Back to Journals » Nanotechnology, Science and Applications » Volume 18
From Nature to Nanotechnology: The Bioactivities of Mangiferin Explored
Authors Al-Madhagi H
Received 27 February 2025
Accepted for publication 4 July 2025
Published 10 July 2025 Volume 2025:18 Pages 277—294
DOI https://doi.org/10.2147/NSA.S525423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Kattesh Katti
Haitham Al-Madhagi
Biochemical Technology Program, Dhamar University, Dhamar, Yemen
Correspondence: Haitham Al-Madhagi, Email [email protected]
Abstract: Mangiferin, a naturally occurring C-glucosylxanthone derived from various parts of the mango tree (Mangifera indica), has gained significant attention for its diverse pharmacological properties, including antioxidant, anti-inflammatory, antimicrobial, anticancer, and anti-diabetic activities. This mini-review provides an updated overview of the phytochemistry, pharmacokinetics, and medicinal properties of mangiferin. However, the low solubility (0.111 mg/mL) and oral bioavailability (less than 2%) of mangiferin pose significant challenges for its clinical application. To address these issues, the development of nanoformulations such as nanoparticles, micelles, and liposomes has been explored, which was proven to improve mangiferin’s solubility, stability, and targeted delivery. These nanocarriers enhance the bioavailability and therapeutic efficacy of mangiferin, making it a promising candidate for various therapeutic applications. The review ends with the discussion of the safety of mangiferin and its formulations in addition to the potential for clinical translation.
Keywords: mangiferin, phytochemistry, anticancer, antioxidant, inflammation, neurodegenerative diseases
Introduction
Mangiferin (2-(β-D-Glucopyranosyl)-1,3,6,7-tetrahydroxy-9H-xanthen-9-one) is a naturally occurring xanthone glycoside that is found in significant quantities in various parts of the mango tree (Mangifera indica L)., including the peel, stalks, leaves, bark, kernel, and stone. This polyphenolic compound has garnered considerable attention due to its wide range of potential health benefits, which have been attributed to its potent antioxidant properties.1,2
In addition to mangiferin, the mango plant also contains two other xanthone derivatives, isomangiferin and homomangiferin, which together account for approximately 10% of the total phenolic content in various tissues, such as the leaves, peel, and twigs. These xanthone compounds are believed to exert their antioxidant effects by chelating iron, thereby preventing the generation of hydroxyl radicals via Fenton-type reactions.3
While mangiferin was initially isolated from the Mangifera indica (mango) plant, it has since been found in the flowers of sixteen other plant families, including Iridaceae, Anacardiaceae, and Gentianaceae. This widespread distribution across different plant species highlights the ecological and evolutionary significance of mangiferin as a secondary metabolite.4,5 Mango tree is the richest source in mangiferin as it contains approximately 42 mg/kg in seed kernel, 1690 mg/kg in mango peel, 4 mg/kg in mango pulp, while the major content is contained in stem bark ca. 71 g/kg.6 The proportion of mangiferin and its derivatives in the total phenolic content of mango extracts varies by source and cultivar but is often substantial. For example, in Thai Bao mango peel extract, mangiferin was the major phenolic compound quantified at 8755.89 mg per 100 g of extract, indicating it constitutes a significant portion of the total phenolics.7 In mango kernels from various cultivars, mangiferin content ranges from 0.05 to 6.4 mg/g, with the Brazilian cultivar “Van Dyke” showing the highest levels.8
Mangiferin is a potent antioxidant with a wide range of health-promoting properties. It has demonstrated antimicrobial, antitumor, antihyperglycemic, antioxidative, analgesic, immunomodulatory, antiaging, and hepatoprotective effects, making it a promising therapeutic agent. In addition to its diverse medicinal applications, mangiferin has also been incorporated into various food supplements.9
The chemical structure of mangiferin, which consists of a xanthone core with a β-D-glucopyranosyl moiety attached at the C2 position, is depicted in Figure 1. This unique structural feature contributes to mangiferin’s ability to scavenge free radicals and modulate various signaling pathways such transcription regulators, serine/threonine kinases, cyclins, growth hormones and factors, cytokines as well as chemokines, in addition to the adhesion proteins, involved in the pathogenesis of numerous diseases including cancer, diabetes, neurodegeneration and infections.10
 |
Figure 1 Chemical structure of mangiferin showing the attached sugar moiety in purple. |
The presence of mangiferin in a variety of mango species, coupled with its outstanding pharmacological activities, has generated considerable interest in the scientific community. Researchers continue to explore the mechanisms underlying mangiferin’s therapeutic potential and its potential applications in the development of novel fortified nutraceuticals and pharmaceuticals. Motivated by these characteristics and potentials, this mini review was sought to provide a brief updated summary of the available literature about mangiferin chemistry, distribution, pharmacokinetics and biological activities including its antioxidant, anti-inflammatory, anti-cancer, anti-neurodegeneration, anti-diabetic and antimicrobial properties. The coupling of nanotechnology for loading mangiferin and its potency are also covered. This mini review is ended with the discussion of the reported toxicity of mangiferin and its nanoformulations.
Biochemistry of Mangiferin
Mangiferin biosynthesis in plants is unique in its acquisition of amino acids, saccharides as well as lipid precursors. The essential amino acid phenylalanine and malonyl CoA are the precursors for the biosynthesis of mangiferin. Then, a successive multi-step pathway ensues to form p-coumaric acid, caffeic acid and maclurin before the synthesis of mangiferin, where it is accomplished via Friedel-Crafts reactions, in which a glycosyl donor and an electron-rich aromatic compound are linked by a glycosidic bond as summarized in Figure 2. The non-functional ninth carbon (C9) of mangiferin was proved fundamental for aryl C-glycosylation process owing to the poor electron density therein.11 Treatment of the aglycone 1,3,6,7-tetrahydroxyxanthone with sodium methoxide (NaOMe) yields mangiferin at 0.1%, followed by the formation of an O-glycosidic bond upon hydrolysis with R-acetobromoglucose. This two-step process involves the initial reaction of 1,3,6,7-tetrahydroxyxanthone with R-acetobromoglucose to form the O-glycosidic intermediate, which is then hydrolyzed to yield mangiferin. Notably, aglycone compounds generally exhibit higher antioxidant potential compared to their corresponding glycosides.12
 |
Figure 2 Biosynthetic scheme of mangiferin within mango plant cells starting from phenylalanine. Data from Kaur et al.13 |
The hallmark distinguishing C-glycosides from O-glycosides is that the corresponding replacement of anomeric oxygen with carbon. In result, such replacement render the glycoside more resistant to both acid and glycosidase hydrolytic reactions.14 Within the human body, such enzymic hydrolytic reactions take place of 2-O-β-D-apioside, puerarin, safflor yellow B, and homoorientin and mangiferin, to name a few, by the gut microbiome. The glucose moiety induces extra potency in terms of bioavailability and antioxidant properties.15
Bioavailability and Pharmacokinetics of Mangiferin
When it comes to pharmacokinetics and bioavailability, mangiferin is hampered by different obstacles including low solubility and diminished oral bioavailability. For instance, studies have reported that the aqueous solubility of mangiferin is relatively low, with a value of only 0.111 mg/mL. Furthermore, the oral bioavailability of mangiferin has been shown to be as low as 1.2%.16 This limited bioavailability can be attributed to mangiferin’s inherent properties, such as reduced hydrophobicity, low absorption via gastrointestinal tract. Such medicinal limitations impede the utilization of the broad proved bioactivities of mangiferin and the subsequent translation of mangiferin as promising therapeutics. Thanks to the modern medicinal as well as pharmaceutical technology that suggested using derivatives with improved pharmacokinetics profile and/or utilizing encapsulation and nanotechnology solutions to address the mentioned obstacles.17
Kammalla et al18 conducted a study on the pharmacokinetic characteristics of mangiferin and a polyherbal formulation containing a similar amount of mangiferin after oral administration in Wistar rats. Samples were collected and analyzed at predefined time intervals using liquid chromatography-mass spectrometry (LC-MS) methodology. The residence time of mangiferin in the polyherbal formulation was observed to be longer compared to mangiferin administered alone, potentially attributed to the presence of other ingredients enhancing its bioavailability.18
In a study by Lin et al,19 the pharmacokinetic behavior of mangiferin calcium salt was investigated using HPLC techniques. The findings revealed a superior pharmacokinetic profile compared to mangiferin alone, highlighting its potential for enhanced therapeutic efficacy in diabetic treatment.19
Mangiferin nanocrystals displayed significantly better pharmacokinetics, stability and even solubility. For example, mangiferin nanocrystals using a fractional factorial design were synthesized to enhance their solubility, stability, and pharmacokinetic profile. The researchers optimized factors such as mangiferin concentration, HPMC, pluronic acid, tween 80, and antisolvent to solvent ratio to achieve desired responses like particle size, PDI, zeta potential, and entrapment efficiency. The optimized nanocrystals had a particle size of around 100 nm, a zeta potential of −12 mV, and high entrapment efficiency up to 86.23%. FTIR and DSC confirmed mangiferin’s stability within the nanocrystals. In vitro release studies showed a good release pattern with up to 99.02% drug release. Stability studies demonstrated the formulation’s stability for 6 months. Pharmacokinetic evaluation in rabbits revealed significantly improved parameters with the nanocrystals compared to the suspension. The peak plasma concentration was 412 ng/mL for nanocrystals vs 367 ng/mL for the suspension. The AUC0-t was 23,567.45 ± 10.876 µg×h/mL for nanocrystals vs 18,976.12 ± 9.765 µg×h/mL for the suspension, indicating greater mangiferin availability from the nanocrystals. Overall, these data highlight the potential of mangiferin nanocrystals in enhancing the solubility, stability, and bioavailability of this poorly soluble drug.20
