Back to Journals » ClinicoEconomics and Outcomes Research » Volume 17
Healthcare Costs and Treatment Patterns of Triplet Therapies in Relapsed/Refractory Multiple Myeloma: Real World Evidence from Italy
Authors Perrone V, Leogrande M , Giacomini E, Cappuccilli M , Degli Esposti L
Received 14 April 2025
Accepted for publication 6 July 2025
Published 19 July 2025 Volume 2025:17 Pages 495—505
DOI https://doi.org/10.2147/CEOR.S529788
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Valentina Perrone, Melania Leogrande, Elisa Giacomini, Maria Cappuccilli, Luca Degli Esposti On behalf of the LHU study group
CliCon S.R.L. Società Benefit, Health Economics and Outcomes Research, Bologna, Italy
Correspondence: Valentina Perrone, CliCon S.R.L. Società Benefit - Health, Economics and Outcomes Research, Via Murri 9, Bologna, 40137, Italy, Tel +3954438393, Fax +39544212699, Email [email protected]
Purpose: This analysis sought to characterize patients with relapsed or refractory multiple myeloma (RRMM) on triplet therapy with immunomodulatory agents/proteasome inhibitors/monoclonal antibodies combined with dexamethasone, describing their demographic and clinical features, therapeutic pathways and the related healthcare costs for the Italian National Health Service (NHS).
Patients and Methods: A retrospective observational analysis was conducted on administrative databases of Italian healthcare entities, covering about 3.6 million health-assisted residents. From 2017 to 2020, patients receiving at least one triplet combination reimbursed by the Italian NHS for the treatment of RRMM were included. RRMM treatment pathways were evaluated in terms of duration of therapy and treatment lines. Healthcare costs per patient were assessed on monthly basis during the therapy period by computing expenditures for drugs, hospitalizations and outpatient specialist services.
Results: A total of 209 RRMM patients on triplet combinations were identified, with a mean age of 67.4 years, 56% males and mild-to-moderate comorbidity profile, with heart disease and renal disease as the most common coexisting conditions (respectively, 13.4% and 7.7% of patients). KRd (carfilzomib/lenalidomide/dexamethasone) was the triplet administrated to the largest proportion of patients (44%), then DaraRd (daratumumab/lenalidomide/dexamethasone) triplet (24%). Treatment duration was on average 7 months for overall patients, 7.7 months for 118 patients with triplet as second line at inclusion, and 6 months for 91 patients with triplet as third or further line at inclusion. The monthly overall costs were € 9,517, with drug expenses accounting for 93% of total expenditures. Progressing to later treatment lines, cost analysis showed comparable trends, being drugs as the most impactive item.
Conclusion: This analysis on RRMM patients under triplet medication in real-life Italian clinical practice confirmed the complex multifaceted profile of this frail population, highlighting a challenging clinical management for the oncologists and a major economic burden for the NHS.
Keywords: triplet combination therapy, immunomodulatory agents, proteasome inhibitors, monoclonal antibodies, duration of therapy, treatment lines, cost analysis
Introduction
Multiple myeloma (MM) is a relatively uncommon disease, characterized by an abnormal proliferation of a single plasma cell clone, resulting in the production of monoclonal immunoglobulins and end-organ damage.1
The latest epidemiological estimates have shown that MM currently accounts for almost 2% of overall cancers and 10% of all hematologic malignancies.2 Anyhow, recent international data indicated that MM numbers are on the rise, with worldwide estimated age-standardized rate of 1.78/100,000 people for incidence and of 1.14/100,000 people for mortality.3
Over the last decades, novel drug classes, like immunomodulators and proteasome inhibitors, have been added to the therapeutic armamentarium available for both frontline and relapsing treatment interventions.4–6 However, in spite of the undeniable advances related to the growing availability of innovative molecules, a substantial number of patients still experience relapsed or refractory MM (RRMM), with a weaker and less durable drug response when receiving successive lines of therapy.7
The therapeutic management of RRMM acquires an even higher complexity in those patients who become refractory to both proteasome inhibitors and immunomodulatory drugs.8 This critical challenge has recently boosted the efforts to search new drug classes or combinations. The advent of monoclonal antibodies directed against CD38 antigen, a transmembrane glycoprotein highly expressed on MM cells, has further improved the disease perspectives, in terms of progression-free survival and overall survival.9 This evolving set of therapeutic options has opened the possibility of using these drugs in various triplets or quadruplets, combining immunomodulatory agents (thalidomide, lenalidomide, and pomalidomide), proteasome inhibitors (bortezomib, carfilzomib, ixazomib), monoclonal antibodies (daratumumab, elotuzumab, isatuximab, belantamab), with the corticosteroid dexamethasone.10–12
