipoprotein lipidation and subsequent lipoprotein deficiency in S. aureus.22 Notably, osteoblasts stimulated with heat-killed lipoprotein-deficient S. aureus did not enhance the mRNA expression of Tnfsf11, Tnfa or the Tnfsf11/Tnfrsf11b ratio compared to vehicle-treated cells. Additionally, the intraarticular injection of S. aureus LPP resulted in a clear increase in TRAP-positive cells compared to PBS-injected knees, strongly suggest that S. aureus LPP induce osteoclast activation (Figure 4E). Our findings highlight the crucial role of S. aureus lipoproteins in the development of osteoporosis associated with S. aureus septic arthritis.
Discussion
There is a subtle relationship between aging, septic arthritis, and osteoporosis. Advanced age is a risk factor for septic arthritis. Aging increases the incidence of osteoporosis, particularly in females. Furthermore, septic arthritis appears to trigger localized bone resorption and stimulates osteoporosis. S. aureus lipoproteins bind TLR2 and have been shown to induce focal bone resorption following intra-articular injections in mouse knees.18 This information led us to hypothesize that the interplay between TLR2 and aging may contribute to bone resorption in murine septic arthritis induced by S. aureus bacteremia.
In the present study, we investigated the potential impact of TLR2 and age on bone loss, both in healthy conditions and during septic arthritis induced by S. aureus bacteremia in female mice. Our findings indicate that regardless of TLR2 expression, aged female mice experienced decreases in both total and trabecular BMD. However, the significance of TLR2 in this process has not been previously reported. Focal septic arthritis directly contributes to total and trabecular bone loss in mice. Remarkably, the absence of TLR2 and the aging process contributed to the preservation of the negative changes in BMD during infection. Our findings demonstrate that bone loss induced by septic arthritis leads to decreased BMD, a process heavily dependent on TLR2 expression and age.
The present study used aged mice ranging from 73 to 89 weeks of age, aligning with the human age range of 56 to 69 years.30,31 Postmenopausal women are notably prone to osteoporosis due to decreased ovarian production of sex steroids, elevated follicle-stimulating hormone (FSH) expression, and estrogen deficiency.32,33 Mice exhibit comparable endocrine changes to human menopause, including follicle decline and irregular cycling, but certain studies have shown that the hormonal environment in aged mice may not perfectly mimic the hormonal changes seen in human menopause.34 Our data revealed a decline in femur BMD – both total and trabecular – in aging female mice, consistent with human observations.35
Considering the absence of TLR2 might confer resistance against osteoporosis, considering TLRs’ role in recognizing microbial danger signals and triggering osteoclastogenesis.36 Throughout life, mice are likely to encounter diverse pathogens, such as staphylococci expressing bacterial lipoproteins. TLR2 deficiency potentially halts osteoclastogenesis induced by TLR2 activation. However, the total and trabecular BMD were similarly reduced in both aging WT and TLR2−/− mice, indicating TLR2’s limited involvement in age-related osteoporosis.
It has been previously demonstrated that both total and trabecular bone mineral density are significantly reduced after three days in a septic arthritis mouse model compared to uninfected controls.4 However, in the current study, no significant differences in trabecular BMD were observed between the control and infection groups. This discrepancy may be explained by the lower bacterial dose used in our study, which was adjusted to accommodate the older and TLR2 deficient mice. The response of BMD to S. aureus bacteremia presents complex dynamics. Total BMD before and after infection appears to be dependent on both TLR2 expression and age. A significant decrease following infection was only observed in young WT mice but not in aged mice or TLR2 deficient mice. Trabecular BMD was not significantly affected by infection in young WT mice. This observation could be attributed to the joints being unaffected by septic arthritis, which maintained an unchanged trabecular BMD. It is important to note that aged TLR2-deficient mice demonstrated a substantial increase in trabecular BMD after infection compared to their respective healthy controls. The reason for this observation remains unexplained, highlighting the need for further studies to validate our findings and elucidate the underlying mechanisms responsible for the observed changes in trabecular BMD in response to infection. These observations indicate that infection stimulates bone formation independently of TLR2.