The metabolism of mangiferin by cytochrome P450 (CYP) enzymes is limited, as mangiferin exhibits poor membrane permeability and low bioavailability, which restricts its extensive metabolism by CYPs. Pharmacokinetic studies indicate that CYP450 enzymes have a minor role in mangiferin’s metabolism, with its poor exposure mainly attributed to low absorption rather than extensive CYP-mediated biotransformation. Inhibiting CYP450 enzymes actually decreases mangiferin and its metabolite norathyriol exposure, suggesting that CYPs may be involved in the metabolic conversion but not as the primary clearance pathway. Instead, gut microbiota plays a significant role in transforming mangiferin into metabolites such as norathyriol. Moreover, mangiferin’s metabolism is also influenced by UDP-glucuronosyltransferases (UGTs) and P-glycoprotein (P-gp) transporters, with P-gp inhibition enhancing mangiferin exposure more markedly than CYP inhibition. Overall, CYP enzymes contribute modestly to mangiferin metabolism, with the compound’s pharmacokinetics largely governed by poor intestinal absorption and microbial metabolism rather than extensive hepatic CYP-mediated metabolism.21
Bioactivities of Mangiferin
After examining the biochemistry and pharmacokinetics of mangiferin, the subsequent bioactivities are introduced in this section. It exhibits a broad spectrum of therapeutic and medicinal effects in vitro as well as in vivo. Its pharmacological actions include antidiabetic, anti-inflammatory, analgesic, anti-tumor, anti-neurodegeneration, antimicrobial, and antioxidant properties (Figure 3). The unique xanthonoid structure of mangiferin enables it to combat free radicals and pro-oxidants, offering protection against various pathophysiological conditions.22 Mangiferin demonstrates significant immunomodulatory activities, influencing immune responses and tumor formation. Studies suggest that mangiferin derived from mango tree leaves may hold promise in diabetes treatment by reducing the progression of diabetic nephropathy and improving renal function. Additionally, mangiferin shows potential in managing hyperlipidemia-related cardiovascular complications in diabetic patients. Its pharmacological profile extends to anti-inflammatory, anti-diabetic, antioxidant, antiatherogenic, and antihyperlipidemic properties.23 Furthermore, mangiferin exhibits antidepressant effects and has been associated with memory enhancement, cognitive improvement, and anxiolytic properties. Research indicates that Mangifera indica extract, containing mangiferin, may offer neuroprotective benefits against excitotoxic neuronal death, suggesting therapeutic potential for neurodegenerative disorders.24 Moreover, mangiferin has shown chemoprotective properties and anticancer effects by enhancing apoptosis through NF-kB down-regulation. Its antibacterial activity and ability to modulate the blood-retina barrier indicate potential for addressing various ophthalmic conditions.25
 |
Figure 3 Various medicinal properties of mangiferin. |
These properties made mangiferin a multifaceted, potent glycoside that has the capability to ameliorate the initiation or, at least, the progression of chronic disorders. The specific mechanisms and pathways through which mangiferin exerts the mentioned effects and the corresponding efficacy are the subject of the following sections.
Free Radicals-Neutralizing Activity
Free radicals are highly reactive molecules. The excess of their levels above the antioxidant capacity of an organism can be of damage to the cell; the state is called oxidative stress. This condition may be implicated in cancer, cardiovascular disorders, and neurodegenerative conditions. Antioxidants are vital substances produced by the body and supplied through dietary sources, fruits, and vegetables that neutralize free radicals and protect the body against oxidative damage. A balance between free radicals and antioxidants is crucial for the maintenance of health, and increased intake of antioxidants may counteract some of the adverse effects of oxidative stress on the body.26
The antioxidant properties of bioactive compounds like mangiferin are attributed to their ability to decrease oxidative stress, which is a major contributing factor to various diseases. Mangiferin and mangiferin-containing formulations have been extensively studied for their potential in preventing and treating diseases through their antioxidant activities. The antioxidant activity of mangiferin is primarily due to its unique C-glycosylxanthone structure, which enables it to scavenge free radicals and mitigate oxidative stress. This activity has been assessed through both in vitro and in vivo methods.27
Recent studies have explored strategies to enhance the antioxidant potential of mangiferin. Lee et al28 reported that glucosyl-α-(1→4)-mangiferin exhibited superior antioxidant activity compared to mangiferin, as demonstrated by ferric reducing ability power (FRAP, 1.19-fold), Oxygen radical antioxidant capacity (ORAC, 1.24-fold), and 1.16-fold DPPH assays. Jo et al27 prepared a mangiferin -cyclodextrin complex using molecular self-assembly and found that the presence of β-cyclodextrin further increased the antioxidant activity of mangiferin.28
It was showed that mangiferin obtained from the leaves of Bombax ceiba possessed strong antioxidant properties. Medina Ramírez et al29 studied the antioxidant potential of tea made from mango leaves and found that due to its high phenolic content, it could serve as a beneficial beverage for disease prevention. Decoction at a 5% (w/v) plant concentration exhibited highest mangiferin concentration and was stable for at least 48 h.29
The structure–antioxidant relationship of mangiferin was investigated using quantum chemistry calculations, specifically density functional theory (DFT). The results highlighted the importance of the catechol moiety over the resorcinol moiety for antioxidant capacity. The sugar component, which forms hydrogen bonds with the resorcinol moiety of the xanthone ring, acts as a strong electron-withdrawing group. The study found that hydrogen transfer is more crucial for antioxidant activity than electron transfer. Additionally, hydrogen abstractions in the ortho positions are more favored than those in the meta positions, indicating a synergistic effect between the xanthone and sugar rings.30
The free radical scavenging activity of mangiferin can be attributed to its structural features. Specifically, the presence of four phenolic hydroxyl groups, two of which are susceptible to abstraction by ROS, leads to the formation of two phenoxyl radicals. These radicals are stabilized by resonance, which enhances their ability to neutralize free radicals.31 This unique structural arrangement allows mangiferin to effectively intercept and neutralize ROS, thereby exhibiting potent antioxidant properties (Figure 4).
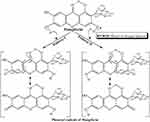 |
Figure 4 Biochemical mechanism of free radicals-scavenging activity of mangiferin. Figure was reused from PalPB, SinhaK, SilPC. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, Tnfα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic Rats. PLoS One. 2014;9:e107220. © 2014 Pal et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License.31 |
The diverse bioactivities of mangiferin can largely be traced to its potent antioxidative actions. Chronic illnesses are often associated with the overgeneration of free radicals, thus creating a highly damaging oxidative stress environment. In this context, mangiferin exerts principal roles in mitigating the effects of oxidative stress and, consequently, may contribute significantly to the amelioration and management of a number of chronic pathologies.
Mangiferin Nanoformulations
As discussed earlier, the potency of mangiferin is hampered by its low pharmacokinetics profile which can be enhanced by utilizing nanoloading technology. For instance, a study by Phuong et al32 developed silk fibroin nanoparticles loaded with mango leaf extract using a simple coacervation approach. The prepared nanoparticles displayed well pharmacokinetic profile and showed three-step controlled release profile. The nanoparticles were demonstrated to be safe against erythrocytes and human embryonic kidney cells, while significantly preserving the antioxidative power of the crude extract (IC50 = 6.872 ± 0.512 μg/mL). The results suggested that silk fibroin nanoparticles could serve a promising delivery agent of mangiferin for medical applications.
Samadarsi and his collaborators encapsulated mangiferin with β-lactoglobulin-chitosan nanoparticles, which were incorporated into milk to create a food product characterized by a low glycemic index (GI) and enhanced antioxidant properties. The fortification process resulted in a significant reduction of the milk’s GI by 34.5%. The bioaccessibility of mangiferin was markedly improved through this fortification, leading to fortified milk exhibiting robust antioxidant activity alongside effective inhibition of lipid peroxidation and protein oxidation. Specifically, the mangiferin-loaded nanoparticles demonstrated substantial potential as a functional food product, providing both high antioxidant capacity and the ability to inhibit oxidative damage, thereby positioning them as an ideal candidate for low GI food applications.33 These findings underscore the efficacy of utilizing mangiferin-loaded nanoparticles in enhancing the nutritional profile of dairy products through its potent antioxidant properties.