In this scenario, the hematologist-oncologist’s decisions towards the best treatment options for RRMM are driven by the results of clinical research and real-world evidence (RWE) data that generally have shown a good degree of reproducibility.13 However, some flaws in randomized clinical trials should be taken into account, above all the stricter eligibility criteria in population selection that might exclude part of the patients for reasons like age, prior treatment lines in patients refractory to previous therapies, and the presence of comorbidities, especially renal failure and cardiovascular disease.14 Besides, several studies in the real-world setting are ongoing, with the goal of achieving patient-tailored treatment approaches, thus pointing out the urgent need for approval and rapid introduction into the routine clinical practice of new potent drugs. Recently, US and European regulatory medicines authorities have approved other drugs for patients with RRMM, including pomalidomide, carfilzomib, elotuzumab, ixazomib, daratumumab, and more recently, selinexor—a first-in-class selective inhibitor of nuclear export (SINE).11
This real-world analysis was then undertaken to analyse the demographic and clinical characteristics of patients with RRMM treated with triplet combinations, to describe their therapeutic pathways in terms of number of treatment lines and duration of therapy, and to evaluate the healthcare direct costs for Italian National Health Service (NHS).
Materials and Methods
Data Source
This retrospective observational analysis was conducted by using data extrapolated from administrative databases of a sample of geographically distributed Italian healthcare entities, covering about 3.6 million health-assisted subjects. The following databases were investigated: i) demographic database, to get information on patients’ demographic data, like gender, age and death; ii) pharmaceuticals database, containing data on medicinal products reimbursed by the Italian NHS, in terms of Anatomical Therapeutic Chemical (ATC) code, number of packages, number of units per package, unit cost per package, and prescription date; iii) hospitalization database, which collects all hospitalizations data, like discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), Diagnosis Related Group (DRG) and DRG-related charge (provided by the Italian Health System); iv) outpatient specialist services (OSS) database, which gathers all data regarding visits and diagnostic tests (date and type of prescription, description activity and laboratory test or specialist visit charge); v) payment exemption database, which includes exemption codes by which patients are exempted from paying the contribution charge for services/treatments in case of specific diseases diagnoses.
In order to guarantee privacy, in full compliance with the European General Data Protection Regulation (GDPR) (2016/679), each study subject was identified by an anonymous univocal numeric code. This code allowed the electronic linkage between the various databases. All the results of the analyses were provided in aggregated form so that any information cannot be attributable, either directly or indirectly, to individual patients.
According to “Opinion 05/2014 on Anonymisation Techniques” drafted by the “European Commission Article 29 Working Party”, the analyses involving less than 3 patients were not reported (and indicated as “NR”), as potentially reconductable to single individuals.
The project was conducted in accordance with the Declaration of Helsinki and approved by the following Ethics Committee: Comitato etico interprovinciale Area I Barletta Andria Trani - BAT (protocol number 68/CE/20, approval date 3/12/2020); Comitato etico interprovinciale Area I Foggia (protocol number 63/CE/20, approval date 3/12/2020); Comitato Etico “Lazio 2” (protocol number 0216084/2020, approval date 16/12/2020); Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana (protocol number 20190211, approval date 12/09/2019); Comitato Etico Regionale Umbria (protocol number 19414/20/ON, approval date 16/09/2020).
According to the pronouncement of the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes – n.9/2014, December 11th – published on the Official Gazette n. 301 on December 30th, 2014) data treatment is authorized without patient informed consent, when collection is impossible for organizational reasons.
Identification of Study Population
From January 2017 to December 2020 (inclusion period) patients receiving at least one triplet combination indicated for the treatment of RRMM were included.
The triplet regimens considered were those approved and reimbursed by the Italian NHS during the study period, specifically:
- DaraRd: daratumumab/lenalidomide/dexamethasone;
- PomVd: pomalidomide/bortezomib/dexamethasone;
- IxaRd: ixazomib/lenalidomide/dexamethasone;
- EloRd: elotuzumab/lenalidomide/dexamethasone;
- KRd: carfilzomib/lenalidomide/dexamethasone;
- DaraVd: daratumumab/bortezomib/dexamethasone;
- EloPd: elotuzumab/pomalidomide/dexamethasone.