Our working hypothesis centers on focal septic arthritis as a primary driver of bone resorption in S. aureus bacteremia. Unlike the symmetrical involvement of numerous small joints observed in rheumatoid arthritis, septic arthritis typically targets larger joints, particularly the knees, often resulting in monoarthritis.37 In our prior study, we noted a predisposition for distal femur involvement in knees in septic arthritis within our murine model.24 Given that our BMD assessments were derived from the femur metaphysis, this location provided an ideal foundation for testing our hypothesis. Indeed, our findings indicate that knees exhibiting erosion in young WT mice are associated with significantly reduced BMD (both total and trabecular) compared with non-eroded knees. This finding supports the notion that focal bone erosion due to septic arthritis is the primary contributor to the BMD reduction in young WT mice. Intriguingly, this pattern was not mirrored in TLR2 deficient mice, both young and old, in which no BMD disparities were observed between the eroded and non-eroded knees. This suggests a pivotal role for TLR2 in mediating BMD reduction resulting from focal S. aureus septic arthritis.23
The scarcity of eroded joints in aged WT mice complicates the drawing of definitive conclusions regarding the role of aging in septic arthritis-induced BMD reduction. However, the statistical significance of the correlation between erosion scores and BMD was remarkably robust in young WT mice (p < 0.0001). This significance decreased to 0.0167 for total BMD and 0.0889 for trabecular BMD in the aged WT mice. This subtle shift strongly implies that advanced age likely diminishes the BMD-reducing effects of focal S. aureus in septic arthritis. Notably, it has been shown that aging impairs the TLR2 signaling pathway38,39 and a dysfunctional innate immune response to TLR2 agonists,13 providing a potential mechanistic link to our observations.
Our in vitro findings were consistent with our in vivo results. Lipopeptides and lipoprotein-containing heat-killed S. aureus robustly upregulated RANKL, a potent stimulator of osteoclast differentiation and activation.40 Conversely, OPG, which acts as a soluble decoy receptor for RANKL,41 was downregulated in response to these substances. Notably, the balance between RANKL and OPG expression levels within osteoblastic cells serves as a pivotal indicator of osteogenesis activation.42,43 Remarkably, the RANKL/OPG ratio exhibited a substantial increase in response to lipopeptides, particularly evident with Pam3 (upregulation exceeding 15-fold) as well as the heat-killed SA113 parent strain rich in lipoproteins. Intriguingly, this effect was absent in the heat-killed SA113∆lgt strain, which lacks proper prelipoprotein lipidation. Heat-killed S. aureus is known to elicit arthritis and provoke bone erosions.44 Lipoproteins, which are key contributors to joint inflammation, mediate their effects through TLR2. Our previous study further substantiated that S. aureus lipoproteins trigger bone loss within a locally induced mouse model, operating through TLR2 and involving monocytes/macrophages.18 Indeed, ex vivo data from UV-inactivated S. aureus stimulation of neonatal mouse parietal bone revealed that S. aureus enhances bone resorption and periosteal osteoclast formation by increasing osteoblast RANKL production through TLR2.45 It has been shown that bacterial DNA and peptidoglycan, both abundant in heat-killed S. aureus, possess arthritogenic and bone-destructive potential.46,47 The RANKL/OPG ratio remained unaffected in the heat-killed SA113∆lgt strain, indicating that S. aureus lipoproteins, rather than peptidoglycan and bacterial DNA, constitute the primary drivers behind the elevation of RANKL/OPG in osteoblasts. This phenomenon triggers osteoclast activation and promotes bone resorption.