Other researchers developed mangiferin-rich extract (MRE) from mango leaves and encapsulate it in nanoparticles using an electrospraying technique for cosmeceutical applications. Proteomic analysis revealed that MRE is involved in actin-filament organization, positive regulation of cytoskeleton organization, and metalloenzyme-activity regulation, suggesting its potential cosmeceutical mechanisms. Nanoparticles were prepared from 0.8% w/v MRE and 2% w/v Eudragit® L100 solution using electrospraying, resulting in a mean size of 247.8 nm, a PDI of 0.271, and an 84.9% entrapment efficiency. Skin-retention studies showed that the mangiferin content in MRE-loaded nanoparticle (MNP)-containing emulsion-gel membranes was higher than in membranes with MRE solution alone, demonstrating that the electrosprayed MNPs enhance transdermal delivery for cosmetic applications owing to strong antioxidative abilities.
These findings highlight the importance of mangiferin and mangiferin -containing formulations as promising antioxidant agents with therapeutic potential in various diseases. The ability to enhance the antioxidant activity of mangiferin through structural modifications and complexation further expands its applications in disease prevention and treatment.
Anti-Inflammatory and Anti-Tumor Activity
Inflammation is a complex overlapped immune reaction involving the stimulation of NF-κB (nuclear factor κB) signaling pathways, upregulation of enzymes producing prostaglandins (cyclooxygenase 2) and nitric oxide (inducible nitric oxide synthase), besides the instigating the biosynthesis of cytokines implicated in inflammation such as tumor-necrosis factor (TNF-α), interleukins (IL-6, IL-8, IL-1β), and other chemokines (CCL2, and CXCL8). Chronic inflammation is a hallmark of various human diseases, including atherosclerosis, cardiovascular disorders, diabetes mellitus, osteoarthritis, and irritable bowel syndrome. In IBD, intestinal barrier dysfunction leads to increased permeability, allowing the passage of toxins, pathogens, and exotoxins, which can contribute to the development of colon cancer.34,35
Inflammation, and the consequent inflammatory response, leads to the liberation of inflammatory cytokines that transforms the fate of recipient cell. These involve cellular hyperplasia, invasion, and metaplasia of normal cells, thereby favoring cancer creation. Such accumulated cytokines and their inflammatory impact (cytokine storm) can ultimately trigger cancer and metastasis if persisted for long without clinical intervention. This is not surprising, as the single cell receives conflicting signals simultaneously disturbing their normal functioning.36 In other simple words, inflammation and cancer are tightly correlated.
For the inflammation reaction to proceed into cancer formation, different players should participate. These include immune cells like macrophage, dendritic cells, and NK cells along with released highly potent cytokines such as TNF-α, IL-6, TGF-β (transforming growth factor β). These factors and cells are usually accompanied by promoting the signaling cascade culminated with the expression of NF-κB, the inflammation master within immune cells, aggravating the situation further. Additionally, other factors, including epigenetics represented by microRNAs, specific membrane receptors, oncogenes and oncoproteins, the tumor microenvironment, and the intestinal microbiome, also influence the progression of cancer.37,38
Given the strong connection between inflammation and cancer, targeting the pathway leading to inflammatory factors blockade and, at the same time, inhibiting cancer initiation, progression and even metastasis, these theragnostic tools can play a crucial role in cancer management.
Various studies have demonstrated the anti-inflammatory properties of mangiferin, although the precise mechanisms remain unclear. A plethora of evidences indicate that mangiferin’s anti-inflammatory effects are associated with its ability to modulate three key signaling pathways: NF-κB, MAPK, and JAK/STAT pathways. Additionally, mangiferin efficiently influences a range of proteins mediating signaling cascades, including transcription regulators, serine/threonine kinases, cyclins, growth hormones and factors, cytokines as well as chemokines, in addition to the adhesion proteins.39 Moreover, mangiferin neutralize and scavenge the produced reactive oxygen species (ROS) and reactive nitrogen species (RNS) in different organs such as the myocardium, renal tissues, liver, and lungs.40 Furthermore, mangiferin shows promise in cancer management, with evidence suggesting its potential in ovarian cancer treatment by regulating Yes-associated protein to inhibit metastasis of OVCAR8 cells. Additionally, mangiferin has been found to suppress the metastasis of epithelial ovarian cancer by down-regulating matrix metalloproteinase 2 (MMP2) and MMP9.41 A plenty of in vivo reports proving the anti-inflammatory as well as the anti-cancer activities of mangiferin were conducted and some which are summarized in Table 1.
 |
Table 1 Some of the Anti-Cancer Evidences of Mangiferin |
Mangiferin Nanoformulations
Researchers from different labs are focusing on preparing and testing the bioactivity of the nanomaterials of mangiferin as a novel approach to combat tumors.
The work by Wang and his et al presented the synthesis and characterization of a targeted nanodrug delivery system comprising hyaluronic acid, mangiferin, and methotrexate (HA-MA-MTX) designed to improve the efficacy of tumor treatment while minimizing off-target toxicity. The HA-MA-MTX nanoparticles were synthesized through a self-assembly approach, with the formation of ester bonds verified using 1H NMR and Fourier-transform infrared spectroscopy (FT-IR). Characterization revealed that the nanoparticles had an average size of approximately 138 nm, which is conducive to cellular uptake. In vitro cytotoxicity assessments indicated that the HA-MA-MTX nanoparticles significantly inhibited the proliferation of K7 cancer cells, while demonstrating reduced cytotoxicity toward normal MC3T3-E1 cells compared to methotrexate administered alone. This selective targeting is attributed to the dual mechanisms of action facilitated by hyaluronic acid and methotrexate, which target CD44 and folate receptors, respectively. Overall, these findings suggest that the HA-MA-MTX nanodrug delivery system has the potential to enhance the therapeutic effectiveness of methotrexate in cancer treatment while mitigating its adverse effects on healthy tissues.47
Also, Razura-Carmona et al48 explored the encapsulation of mangiferin in zinc oxide nanoparticles (ZnO NPs) synthesized from different precursors, specifically zinc nitrate and zinc acetate, to evaluate their biological effects and toxicity. The results indicated that the encapsulation rate was between 55% and 60%, morphology of ZnO NPs varied depending on the precursor used, but there was no significant difference in their antitopoisomerase activity. Notably, the ZnO NPs synthesized from zinc acetate with mangiferin (ZnOA-MG) exhibited a significant reduction in the production of COX-II prostaglandins (97.38 ± 7.09%) compared to COX-I (68.02 ± 2.14%), although it was not a selective treatment. Furthermore, ZnOA-MG demonstrated the lowest hepatotoxicity, with an IC50 of 140.19 ± 13.10 µg/mL, while the nanoparticles from zinc nitrate (ZnON) were more cytotoxic to liver cancer cells (HEP-G2) and lung cells (BEAS-2B), with IC50 values of 51.27 ± 4.72 and 26.91 ± 3.21 µg/mL, respectively. All treatments affected erythrocyte morphology at low concentrations (25 µg/mL).
Another study prepared mangiferin-loaded polymeric nanoparticles using poly(lactic-co-glycolic acid) (PLGA) to evaluate their optical properties, anti-topoisomerase I activity, and cytotoxicity. The nanoparticles were synthesized via the emulsion solvent evaporation method, with an optimal formulation achieved through response surface methodology, resulting in a mean particle size of 176.7 ± 1.021 nm and a polydispersity index (PDI) of 0.153, alongside a mangiferin encapsulation efficiency of approximately 55%. The formulation displayed a gradual release profile of mangiferin under acidic conditions (pH 1.5), with significant modifications in the maximum absorption wavelength observed in UV-Vis spectroscopy. The anti-topoisomerase assay indicated that the optimal formulation (MG4 at 25 µg/mL) exhibited antiproliferative activity, while high concentrations (2500 µg/mL) showed no cytotoxic effects on BEAS-2B and HEPG2 cell lines.49 Overall, the study demonstrates that the encapsulation of mangiferin in PLGA nanoparticles enhances its stability and biological activity without compromising the viability of healthy cells, highlighting the potential of these nanoparticles for therapeutic applications.
In a pre-clinical study, mangiferin was encapsulated with 198Au which emits two types of rays, and ɣ. While β emissions were used to target the tumor, ɣ-rays were employed to monitor the distribution of the gold nanoparticles. The nanoformulation was tested against prostate cancer in experimental animals. Upon transtumoral injection of this nanoformulation, it was found that 80% of dose is retained within the tumor microenvironment. Also, the tumor volume was shown to be significantly diminished (5-fold) after 3 weeks of injection.50
The same team utilized the same formulation to assess its potency against breast tumor in mice. It turned out that after the fifth week post-injection, the tumor volume of the treated group was smaller than the control group.51
Also, in terms of immunomodulatory properties of mangiferin-loaded gold nanoparticles were investigated in prostate cancer. Researchers found that the anti-tumor cytokines IL-12 (10-fold) and TNF-α (50-fold) were upregulated whilst reducing the pro-tumor cytokines IL-10 and IL-6 (2-fold).52
These findings emphasize the efficacy of nanotechnology for addressing the weak activity, limited bioavailability of mangiferin in its free state. In addition, these studies emphasize the applicability of mangiferin nanoformulations to treat tumors in pre-clinical as well as pilot clinical trials.