The triplet schemes isatuximab/carfilzomib/dexamethasone (IsaKd), isatuximab/pomalidomide/dexamethasone (IsaPd) and daratumumab/pomalidomide/dexamethasone (DPd) were not analysed because they were not yet among the reimbursable therapies in Italy during the study period. Similarly, although the RVd (lenalidomide, bortezomib, and dexamethasone) combination was approved in Italy in 2019, the number of patients treated with this regimen within our study window was too small to allow for meaningful analysis.15–18
Among the included patients, the index date was defined as the time of the first drug prescription of triplet therapy for RRMM during the inclusion period. Considering the availability of the data from January 2009 to December 2021, the characterization period started at least 12 months before the index date (all patients had at least 1 year of data availability period before the index date) and follow-up included all the available period after the index date (all patients had at least 1 year of data availability period after the index date). Patients were analysed overall and stratified according to the number of treatments used for RRMM tracked within the database before the line at index date. For each patient, only one treatment regimen was included in the analysis, defined as the first reimbursed triplet regimen for RRMM received during the inclusion period. This regimen was considered the index treatment. Patients were then categorized according to the line of therapy (second line or third line or beyond) in which the index regimen was initiated. This approach was adopted to ensure consistency in the evaluation of treatment duration and healthcare resource utilization and to avoid duplication of patient data across multiple treatment lines.
For the whole sample included in the study, the following demographic and clinical characteristics were collected: age at the index date, distribution of age classes (35–44 years, 45–54 years, 55–64 years, 65–74 years, 75–84 years, ≥85 years), gender (expressed as percentage of male sex), and comorbidly profile evaluated through the Charlson Comorbidity Index (CCI), a scoring system that sums the weight of each concurrent disease.19 Besides, the most common previously described concomitant conditions in MM20,21 were recorded, namely: (ii) chronic kidney disease (CKD), identified by at least 1 hospitalization with a discharge diagnosis for CKD (ICD-9-CM code: 585); (iii) heart disease, identified by at least 1 hospitalization with a discharge diagnosis for ischemic heart disease (ICD-9-CM codes: 410, 411, 413, 414), heart failure (ICD-9-CM code: 428), cerebrovascular disease (ICD-9-CM codes: 430, 431, 432, 433, 434, 435, 436, 437, 438), atherosclerosis and aneurysm (ICD-9-CM code: 440–442).
Definition of RRMM Treatment Pathways: Duration of Therapy and Treatment Lines
To evaluate RRMM treatment pathways, the duration of therapy (DOT) was defined by the months from the initiation to the end of prescriptions of the therapy presented at index date. The profiles of treatments used for line definition are reported in Table S1. Briefly, if a treatment without corticosteroids (prednisone or dexamethasone) was found, it was reconducted to its defined treatment regimen (ie, VM was considered as VMP, VT as VTd, V as Vd; R was considered as Rd in the absence of autologous stem cell transplantation-ASCT). This is because the hospital-dispensing of corticosteroids could be not traceable among the databases. Data on ASCT were also computed (procedure codes 41.01, 41.04, 41.07, 41.09).
Analysis of Monthly Healthcare Costs per Patient
The mean monthly healthcare costs per patient were assessed as overall expenditures and by cost item, namely drugs, hospitalizations (ordinary and day hospital), and outpatient specialist service (specialist visits, diagnostic and laboratory tests). Specifically, hospitalisation costs were determined by using the DRG tariffs, drug costs were calculated for drugs reimbursed by the Italian NHS using the Italian NHS purchase price with net discount, and outpatient specialist service costs (related to all services) were based on Regional tariffs. Among the dataset, outliers (the values that exceed more than three standard deviations the mean value) were excluded. Given the dynamic context of the availability of therapeutic options for RRMM, a sensitivity analysis is presented with the aim to estimate an up-to-date scenario, by replacing the cost of original brand lenalidomide with the price ex-factory of the generic equivalent (available from 2022). To perform the analysis, the cost of the generic equivalent corresponding to the originator lenalidomide package dispensed was considered. The packages dispensed were 2.5 mg (packages with 7 and 21 pills), 5 mg (package with 21 pills), 2.5 mg (package with 21 pills) 10 mg (packages with 7 and 21 pills), 15 mg (packages with 7 and 21 pills), 20 mg (packages with 7 and 21 pills), 25 mg (package with 21 pills).