The systemic inflammation triggered by bacteremia can exert an impact on bone resorption, consequently leading to a reduction in BMD.48 Here, our study presents compelling evidence that in cases of bacteremia, focal septic arthritis plays a pivotal role in inducing significant localized bone loss within the affected joints, affecting both cortical and trabecular bone structures. Of particular interest is the observation that this effect is prominently influenced by TLR2 and, to some extent, by the aging process in the context of S. aureus infection, as illustrated in Figure 5. In future experiments, testing TLR2 inhibitors in murine models of S. aureus septic arthritis could reveal whether targeted suppression of TLR2 can mitigate infection-related bone loss, potentially opening new avenues for therapeutic interventions that address both joint inflammation and osteolytic damage without compromising infection control.
It is important to note that septic arthritis can also be induced by gram-negative bacteria, with lipopolysaccharides (LPS) potentially serving as the most potent bacterial component driving joint inflammation. Consequently, in instances of septic arthritis caused by Gram-negative bacteria, the impact of BMD reduction due to septic arthritis could be contingent on TLR4 rather than TLR2. From a clinical standpoint, our findings underscore the necessity of incorporating joints affected by septic arthritis into BMD measurements of younger patients who have recently experienced this condition. This practice is essential for understanding the fracture risks associated with these specific joints. Conversely, this inclusion might be less significant in patients with advanced age.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. The original contributions of this study are included in the article/supplementary material, and further inquiries can be directed to the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Swedish Medical Research Council (grants 523-2013-2750, 2019-01135, 2024-02667 to T. J., and 2020-02181 to U.H.L.); ALF grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (grants ALFGBG-823941, ALFGBG-933787, ALFGBG-965074, and ALFGBG-1005076 to T. J., ALFGBG-965793 to U.H.L.); National Natural science foundation of China (Grant number 82360396) to Z.H.; E och K.G. Lennanders stipendiestiftelse to (M. M.); Inger Bendix Foundation for Medical Research to (M. M.); Magnus Bergvalls Stiftelse (grants 2022-426 to M.M.); Petrus och Augusta Hedlunds Stiftelse (grants M-2023-2079 to M.M.); Stiftelsen Konung Gustaf V:s 80 årsfond (grants SGI-2022-0880 and SGI-2023-1043 to M.M.); Rune och Ulla Amlövs Stiftelse för Neurologisk och Reumatologisk Forskning to (M. M., T. J., and Z. H.); the Gothenburg Society of Medicine (grants GLS-002/02 to M.S.); Stiftelsen Erik & Lily Philipson Minnesfond to (M.S.); Stiftelsen Wilhelm och Martina Lundgrens Vetenskapsfond (grants 2023-SA-4253 to M.M.); Sahlgrenska University Hospitals Research Foundations (grants SU-998178 and SU-984612 to Z.H., SU-984324 and SU-998197 to M.D.; SU-971990, SU-984308, and SU-998128 to M. M.).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang J, Wang L. Novel therapeutic interventions towards improved management of septic arthritis. BMC Musculoskelet Disord. 2021;22(1):530. doi:10.1186/s12891-021-04383-6
2. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423–428. doi:10.1017/s0950268800059070
3. Abram SGF, Alvand A, Judge A, Beard DJ, Price AJ. Mortality and adverse joint outcomes following septic arthritis of the native knee: a longitudinal cohort study of patients receiving arthroscopic washout. Lancet Infect Dis. 2020;20(3):341–349. doi:10.1016/S1473-3099(19)30419-0
4. Verdrengh M, Carlsten H, Ohlsson C, Tarkowski A. Rapid systemic bone resorption during the course of Staphylococcus aureus-induced arthritis. J Infect Dis. 2006;194(11):1597–1600. doi:10.1086/508751
5. McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3(2):57–63. doi:10.1007/s11914-005-0005-y
6. Nuti R, Brandi ML, Checchia G, et al. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med. 2019;14(1):85–102. doi:10.1007/s11739-018-1874-2
7. Mi B, Xiong Y, Knoedler S, et al. Ageing-related bone and immunity changes: insights into the complex interplay between the skeleton and the immune system. Bone Res. 2024;12(1):42. doi:10.1038/s41413-024-00346-4
8. Nguyen MT, Götz F. Lipoproteins of gram-positive bacteria: key players in the immune response and virulence. Microbiol Mol Biol Rev. 2016;80(3):891–903. doi:10.1128/mmbr.00028-16
9. Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285(5428):736–739. doi:10.1126/science.285.5428.736
10. Ghosh R, Dey R, Sawoo R, Bishayi B. Neutralization of IL-17 and treatment with IL-2 protects septic arthritis by regulating free radical production and antioxidant enzymes in Th17 and Tregs: an immunomodulatory TLR2 versus TNFR response. Cellular Immunology. 2021;370:104441. doi:10.1016/j.cellimm.2021.104441
11. Ghosh R, Bishayi B. Endogenous blocking of TLR2 along with TNF-α and IL-1β ameliorates the severity of the S. aureus arthritis via modulating STAT3/SOCS3 expressions in tissue resident macrophages. Microb Pathogenesis. 2024;187:106518. doi:10.1016/j.micpath.2023.106518
12. Kline KA, Bowdish DM. Infection in an aging population. Curr Opin Microbiol. 2016;29:63–67. doi:10.1016/j.mib.2015.11.003
13. Bailey KL, Smith LM, Heires AJ, Katafiasz DM, Romberger DJ, LeVan TD. Aging leads to dysfunctional innate immune responses to TLR2 and TLR4 agonists. Aging Clin Exp Res. 2019;31(9):1185–1193. doi:10.1007/s40520-018-1064-0
14. Elsissy JG, Liu JN, Wilton PJ, Nwachuku I, Gowd AK, Amin NH. Bacterial septic arthritis of the adult native knee joint: a review. JBJS Rev. 2020;8(1):e0059. doi:10.2106/jbjs.Rvw.19.00059
15. Alexandersson H, Dehlin M, Jin T. Increased incidence and clinical features of septic arthritis in patients aged 80 years and above: a comparative analysis with younger cohorts. Pathogens. 2024;13(10):891. doi:10.3390/pathogens13100891
16. Hu Z, Kopparapu PK, Deshmukh M, et al. The impact of aging and toll-like receptor 2 deficiency on the clinical outcomes of Staphylococcus aureus bacteremia. J Infect Dis. 2023;228(3):332–342. doi:10.1093/infdis/jiad046
17. Mohammad M, Nguyen MT, Engdahl C, et al. The YIN and YANG of lipoproteins in developing and preventing infectious arthritis by Staphylococcus aureus. PLoS Pathog. 2019;15(6):e1007877. doi:10.1371/journal.ppat.1007877
18. Schultz M, Mohammad M, Nguyen MT, et al. Lipoproteins cause bone resorption in a mouse model of Staphylococcus aureus septic arthritis. Front Microbiol. 2022;13:843799. doi:10.3389/fmicb.2022.843799
19. Dey R, Bishayi B. Ciprofloxacin and dexamethasone in combination attenuate S. aureus induced brain abscess via neuroendocrine-immune interaction of TLR-2 and glucocorticoid receptor leading to behavioral improvement. Int Immunopharmacol. 2021;97:107695. doi:10.1016/j.intimp.2021.107695
20. Dey R, Bishayi B. Dexamethasone exhibits its anti-inflammatory effects in S. aureus induced microglial inflammation via modulating TLR-2 and glucocorticoid receptor expression. Int Immunopharmacol. 2019;75:105806. doi:10.1016/j.intimp.2019.105806
21. Jarneborn A, Mohammad M, Engdahl C, et al. Tofacitinib treatment aggravates Staphylococcus aureus septic arthritis, but attenuates sepsis and enterotoxin induced shock in mice. Sci Rep. 2020;10(1):10891. doi:10.1038/s41598-020-67928-0
22. Stoll H, Dengjel J, Nerz C, Götz F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun. 2005;73(4):2411–2423. doi:10.1128/iai.73.4.2411-2423.2005