Anti-Neurodegeneration
The mammalian brain has a high metabolic rate which necessitate ongoing O2 to fuel the energy-producing metabolic pathways and is susceptible to oxidative stress triggered by reactive oxygen and reactive nitrogen radicals. Oxidative stress and its drivers (free radical) can lead to the depletion of antioxidant system within the brain, impeding fundamental metabolic and signaling routes of the neurons. Of note, the antioxidant system is under the control of a transcription regulator known as Nuclear factor erythroid 2-like 2 (Nrf2). It regulates antioxidant pathways and mediates the subsequent activation of downstream and related proteins network like heme oxygenase 1 (HO-1). The experimental evidence suggested the essential role of Nrf2/HO-1 pathway as it was able to protects the ischemic injury induced in the brain via reducing oxidative damage.53
On the other hand, neuroinflammation, mediated by the activated transcriptional factor NF-κB signaling network. The latter is, in turn, stimulated by IL-6 and TNF-α (pro-inflammatory inducers) participating in progression of neurological diseases and exacerbates CNS injury.54 Mangiferin has been shown to play a crucial role in neuroprotection by enhancing free radical scavenging through its C-glucosyl linkage and polyhydroxy component. It can inhibit brain MAO-B activity in Parkinson’s disease models, improve glutathione levels, and reduce malondialdehyde and ROS levels, thereby enhancing antioxidant status.55 Mangiferin demonstrates superior scavenging of NO compared to curcumin. By forming iron complexes, mangiferin protects mitochondria from ferrous citrate, inhibiting lipid peroxidation and preventing mitochondrial swelling induced by Fe2+-citrate in rat liver mitochondria.56 Oxidative stress, disintegrated membrane and ferroptosis all result in neuronal cell damage as neurons are very sensitive to ROS and Fe2+ levels, albeit it still not clear whether iron imbalance is a result or just a consequence of neurodegeneration.57 Moreover, mangiferin elevates glutathione levels, enhances endogenous antioxidant activities, and mitigates lipid peroxidation in neurons exposed to amyloid β protein, thereby improving cellular resilience. Additionally, mangiferin increases the levels of antioxidants like superoxide dismutase and catalase in response to oxidative stress, and it inhibits neurotoxicity induced by 6-OHDA and the free radical-mediated pathway in a ketamine model of schizophrenia.58 Beneficial effects of mangiferin in the context of neurodegenerative disorders are listed in Table 2.
 |
Table 2 Beneficial Effects of Mangiferin on Neurodegenerative Disorders Along with the Mechanism of Action |
The potential actions of mangiferin on neurodegeneration extend beyond its antioxidant properties, encompassing several mechanisms that may contribute to neuronal protection and improved outcomes in neurodegenerative conditions. One key mechanism is its anti-inflammatory action, where mangiferin may inhibit the activation of microglia, the immune cells of the central nervous system, thereby reducing neuroinflammation—a significant contributor to neuronal damage in diseases like Alzheimer’s and Parkinson’s. This reduction in inflammation can help preserve neuronal integrity and function.66 The other mechanism lies in the capability of mangiferin to promote neurogenesis and enhance synaptic plasticity by stimulating the expression of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which are crucial for neuronal survival and growth. Furthermore, it could improve mitochondrial dynamics by enhancing mitochondrial biogenesis and fusion, thereby supporting energy metabolism and reducing the risk of mitochondrial dysfunction.58 These combined effects suggest that mangiferin may offer a multifaceted approach to mitigating neurodegeneration through inflammation modulation, neurotrophic support, and mitochondrial health.
Mangiferin Nanoformulations
Researchers are racing for developing nanocarriers for the purpose of delivery of mangiferin to manage a broad range of chronic illnesses, but the studies on neurodegenerative disorders are scarce. For instance, Ahmed and his collaborators prepared polysorbate-80 (P80) coated mangiferin (MNG) loaded PLGA nanoparticles (NPs) to enhance MNG’s brain bioavailability for ischemic stroke treatment. The solvent evaporation method was used to prepare MNG-PLGA NPs, followed by P80 coating. The optimized P80-MNG-PLGA NPs had a particle size of 103.4 ± 2.66 nm, PDI of 0.201 ± 0.008, zeta potential of −35.8 ± 2.48 mV, and 37.16 ± 2.09% drug loading with 76.08 ± 4.91% entrapment efficiency. The NPs demonstrated sustained drug release (83.43 ± 6.47%) and excellent intranasal permeation (>83%). A novel LC-MS/MS method was developed to quantify MNG in the ischemic rat brain, showing good linearity (10.0–1000.0 ng/mL) and retention times of 0.756 min for MNG and 1.258 min for the internal standard. The NPs improved neurobehavioral and biochemical parameters and showed favorable histopathological outcomes in the ischemic rat model, suggesting their potential for enhancing MNG’s therapeutic efficacy in cerebral ischemia.67
Antidiabetic Activity
Diabetes mellitus, particularly type 2, still constitutes untreatable health problem. Its incidence is on the rise annually worldwide. Type 2 diabetes, which accounts for >80% among the three diabetic types, is characterized by a limited utilization of the absorbed glucose, particularly in the two major peripheral tissues, ie skeletal musculature and the adipocytes. Individuals displaying chronic hyperinsulinemia as well as hyperglycemia are at a greater risk of developing cardiovascular diseases.68
Mangiferin has been shown to possess antidiabetic properties, at least its potency to alleviate hyperlipidemia in type 2 diabetics. For example, mangiferin promoted hyperinsulinemia and lowered glycemic levels in an insulin tolerance test in experimental animal models, suggesting that it exerts its antidiabetic effects by improving insulin sensitivity.69
Further studies have confirmed the antidiabetic activity of mangiferin in mice. For instance, mice orally given mangiferin showed lowered concentrations of plasma glucose, while normal mice showed no significant changes, indicating the selective efficacy of mangiferin in treating type 2 diabetes.70
Mangiferin was also exhibited efficacy in managing diabetic complications like nephropathies. In a study on diabetic rats, mangiferin therapy in the range 15 till 60 mg/kg for a testing period of 9 weeks, ameliorated chronic renal insufficiency that has established in these rats. The examined therapeutic mechanisms involved mitigating blood urea nitrogen, albuminuria, glomerular extracellular matrix, and renal weight index. Additionally, mangiferin declined advanced glycation end products (AGEs) through its strong antioxidant activities whilst epigenetically downregulate the mRNA and protein expression of the membrane receptors of AGEs in the rat renal cortex.31
A recent meta-analysis and systematic review culminated with the conclusion that the oral administration of mangiferin has proved a potent antidiabetic impact as tested in the experimental animal models. Indeed, this hypoglycemic action could be attributable to its combined anti-inflammatory and antioxidative actions, as well as to its beneficial role in improving glycolipid metabolism and alleviating the insulin resistance.71
Mangiferin Nanoformulations
Wang et al72 investigated the development and application of mangiferin-loaded N-succinyl chitosan-alginate grafted nanoparticles aimed at targeting atherosclerosis in a rat model of diabetes-mediated hyperlipidemia. Mangiferin, a polyphenolic compound derived from Mangifera indica, is known for its hypoglycemic and hypolipidemic properties but suffers from poor solubility and bioavailability. To enhance its therapeutic efficacy, mangiferin was incorporated into a nanomatrix of N-succinyl chitosan grafted with alginate. Characterization techniques, FTIR, and two-dimensional nuclear magnetic resonance (2D-NMR), confirmed strong interactions between mangiferin and the N-succinyl chitosan matrix. The resulting nanoparticles exhibited a spherical morphology with sizes ranging from 100 to 200 nm and demonstrated a complete in vitro drug release profile. In vivo studies revealed significant therapeutic effects, with blood glucose levels decreasing from approximately 300 mg/dL to around 90 mg/dL following treatment with the nanoparticles. Additionally, the formulation led to a 37% reduction in total plasma cholesterol and a 61% decrease in serum triglycerides, which are notably higher reductions compared to previous studies that reported only 1–36% and 10–40% reductions, respectively, with mangiferin alone.72 These findings suggest that N-succinyl chitosan-alginate grafted nanoparticles could serve as an effective delivery system for mangiferin, enhancing its bioavailability and therapeutic potential against atherosclerosis in diabetic conditions, while also mitigating oxidative stress and inflammatory responses associated with hyperlipidemia.
Another work aimed to develop targeted polymeric nanoparticles based on mangiferin for enhanced protection of pancreatic β-cells and improved efficacy against type 1 diabetes mellitus (T1DM). The researchers constructed MGF-loaded nanoparticles using a biodegradable and biocompatible polymer, poly(lactic-co-glycolic acid) (PLGA), and functionalized them with a β-cell-targeting peptide. The optimized nanoparticles had a size of around 150 nm, a narrow size distribution, and a high mangiferin encapsulation efficiency (6.86 ± 0.60%). In vitro studies demonstrated that the targeted nanoparticles effectively protected pancreatic β-cells from cytokine-induced apoptosis and preserved insulin secretion. In a mouse model of T1DM, a single intravenous injection of the targeted nanoparticles significantly reduced hyperglycemia, increased insulin levels, and preserved pancreatic β-cell mass compared to non-targeted nanoparticles or free MGF as summarized in Figure 5. The findings suggest that this targeted MGF nanoparticle system could be a promising therapeutic approach for protecting pancreatic β-cells and managing T1DM.73
 |
Figure 5 In vivo injection of polymeric nanoparticles containing GLP-1 for targeted delivery of mangiferin into β-islets of pancreas for the treatment of T1DM along with the obtained findings. Figure was reproduced from WangM, ZhangZ, HuoQ, et al.Targeted polymeric nanoparticles based on mangiferin for enhanced protection of pancreatic β-cells and type 1 diabetes mellitus efficacy. ACS Appl. Mater. Interfaces. 2022;14:11092–11103. Copyright 2022 American Chemical Society.73 |
These data glorify the potential of mangiferin as a therapeutic agent in the management of type 2 diabetes and its associated complications, particularly diabetic nephropathy. Further research is warranted to elucidate the precise mechanisms underlying the antidiabetic effects of mangiferin and to explore its clinical applications.