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) and categorical variables as numbers and percentages. Multiple regression analysis was performed to examine the association between the triplet regimen received and DOT, adjusting for covariates, including age, gender and comorbidities. The triplet group with larger sample size has been considered as the reference group. A P value <0.05 was considered as statistically significant. All analyses were carried out using STATA SE, version 17.0 (StataCorp LLC, College Station, TX, USA).
Results
Identification of Study Population and Distribution of Patients by Triplet Combination
Among the study sample covering 3,605,876 health-assisted individuals, a total of 209 RRMM patients treated with triplet combinations were identified (Figure 1A). At the index date, KRd was the most frequently prescribed combination (44%), followed by DaraRd (24%) (Figure 1B).
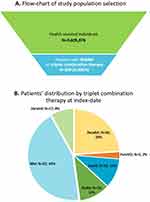 |
Figure 1 (A) Flow-chart of study population for the selection of RRMM patients; (B) Patient distribution by triplet combination at index date. |
The demographic and clinical characteristics of overall RRMM patients are described in Table 1. Among the 209 RRMM patients with triplet therapy, the mean age was 67.4 years, and 56% were male. The average CCI was 0.9, with 40.2% patients scoring 0, 37.3% scoring 1 and 22.5% with a CCI above 2, thus revealing an overall mild-to-moderate comorbidity profile. Heart disease was observed in 13.4% of patients and CKD in 7.7%.
 |
Table 1 Demographic and Clinical Characteristics of Overall RRMM Patients |
Duration of Treatment
As shown in Table 2A, the mean duration of treatment at index date was 7 months for all included patients, 7.7 months for 118 patients with triplet as second line at index date (2L), and 6 months for 91 patients with triplet as third or further line at index date (3L+).
 |
Table 2 Duration of Treatment (Months) in the Overall Included 209 RRMM Patients and by Number of Lines at Inclusion (A) and the 114 Patients with at Least 2 years of Follow-Up (B) |
Focusing on therapies based on proteasome inhibitors in those patients with at least 2 years of follow-up (Table 2B), the DOT overall was on average 10.8 months in 4 patients under IxaRd treatment, and 6.2 months in 55 patients under KRd treatment. IxaRd in 2L (N=4) had a mean DOT of 10.8 months, KRd (N=31) a mean DOT of 6.0 months. Among the RRMM patients with at least 2 years of follow-up, the only triplet with proteasome inhibitor found in 3L+ was KRd, administered in 24 patients with a mean DOT of 6.6 months.
The regression model, by adjusting for baseline covariates, namely age, gender and comorbidities, is described in Table 3. KRd was considered as the reference group, since it included most patients. DaraRd and EloRd triplets have a duration 3.9 and 4.1 months higher compared to that of KRd, while PomVd triplet has a duration of 2.79 months lower than KRd DOT (Table 3). IxaRd triplet also showed a trend towards a higher DOT (3.3 months higher than KRd), although nearly significant (P=0.058). The differences in DOT reported above have been computed using a regression model that considers adjustment for covariates, so it does not coincide with the exact difference between the means of DOT between the various triplets.
 |
Table 3 Regression Analysisa to Evaluate the Relationship Between Index Triplets Administrated and Duration of Index Treatment |
Analysis of Monthly Healthcare Costs
The pattern of mean monthly healthcare costs per patient (calculated for the duration of therapy) is illustrated in Figure 2. After eliminating outliers, the analysis performed on 206 patients showed that the monthly overall costs were € 9,517 during the treatment, with drug expenses as the most impactive item, accounting for € 8,837. The expenses for hospitalizations and outpatient specialist services were lower, € 413 and € 266, respectively. The monthly healthcare costs per patient in 2L are shown in Figure 2. After eliminating outliers, the remaining 115 patients showed a monthly overall cost of € 9,476 of which drug expenses as the weightiest item, accounting for € 8,771, followed by hospitalizations (€ 431) and outpatient specialist services (€ 274). In 3L+, the analysis was conducted on 91 patients (excluding outliers), and the total monthly expenses were € 9,578, those for drugs € 8,939, and then hospitalizations (€ 386) and outpatient specialist services (€ 254). The sensitivity analysis performed considering the ex-factory cost of generic equivalent of lenalidomide yielded to a delta of around € 1,300–1,400 in the different population analysed.
The weights of each cost item in the overall monthly healthcare costs per patient stratified by triplet and lines of therapy are shown in Supplementary Figures 1–3. Only subgroups with more than 4 patients were presented.