23. Mohammad M, Hu Z, Ali A, et al. The role of Staphylococcus aureus lipoproteins in hematogenous septic arthritis. Sci Rep. 2020;10(1):7936. doi:10.1038/s41598-020-64879-4
24. Fatima F, Fei Y, Ali A, et al. Radiological features of experimental staphylococcal septic arthritis by micro computed tomography scan. PLoS One. 2017;12(2):e0171222. doi:10.1371/journal.pone.0171222
25. Ali A, Welin A, Schwarze JC, et al. CTLA4 immunoglobulin but not anti-tumor necrosis factor therapy promotes Staphylococcal septic arthritis in mice. J Infect Dis. 2015;212(8):1308–1316. doi:10.1093/infdis/jiv212
26. Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol. 2012;816:19–29. doi:10.1007/978-1-61779-415-5_2
27. Granholm S, Henning P, Lindholm C, Lerner UH. Osteoclast progenitor cells present in significant amounts in mouse calvarial osteoblast isolations and osteoclastogenesis increased by BMP-2. Bone. 2013;52(1):83–92. doi:10.1016/j.bone.2012.09.019
28. Mohammad M, Ali A, Nguyen MT, Götz F, Pullerits R, Jin T. Staphylococcus aureus lipoproteins in infectious diseases. Front Microbiol. 2022;13:1006765. doi:10.3389/fmicb.2022.1006765
29. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1. doi:10.1186/ar2165
30. Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol a Biol Sci Med Sci. 2013;68(10):1209–1217. doi:10.1093/gerona/glt046
31. Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith AThe Mouse in Biomedical Research. Elsevier Academic Press; 2006.
32. Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006;103(40):14925–14930. doi:10.1073/pnas.0606805103
33. Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi:10.1016/j.cell.2006.01.051
34. Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153(8):3571–3578. doi:10.1210/en.2012-1340
35. Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4(2):61–76. doi:10.1177/1759720x11430858
36. Yim M. The role of toll-like receptors in osteoclastogenesis. J Bone Metab. 2020;27(4):227–235. doi:10.11005/jbm.2020.27.4.227
37. Mathews CJ, Weston VC, Jones A, Field M, Coakley G. Bacterial septic arthritis in adults. Lancet. 2010;375(9717):846–855. doi:10.1016/S0140-6736(09)61595-6
38. Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126(12):1305–1313. doi:10.1016/j.mad.2005.07.009
39. Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012;47(7):507–518. doi:10.1016/j.exger.2012.04.004
40. Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–3602. doi:10.1073/pnas.95.7.3597
41. Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi:10.1016/s0092-8674(00)80209-3
42. Horwood NJ, Elliott J, Martin TJ, Gillespie MT. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology. 1998;139(11):4743–4746. doi:10.1210/endo.139.11.6433
43. Nagai M, Sato N. Reciprocal gene expression of osteoclastogenesis inhibitory factor and osteoclast differentiation factor regulates osteoclast formation. Biochem Biophys Res Commun. 1999;257(3):719–723. doi:10.1006/bbrc.1999.0524
44. Ali A, Zhu X, Kwiecinski J, et al. Antibiotic-killed Staphylococcus aureus induces destructive arthritis in mice. Arthritis Rheumatol. 2015;67(1):107–116. doi:10.1002/art.38902
45. Kassem A, Lindholm C, Lerner UH. Toll-like receptor 2 stimulation of osteoblasts mediates staphylococcus aureus induced bone resorption and osteoclastogenesis through enhanced RANKL. PLoS One. 2016;11(6):e0156708. doi:10.1371/journal.pone.0156708
46. Deng GM, Nilsson IM, Verdrengh M, Collins LV, Tarkowski A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat Med. 1999;5(6):702–705. doi:10.1038/9554
47. Liu ZQ, Deng GM, Foster S, Tarkowski A. Staphylococcal peptidoglycans induce arthritis. Arthritis Res. 2001;3(6):375–380. doi:10.1186/ar330
48. Caetano-Lopes J, Canhão H, Fonseca JE. Osteoimmunology--The hidden immune regulation of bone. Autoimmun Rev. 2009;8(3):250–255. doi:10.1016/j.autrev.2008.07.038
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.