Antimicrobial Properties
It is obvious from the molecular structure of mangiferin that it would constitute a strong bioactivity against microbes (as antimicrobial agent). This conclusion can be drawn from the highly polar nature of the molecule gained by the multiple hydroxyl moieties, in addition to the carbonyl as well as ether bridges (see Figure 1) as in the case of other plant bioactive flavonoids and glycosides.74 The antimicrobial properties of mangiferin are well established against viruses and bacteria as depicted in Figure 6.
 |
Figure 6 Antimicrobial activity of mangiferin against different classes of bacteria and viruses. |
For instance, Anemarrhena asphodeloides, a plant commonly used in traditional Chinese medicine, is known for its antiviral and antibacterial potency through its primary active component mangiferin.75 Studies have shown that mangiferin effectively combats Staphylococcus aureus and Salmonella typhi bacteria.76 Furthermore, in tissue culture experiments, both mangiferin and isomangiferin demonstrated antiviral effects against herpes simplex virus-1 (HSV-1). These two compounds displayed a substantial reduction of the plaques at rates of 56.8% and 69.5%, respectively.76 Similarly, in a rigorous in vitro investigation, mangiferin demonstrated notable antiviral efficacy against herpes simplex virus-2 (HSV-2) in HeLa cell cultures. Quantitative analysis revealed a half-maximal effective concentration (EC50) of 111.7 mg for inhibiting HSV-2 plaque formation, with a corresponding therapeutic index of 8.1.77 These findings underscore the importance of natural compounds like mangiferin in the ongoing search for novel antiviral strategies, particularly in addressing the challenge of drug-resistant viral strains.
Previous studies have extensively evaluated the antimicrobial potency of mango kernel extracts. It turned out that the extract holds antibacterial activity against Escherichia coli and other Enterobacteriaceae spp. Notably, mangiferin, a bioactive compound found in mango, has demonstrated remarkable antibacterial activity even against highly resistant strains including methicillin-resistant Staphylococcus aureus.78,79
Thai mango (M. indica) seed kernel extract has been demonstrated to possess strong bactericidal actions. Additionally, the chloroform as well as aqueous extract of mango leaves succeeded to break methicillin-resistant Staphylococcus aureus (MRSA) with an inhibition zone of 16 mm.80
Interestingly, the mangiferin’s antibacterial activity extends to in vivo as well, particularly against specific periodontal pathogens, such as Actinobacillus actinomycete mcomitans, Prevotella intermedia, Porphyromonas gingivalis, Fusobacterium nucleatum, and Peptostreptococcus species. This has led to the suggestion that mangiferin could be marketed as an alternative remedial agent or an adjunctive treatment alongside traditional antibiotics.81
Biofilm formation is a critical factor in urinary tract infections, as it provides uropathogenic bacteria with resistance to antimicrobials. A recent study evaluated the efficacy of a methanolic extract of Mangifera indica seed kernels against 17 biofilm-producers strains of uropathogenic E. coli (UPEC) identified from urine samples. The extract demonstrated significant antibacterial and anti-biofilm activity at concentrations of 50 μg/mL and 100 μg/mL. Phytochemical analysis revealed the presence of bioactive compounds, mainly mangiferin, which was confirmed through FTIR, and Gas chromatography analysis. In silico exploration indicated that mangiferin can bind to active sites of LpxC in E. coli, suggesting it may be the key compound responsible for the observed effects.82 Overall, this confirms the anti-biofilm activity of mangiferin which is favorable strategy to combat antibiotic resistance.
Mangiferin Nanoformulations
In a pioneering work, Sicurella et al synthesized mangiferin-loaded smart semisolid gels based on phosphatidylcholine and Pluronic as topical delivery systems to treat HSV-1 infections. Lecithin organogels, Pluronic gels, and Pluronic lecithin organogels were formulated and characterized, with mangiferin efficiently incorporated into all gel types. In vitro diffusion studies demonstrated the gels’ ability to control drug release, especially the Pluronic organogel. Cutaneous administration of the gels was found to be safe in human volunteers. Importantly, a plaque reduction assay revealed the virucidal effect of mangiferin-loaded Pluronic gel and Pluronic lecithin organogel against the HSV-1 KOS strain.83
Another research focused on the creation of mangiferin-loaded core-shell hydrogel beads made from carrageenan and chitosan, utilizing a one-step gelling technique to enhance the solubility and stability of mangiferin. The resulting beads were characterized by favorable physicochemical attributes, high encapsulation efficiency (85%), and a controlled release profile. Safety assessments indicated low cytotoxicity, while antioxidant and antimicrobial tests highlighted their potential for use in food preservation. Overall, the findings suggest that these innovative hydrogel beads could effectively improve the bioavailability and stability of mangiferin in functional foods and nutraceutical applications.84
This study aimed to develop mangiferin-loaded β-lactoglobulin (βLG) nanoparticles for oral delivery. The nanoparticles were prepared using a desolvation technique and characterized for particle size, zeta potential, encapsulation efficiency, and in vitro release. The optimized nanoparticles had a size of 220 nm, a zeta potential of −25 mV, and an encapsulation efficiency of 80%. In vitro release studies showed a biphasic-release pattern with an initial burst release followed by sustained release over 24 hours. The nanoparticles demonstrated good stability when stored at 4°C for 30 days. DPPH assay confirmed that nanoencapsulation retained the antioxidant activity of mangiferin. The study suggests that βLG nanoparticles could be a promising carrier for oral delivery of mangiferin, potentially enhancing its bioavailability and therapeutic efficacy. These nanoparticles displayed potent antibacterial activities against Staphylococcus aureus (G+) and Escherichia coli (G-) strains, while showing no toxicity to beneficial probiotic strains found in the gastrointestinal tract. The findings suggest that mangiferin/β-lactoglobulin nanoparticles could serve as a promising candidate for oral delivery applications.85 These findings confirm the antibacterial properties of mangiferin, suggesting its potential as an antibacterial agent.
Toxicity Concerns of Mangiferin
After examining the various pharmaceutical activities involving anti-cancer, antioxidant, anti-neurodegeneration, anti-diabetic and antimicrobial properties of mangiferin, one should assess its potential toxicity in its free and nanoparticles-loaded formulations before being applied in the clinical context.
Mangiferin, a natural compound derived from various parts of the mango tree, is generally considered safe for human use. Several studies have investigated the toxicity profile of mangiferin, and the findings suggest that it has a wide safety margin. Recent pharmacokinetic research has unveiled promising insights into mangiferin’s safety profile and physiological impact. A human trial involving oral administration of 0.9 g mangiferin yielded no observable toxic effects, corroborating a growing consensus on its safety. This finding aligns with a multitude of studies that have consistently demonstrated mangiferin’s dual capacity for safety and cellular function enhancement. Particularly noteworthy are the results from in vitro experiments on vascular smooth muscle cells, where mangiferin exhibited remarkable vascular protective properties across a wide concentration range (0.1 to 100 μM) without inducing cytotoxicity.86 These findings collectively underscore mangiferin’s potential as a safe and efficacious compound for improving vascular health, warranting further investigation into its therapeutic applications in cardiovascular medicine and beyond.
Comprehensive toxicological evaluations of mangiferin have yielded reassuring results across multiple experimental models. In vivo studies utilizing NMRI mice demonstrated that oral administration of mangiferin at a dose of 2 g/kg did not induce genotoxic effects in bone marrow erythrocytes. Complementary in vitro assessments, including the Ames test and Escherichia coli PQ37 assay, revealed no significant increase in reverse mutations or primary DNA damage when mangiferin was applied at concentrations ranging from 0.050 to 5 mg/plate and 0.005 to 1 mg/mL, respectively.87 Furthermore, the Comet assay showed no evidence of single-strand DNA breaks or alkali-labile sites in blood peripheral lymphocytes or hepatocytes exposed to mangiferin concentrations between 0.010 and 0.500 mg/mL for a duration of 1 hour.88 These findings collectively support the safety profile of mangiferin, suggesting minimal genotoxic potential across various biological systems and concentration ranges.
Furthermore, a 3-month oral administration of mango leaf extract containing 60% mangiferin at a dose of 2 g/kg BW/day to rats did not result in any mortality or toxic effects.89 Similarly, the aqueous extract from Mangifera indica leaves and its biotransformation metabolites did not show measurable genotoxicity or mutagenicity.90
Moreover, a recent investigation explored the toxicological profile of novel hybrid nanoparticles incorporating PLGA and ZnO, loaded with the phytochemicals lupeol and mangiferin. Utilizing an ex vivo model of human peripheral blood mononuclear cells (PBMCs) and erythrocytes, researchers evaluated the biocompatibility of these engineered particles. The study revealed a concentration-dependent toxicity profile, with sol-gel synthesized ZnO exhibiting adverse effects at 1 mg/mL for both cell types. Conversely, PLGA-based particles demonstrated superior biocompatibility, inducing no significant apoptosis in PBMCs after 72 hours of exposure. Notably, the lupeol standard showed comparable efficacy to the chemotherapeutic agent etoposide in mononuclear cells. These findings highlight the potential of PLGA as a safe encapsulation material for bioactive compounds at concentrations up to 1 mg/mL, suggesting its viability for applications in food science and pharmaceutical development.91
Based on the available cellular and animal studies, mangiferin and the corresponding nanoformulations can be considered a safe natural compound. However, it is important to note that more human studies are needed to further establish its safety profile in clinical settings.
Conclusions and Future Prospects
In conclusion, mangiferin’s diverse pharmacological properties, including its antioxidant, anti-inflammatory, anticancer, antimicrobial, and anti-diabetic activities, make it a promising candidate for the development of novel therapeutic agents and nutraceuticals. Its natural origin and relatively low toxicity further support its potential for clinical applications. The comprehensive overview of mangiferin’s phytochemistry, pharmacokinetics, and medicinal properties presented in this mini-review highlights the significance of this compound in the discovery of novel treatments for various diseases, particularly in its nanoformulations as it offers broader distribution, higher bioavailability, and superior potency when compared to the free form. Future prospects for mangiferin include developing novel therapeutic agents by optimizing bioavailability, efficacy, and safety for various disease indications, as well as exploring its potential as a dietary supplement for preventive and therapeutic purposes. Synthesizing and optimizing derivatives with enhanced bioactivities is also a promising area of research. Clinical trials should assess safety, efficacy, and optimal dosing for various disease indications, and combination therapies with other therapeutic agents should be explored. Elucidating the molecular mechanisms underlying mangiferin’s bioactivities is crucial, as is establishing robust quality control measures.92 Pharmacokinetic and pharmacodynamic modeling can help optimize dosing regimens and treatment durations, and investigating therapeutic potential in new disease areas is also warranted. Finally, fostering interdisciplinary collaboration and knowledge sharing is essential for translating research findings into clinical practice.
Third-Party Material
All required permissions have been taken from the respective sources for the use of their data in the current publication.
Funding
This paper received no funding.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Dar A, Faizi S, Naqvi S, et al. Analgesic and antioxidant activity of mangiferin and its derivatives: the structure activity relationship. Biol Pharm Bull. 2005;28:596–600. doi:10.1248/bpb.28.596
2. Ali A, Asgher Z, Cottrell JJ, Dunshea FR. Screening and characterization of phenolic compounds from selected unripe fruits and their antioxidant potential. Molecules. 2023;29:167. doi:10.3390/molecules29010167
3. Matkowski A, Kus P, Goralska E, Wozniak D. Mangiferin – a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Reviews in Med Chem. 2013;13:439–455. doi:10.2174/138955713804999838
4. Ahmad R, Alqathama A, Aldholmi M, et al. Antidiabetic and anticancer potentials of Mangifera indica L. from different geographical origins. Pharmaceuticals. 2023;16(350):350. doi:10.3390/ph16030350
5. Lebaka VR, Wee Y-J, Ye W, Korivi M. Nutritional composition and bioactive compounds in three different parts of mango fruit. IJERPH. 2021;18(741):741. doi:10.3390/ijerph18020741
6. Tacias-Pascacio VG, Castañeda-Valbuena D, Fernandez-Lafuente R, et al. Phenolic compounds in mango fruit: a review. Food Meas. 2022;16:619–636. doi:10.1007/s11694-021-01192-2
7. Thangsiri S, Suttisansanee U, Koirala P, et al. Phenolic content of Thai Bao mango peel and its in-vitro antioxidant, anti-cholinesterase, and antidiabetic activities. Saudi J Biol Sci. 2024;31:104033. doi:10.1016/j.sjbs.2024.104033
8. Castro-Vargas HI, Ballesteros Vivas D, Ortega Barbosa J, Morantes Medina SJ, Aristizabal Gutiérrez F, Parada-Alfonso F. Bioactive phenolic compounds from the agroindustrial waste of Colombian mango cultivars “sugar mango” and ‘tommy atkins’-an alternative for their use and valorization. Antioxidants. 2019;8:41. doi:10.3390/antiox8020041
9. Castro-Muñoz R, Cabezas R, Plata-Gryl M. Mangiferin: a comprehensive review on its extraction, purification and uses in food systems. Adv. Colloid InterfaceSci. 2024;329:103188. doi:10.1016/j.cis.2024.103188
10. Imran M, Arshad MS, Butt MS, Kwon J-H, Arshad MU, Sultan MT. Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017;16:84. doi:10.1186/s12944-017-0449-y
11. Ehianeta TS, Laval S, Yu B. Bio- and chemical syntheses of mangiferin and congeners. BioFactors. 2016;42:445–458. doi:10.1002/biof.1279
12. López-Cárdenas FG, Pérez-Jiménez J, Mateos-Briz R, Zamora-Gasga VM, Sánchez-Burgos JA, Sáyago-Ayerdi SG. Advances in mangiferin: biosynthetic pathways, bioavailability and bioactivity. In: Xiao J, editor. ISBN 978-3-030-94753-8. Handbook of Dietary Flavonoids. Cham: Springer International Publishing; 2023:1–37.
13. Kaur P, Pandey DK, Gupta RC, et al. Biotechnological interventions and genetic diversity assessment in swertia sp.: a myriad source of valuable secondary metabolites. Appl Microbiol Biotechnol. 2021;105:4427–4451. doi:10.1007/s00253-021-11345-4
14. Kumar M, Saurabh V, Tomar M, et al. Mango (Mangifera indica L.) leaves: nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants. 2021;10:299. doi:10.3390/antiox10020299
15. Dutta T, Das T, Gopalakrishnan AV, et al. Mangiferin: the miraculous xanthone with diverse pharmacological properties. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;396:851–863. doi:10.1007/s00210-022-02373-6
16. Ma H, Chen H, Sun L, Tong L, Zhang T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia. 2014;93:54–61. doi:10.1016/j.fitote.2013.10.016
17. Du S, Liu H, Lei T, et al. Mangiferin: an effective therapeutic agent against several disorders (review). Mol Med Repo. 2018;18:4775–4786. doi:10.3892/mmr.2018.9529
18. Kammalla AK, Ramasamy MK, Inampudi J, Dubey GP, Agrawal A, Kaliappan I. Comparative pharmacokinetic study of mangiferin after oral administration of pure mangiferin and us patented polyherbal formulation to rats. AAPS Pharm Sci Tech. 2015;16:250–258. doi:10.1208/s12249-014-0206-8
19. Lin H, Teng H, Wu W, et al. Pharmacokinetic and metabolomic analyses of mangiferin calcium salt in rat models of type 2 diabetes and non-alcoholic fatty liver disease. BMC Pharmacol Toxicol. 2020;21:59. doi:10.1186/s40360-020-00438-x
20. Sarwar AR, Iqbal FM, Jamil MA, Abbas K. Nanocrystals of mangiferin using design expert: preparation, characterization, and pharmacokinetic evaluation. Molecules. 2023;28:5918. doi:10.3390/molecules28155918
21. Tian X, Xu Z, Li Z, et al. Pharmacokinetics of mangiferin and its metabolite—norathyriol, part 2: influence of UGT, CYP450, P‐gp, and enterobacteria and the potential interaction in rhizoma anemarrhenae decoction with timosaponin b2 as the major contributor. BioFactors. 2016;42:545–555. doi:10.1002/biof.1290
22. Kaurav M, Kanoujia J, Gupta M, et al. In-depth analysis of the chemical composition, pharmacological effects, pharmacokinetics, and patent history of mangiferin. Phytomedicine Plus. 2023;3:100445. doi:10.1016/j.phyplu.2023.100445
23. Aswal S, Kumar A, Chauhan A, Semwal RB, Kumar A, Semwal DK. A molecular approach on the protective effects of mangiferin against diabetes and diabetes-related complications. Current Diabetes Rev. 2020;16:690–698. doi:10.2174/1573399815666191004112023
24. Mittal S, Iqubal MK, Iqbal B, Gupta MM, Ali J, Baboota S. A pervasive scientific overview on mangiferin in the prevention and treatment of various diseases with preclinical and clinical updates. J Complement Integr Med. 2021;18:9–21. doi:10.1515/jcim-2019-0250
25. Yehia RS, Altwaim SA. An insight into in vitro antioxidant, antimicrobial, cytotoxic, and apoptosis induction potential of mangiferin, a bioactive compound derived from Mangifera indica. Plants. 2023;12:1539. doi:10.3390/plants12071539
26. Chaudhary P, Janmeda P, Docea AO, et al. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023;11:1158198. doi:10.3389/fchem.2023.1158198
27. Jo Y-J, Cho H-S, Chun J-Y. Antioxidant activity of β-cyclodextrin inclusion complexes containing trans-cinnamaldehyde by DPPH, ABTS and FRAP. Food Sci Biotechnol. 2021;30:807–814. doi:10.1007/s10068-021-00914-y
28. Lee JY, Kim H, Moon Y, et al. Enhancement of the water solubility and antioxidant capacities of mangiferin by transglucosylation using a cyclodextrin glycosyltransferase. Enzyme Microb Technol. 2022;159:110065. doi:10.1016/j.enzmictec.2022.110065
29. Medina Ramírez N, Monteiro Farias L, Apolonio Santana F, et al. Extraction of mangiferin and chemical characterization and sensorial analysis of teas from Mangifera indica L. leaves of the ubá variety. Beverages. 2016;2(33):33. doi:10.3390/beverages2040033
30. Da Veiga AAS, De Jesus Chaves Neto AM, Da Silva ABF, Herculano AM, Oliveira KRM, Dos Santos Borges R. Sugar moiety has a synergistic effect on hydroxylated xanthone for better antioxidant activity of mangiferin. Med Chem Res. 2018;27:1276–1282. doi:10.1007/s00044-018-2147-3
31. Pal PB, Sinha K, Sil PC. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, Tnfα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic Rats. PLoS One. 2014;9:e107220. doi:10.1371/journal.pone.0107220
32. Phuong NTN, Ha MT, Nguyen DXT, et al. Development and antioxidant evaluation of mango leaf (Mangifera Indica L.) extract loaded silk fibroin nanoparticles. Front Mater. 2024;11:1419697. doi:10.3389/fmats.2024.1419697
33. Samadarsi R, Mishra D, Dutta D. Mangiferin nanoparticles fortified dairy beverage as a low glycemic food product: its quality attributes and antioxidant properties. Int J of Food Sci Tech. 2020;55:589–600. doi:10.1111/ijfs.14310
34. Liang Q, Ren X, Chalamaiah M, Ma H. Simulated gastrointestinal digests of corn protein hydrolysate alleviate inflammation in caco-2 cells and a mouse model of colitis. J Food Sci Technol. 2020;57:2079–2088. doi:10.1007/s13197-020-04242-7
35. Allaire JM, Crowley SM, Law HT, Chang S-Y, Ko H-J, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi:10.1016/j.it.2018.04.002
36. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi:10.1016/j.immuni.2019.06.025
37. Fernández M, Reina-Pérez I, Astorga J, Rodríguez-Carrillo A, Plaza-Díaz J, Fontana L. breast cancer and its relationship with the microbiota. IJERPH. 2018;15:1747. doi:10.3390/ijerph15081747
38. Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834:188–196. doi:10.1016/j.ejphar.2018.07.034
39. Mei S, Ma H, Chen X. Anticancer and anti-inflammatory properties of mangiferin: a review of its molecular mechanisms. Food and Chemical Toxicology. 2021;149:111997. doi:10.1016/j.fct.2021.111997
40. Siswanto S, Arozal W, Juniantito V, Grace A, Agustini FD. Nafrialdi the effect of mangiferin against brain damage caused by oxidative stress and inflammation induced by doxorubicin. HAYATI J Biosci. 2016;23:51–55. doi:10.1016/j.hjb.2016.02.001
41. Zeng Z, Lin C, Wang S, et al. Suppressive activities of mangiferin on human epithelial ovarian cancer. Phytomedicine. 2020;76:153267. doi:10.1016/j.phymed.2020.153267
42. Szandruk M, Merwid-Ląd A, Szeląg A. The impact of mangiferin from belamcanda chinensis on experimental colitis in rats. Inflammopharmacol. 2018;26:571–581. doi:10.1007/s10787-017-0337-0
43. Li Y, Wu Y, Jiang K, et al. Mangiferin prevents TBHP-induced apoptosis and ECM degradation in mouse osteoarthritic chondrocytes via restoring autophagy and ameliorates murine osteoarthritis. Oxid Med Cell Longev. 2019;2019:1–17. doi:10.1155/2019/8783197
44. Li M, Wu C, Guo H, Chu C, Hu M, Zhou C. Mangiferin improves hepatic damage-associated molecular patterns, lipid metabolic disorder and mitochondrial dysfunction in alcohol hepatitis rats. Food Funct. 2019;10:3514–3534. doi:10.1039/C9FO00153K
45. Toledo RCL, Brito LF, Caetano MMM, et al. Acute treatment with Mangifera indica L. leaf extract attenuates liver inflammation in rats fed a cafeteria diet. Food Funct. 2019;10:4861–4867. doi:10.1039/C9FO00651F
46. Jia L, Sun P, Gao H, et al. Mangiferin attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting TLR4/P65 and TGF-Β1/Smad2/3 Pathway. J Pharm Pharmacol. 2019;71:1017–1028. doi:10.1111/jphp.13077
47. Wang H, Shao W, Lu X, et al. Characterization, and in vitro anti-tumor activity studies of the hyaluronic acid-mangiferin-methotrexate nanodrug targeted delivery system. Int J Biol Macromol. 2023;239:124208. doi:10.1016/j.ijbiomac.2023.124208
48. Razura-Carmona FF, Herrera-Martínez M, Sáyago-Ayerdi SG, et al. Nanoparticles of two ZnO precursors as an encapsulating matrix of mangiferin: associated studies to cytotoxic effects on liver cancer cells Hep-G2 and healthy lung cell beas-2B. J Clust Sci. 2022;33:163–171. doi:10.1007/s10876-020-01957-7
49. Razura-Carmona FF, Pérez-Larios A, González-Silva N, et al. Mangiferin-loaded polymeric nanoparticles: optical characterization, effect of anti-topoisomerase i, and cytotoxicity. Cancers. 2019;11:1965. doi:10.3390/cancers11121965
50. Al-Yasiri AY, Khoobchandani M, Cutler CS, et al. Mangiferin functionalized radioactive gold nanoparticles (MGF-198 AuNPs) in prostate tumor therapy: green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017;46:14561–14571. doi:10.1039/C7DT00383H
51. Khoobchandani M, Katti KK, Karikachery AR, et al. New approaches in breast cancer therapy through green nanotechnology and nano-ayurvedic medicine – pre-clinical and pilot human clinical investigations. IJN. 2020;15:181–197. doi:10.2147/IJN.S219042
52. Khoobchandani M, Khan A, Katti KK, et al. Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci Rep. 2021;11:16797. doi:10.1038/s41598-021-96224-8
53. Ge X-H, Shao L, Zhu G-J. Oxymatrine attenuates brain hypoxic-ischemic injury from apoptosis and oxidative stress: role of p-Akt/GSK3β/HO-1/Nrf-2 signaling pathway. Metab Brain Dis. 2018;33:1869–1875. doi:10.1007/s11011-018-0293-4
54. Avila JA, Kiprowska M, Jean‐Louis T, Rockwell P, Figueiredo‐Pereira ME, Serrano PA. PACAP27 mitigates an age‐dependent hippocampal vulnerability to PGJ2‐induced spatial learning deficits and neuroinflammation in mice. Brain and Behavior. 2020;10:e01465. doi:10.1002/brb3.1465
55. Alberdi E, Sánchez-Gómez MV, Ruiz A, et al. Mangiferin and Morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid beta oligomers. Oxid Med Cell Longev. 2018;2018:1–13. doi:10.1155/2018/2856063
56. Agarwala S, B NR, Mudholkar K, Bhuwania R, Satish Rao BS. Mangiferin, a dietary xanthone protects against mercury‐induced Toxicity in HepG2 cells. Environ. Toxicol. 2012;27:117–127. doi:10.1002/tox.20620
57. Levi S, Ripamonti M, Moro AS, Cozzi A. Iron Imbalance in Neurodegeneration. Mol Psychiatry. 2024;29:1139–1152. doi:10.1038/s41380-023-02399-z
58. Liu T, Song Y, Hu A. Neuroprotective mechanisms of mangiferin in neurodegenerative diseases. Drug Dev. Res. 2021;82:494–502. doi:10.1002/ddr.21783
59. Du Z, Fanshi F, Lai Y-H, et al. Mechanism of anti-dementia effects of mangiferin in a senescence accelerated mouse (SAMP8) model. Biosci Rep. 2019;39:BSR20190488. doi:10.1042/BSR20190488
60. Infante-Garcia C, Ramos-Rodriguez JJ, Delgado-Olmos I, et al. Long-term mangiferin extract treatment improves central pathology and cognitive deficits in APP/PS1 Mice. Mol Neurobiol. 2017;54:4696–4704. doi:10.1007/s12035-016-0015-z
61. Amazzal L, Lapôtre A, Quignon F, Bagrel D. Mangiferin protects against 1-Methyl-4-phenylpyridinium toxicity mediated by oxidative stress in N2A Cells. Neurosci Lett. 2007;418:159–164. doi:10.1016/j.neulet.2007.03.025
62. Kavitha M, Manivasagam T, Essa MM, et al. Mangiferin antagonizes rotenone: induced apoptosis through attenuating mitochondrial dysfunction and oxidative stress in SK-N-SH neuroblastoma cells. Neurochem Res. 2014;39:668–676. doi:10.1007/s11064-014-1249-7
63. Rao VS, Carvalho AC, Trevisan MTS, et al. Mangiferin ameliorates 6-hydroxydopamineinduced cytotoxicity and oxidative stress in ketamine model of schizophrenia. Pharmacol Rep. 2012;64(4):848–856. doi:10.1016/s1734-1140(12)70879-4
64. Cao C, Su M, Zhou F. Mangiferin Inhibits Hippocampal NLRP3 Inflammasome and exerts antidepressant effects in a chronic mild stress mice model. Behav Pharmacol. 2017;28:356–364. doi:10.1097/FBP.0000000000000305
65. Luo G-Q, Liu L, Gao Q-W, Wu X-N, Xiang W, Deng W-T. Mangiferin prevents corticosterone-induced behavioural deficits via alleviation of oxido-nitrosative stress and down-regulation of indoleamine 2,3-Dioxygenase (IDO) activity. Neurol Res. 2017;39:709–718. doi:10.1080/01616412.2017.1310705
66. Feng S-T, Wang -Z-Z, Yuan Y-H, Sun H-M, Chen N-H, Zhang Y. Mangiferin: a multipotent natural product preventing neurodegeneration in alzheimer’s and parkinson’s disease models. Pharmacol Res. 2019;146:104336. doi:10.1016/j.phrs.2019.104336
67. Ahmad N, Khan MF, Ullah Z, et al. Development and evaluation of polysorbate-80 coated mangiferin PLGA nanoparticles used in the treatment of cerebral ischemia. Polym Bull. 2024;81:7035–7069. doi:10.1007/s00289-023-05030-x
68. Wang M, Liang Y, Chen K, et al. The management of diabetes mellitus by mangiferin: advances and prospects. Nanoscale. 2022;14(6):2119–2135. doi:10.1039/D1NR06690K
69. Mistry J, Biswas M, Sarkar S, Ghosh S. Antidiabetic activity of mango peel extract and mangiferin in alloxan-induced diabetic rats. Futur J Pharm Sci. 2023;9:22. doi:10.1186/s43094-023-00472-6
70. Sekar V, Mani S, Malarvizhi R, Barathidasan R, Vasanthi HR. positive interaction of mangiferin with selected oral hypoglycemic drugs: a therapeutic strategy to alleviate diabetic nephropathy in experimental rats. Mol Biol Rep. 2020;47:4465–4475. doi:10.1007/s11033-020-05517-0
71. Wu Y, Liu W, Yang T, et al. Oral administration of mangiferin ameliorates diabetes in animal models: a meta-analysis and systematic review. Nutr Res. 2021;87:57–69. doi:10.1016/j.nutres.2020.12.017
72. Wang Y, Karmakar T, Ghosh N, Basak S, Gopal Sahoo N. Targeting mangiferin loaded n-succinyl chitosan-alginate grafted nanoparticles against atherosclerosis – a case study against diabetes mediated hyperlipidemia in rat. Food Chem. 2022;370:131376. doi:10.1016/j.foodchem.2021.131376
73. Wang M, Zhang Z, Huo Q, et al. Targeted polymeric nanoparticles based on mangiferin for enhanced protection of pancreatic β-cells and type 1 diabetes mellitus efficacy. ACS Appl. Mater. Interfaces. 2022;14:11092–11103. doi:10.1021/acsami.1c22964
74. Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. 2019;18:241–272. doi:10.1007/s11101-018-9591-z
75. Biswas T, Sen A, Roy R, Maji S, Maji HS. Isolation of mangiferin from flowering buds of Mangifera indica L and its evaluation of in vitro antibacterial activity. Res Rev. 2015;4.
76. Singh SK, Tiwari RM, Sinha SK, Danta CC, Prasad SK. Antimicrobial evaluation of mangiferin and its synthesized analogues. Asian Pac J Trop Biomed. 2012;2:S884–S887. doi:10.1016/S2221-1691(12)60329-3
77. Rechenchoski DZ, Agostinho KF, Faccin-Galhardi LC, et al.Mangiferin: a promising natural xanthone from Mangifera indica for the control of acyclovir – resistant herpes simplex virus 1 infection. Bioorg. Med. Chem. 2020;28:115304. doi:10.1016/j.bmc.2020.115304
78. de Oliveira CS, Falcão-Silva VDS, Siqueira JP, et al. Drug resistance modulation in staphylococcus aureus, a new biological activity for mesoionic hydrochloride compounds. Molecules. 2011;16:2023–2031. doi:10.3390/molecules16032023
79. Jiamboonsri P, Pithayanukul P, Bavovada R, Chomnawang MT. The inhibitory potential of Thai mango seed kernel extract against methicillin-resistant staphylococcus aureus. Molecules. 2011;16:6255–6270. doi:10.3390/molecules16086255
80. Diso SU, Ali M, Mukhtar SI, Garba M. Antibacterial activity and phytochemical screening of Mangifera indica (mango) stem and leaf extracts on clinical isolates of methicillin resistant staphylococcus aureus. J Adv Med Pharm Sci. 2017:1–6. doi:10.9734/JAMPS/2017/31127
81. Al Bshabshe A, Joseph MRP, Awad El-Gied AA, Fadul AN, Chandramoorthy HC, Hamid ME. Clinical relevance and antimicrobial profiling of Methicillin-Resistant Staphylococcus Aureus (MRSA) on routine antibiotics and ethanol extract of mango kernel (Mangifera Indica L.). Biomed Res Int. 2020;2020:4150678. doi:10.1155/2020/4150678
82. Prabhu K, Prasathkumar M, Sivaraman J, et al. Phytochemical Characterization, antibacterial, and anti-biofilm efficacy of Mangifera indica seed kernel: a preliminary study using in vitro and in silico approaches. J King Saud Univ Sci. 2023;35:102688. doi:10.1016/j.jksus.2023.102688
83. Sicurella M, Sguizzato M, Cortesi R, et al. Mangiferin-loaded smart gels for HSV-1 Treatment. Pharmaceutics. 2021;13:1323. doi:10.3390/pharmaceutics13091323
84. Athipornchai A, Pabunrueang P, Trakulsujaritchok T. Mangiferin loaded carrageenan/chitosan core-shell hydrogel beads: preparation, characterization and proposed application. Food Hydrocoll. 2024;147:109394. doi:10.1016/j.foodhyd.2023.109394
85. Samadarsi R, Dutta D. Design and characterization of mangiferin nanoparticles for oral delivery. Journal of Food Engineering. 2019;247:80–94. doi:10.1016/j.jfoodeng.2018.11.020
86. Wisutthathum S, Kamkaew N, Inchan A, et al. Extract of aquilaria crassna leaves and mangiferin are vasodilators while showing no cytotoxicity. J Traditional Complementary Med. 2019;9:237–242. doi:10.1016/j.jtcme.2018.09.002
87. Rodeiro I, Hernandez S, Morffi J, et al. Evaluation of genotoxicity and DNA protective effects of mangiferin, a glucosylxanthone isolated from Mangifera indica L. stem bark extract. Food and Chemical Toxicology. 2012;50:3360–3366. doi:10.1016/j.fct.2012.06.032
88. Mei S, Perumal M, Battino M, et al. Mangiferin: a review of dietary sources, absorption, metabolism, bioavailability, and safety. Crit. Rev. Food Sci. Nutr. 2023;63:3046–3064. doi:10.1080/10408398.2021.1983767
89. Reddeman RA, Glávits R, Endres JR, et al. A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. J Toxicol. 2019;2019:1–14. doi:10.1155/2019/4763015
90. Villas Boas GR, Rodrigues Lemos JM, De Oliveira MW, et al. Preclinical safety evaluation of the aqueous extract from Mangifera indica Linn. (Anacardiaceae): genotoxic, clastogenic and cytotoxic assessment in experimental models of genotoxicity in rats to predict potential human risks. J Ethnopharmacol. 2019;243:112086. doi:10.1016/j.jep.2019.112086
91. Razura-Carmona FF, Díaz-Reséndiz KJG, Covantes-Rosales CE, et al. Toxicity of hybrid particles (PLGA-ZnO) loaded with lupeol and mangiferina: ex vivo model of peripheral cells. ACS Food Sci Technol. 2023;3:675–682. doi:10.1021/acsfoodscitech.2c00417
92. Jamwal D, Saini P, Tomar PC, Ghosh A. Perspectives on the Potential of Mangiferin as a Nutraceutical: a Review. NFS. 2023;53:249–264. doi:10.1108/NFS-01-2022-0013
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
From Citrus to Clinic: Limonene’s Journey Through Preclinical Research, Clinical Trials, and Formulation Innovations

S, Devi N, Bhattacharya B, Sharma A, Singh I, Kumar P, Huanbutta K, Sangnim T
International Journal of Nanomedicine 2025, 20:4433-4460
Published Date: 11 April 2025