Discussion
This real-world analysis depicted the baseline characteristics, treatment patterns and healthcare resource costs in RRMM patients under triplet medications. Our focus on triplet regimens reflects their predominant role in the current RRMM management, particularly for patients previously exposed to all three main drug classes. This subgroup represents a clinically challenging and heavily pretreated population, for whom triplet-based strategies are most commonly employed and studied in both real-world practice and clinical research. Overall, 209 patients were identified, with a mean age of 67.4 years, and a mild comorbidity profile, mostly related to heart and kidney involvement, in line with literature.20–23
The most represented treatments at index date were KRd administered to 44% of patients and DaraRd in 24%. The duration of treatment ranged between 6 and 7 months and the longest DOT was found with EloRd regimen followed by DaraRd and IxaRd in all treatment lines. Among the RRMM patients receiving triplets including proteasome inhibitors and with at least 2 years of follow-up, the regression model highlighted for IxaRd triplet a trend towards an increased DOT of above 3 months. A US study by Chari et al evaluated DOT and time to next therapy (TTNT) in RRMM patients under treatment with bortezomib, carfilzomib and ixazomib combined with lenalidomide/dexamethasone (VRd, KRd and IxaRd, respectively.) and browsed the electronic medical records database to select 531 RRMM patients, 343 of them starting VRd,139 KRd and 49 IxaRd, as frontline regimens. The three cohorts were then followed from 2008 to 2016 in 2L, 3L, or above, discriminating between switchers or retreatments with respect to 1L after at least a 6-month interval. Although the US patients have a similar age at index date compared to our population (median age respectively 69, 65 and 73 years in the mentioned triplet groups), they appeared to have a worse comorbidity profile as demonstrated by a median CCI around 2, regardless of the therapy received. Analysing the discontinuation rates, the IxaRd combination was associated with longer DOT and TTNT compared to the other triplets. Moreover, the patients under IxaRd were older, treated in later lines, had a lower frequency of previous ASCT and less renal complications. These findings, emerging from a real-world clinical setting in United States, are generally consistent with those found in the present analysis. The discrepancy regarding the larger utilization of IxaRd in subsequent lines of therapy after 1L is feasibly due to the substantially longer follow-up (8 years) of the US study,24 while the different value of CCI could be due to the fact that in our analysis a modified version of CCI, not counting for cancer, has been applied.
The healthcare costs per patient, analysed monthly, showed that the burden for frontline treatment of RRMM are high, mostly driven by drug expenses that represented about 93% of the overall expenditures. These findings agree with pharmacoeconomic studies in other countries. A recent retrospective analysis using German claims data by Kocaata et al on 2523 with an existing diagnosis of MM and 1673 newly treated patients between January 2010 and March 2019 found that total direct costs increased as patients moved to later lines of treatment, being primarily burdened by drug prescriptions.25 Similarly, a retrospective study on US claims-database to evaluate the healthcare costs of MM patients with ≥4 prior lines of therapy requiring multiple drug classes reported that expenses for therapies and their administration contributed on average to 88.5% of the total all-cause healthcare expenditures.26 These findings highlight not only the significant economic burden for the healthcare system but also raise concerns about sustainability and equitable access to innovative therapies for RRMM. In real-world settings, high drug costs may impact treatment availability and adherence, potentially influencing patient outcomes. These considerations underscore the importance of cost-effectiveness assessments and policy efforts aimed at ensuring long-term affordability of myeloma care. Besides, the sensitivity analysis that using of the introduction of generic equivalent leads to a decrease of costs could have been strongly underestimated since the ex-factory price was considered instead of the net discount for Italian NHS.
When analyzing the monthly healthcare costs in 2L and 3L+ for each triplet regimen, drug expenses confirmed to be the most impactive cost item, regardless of the treatment line. Although the costs for RRMM management with proceeding of therapy sequences remained elevated, IxaRd, KRd, and DaraRd triplets were associated with lower overall and drug-related expenses. To the best of our knowledge, this is the first Italian real word analysis describing the economic burden associated with each triplet used in different lines of treatment for RRMM and based on duration of treatment, as the currently published studies have investigated drug expenses as an aggregated cost item.27
The results of this analysis should be viewed in consideration of some limitations, above all its retrospective observational design and the use of administrative databases, the small sample size of certain patient subgroups, especially with progression of treatment lines at each disease relapse. Thus, some data could not be disclosed in case of groups consisting of less than 4 patients, in compliance with the law on privacy protection. Since the administrative database does not provide clinical information, it was not possible to identify the “high-risk myeloma” (HRC+) patients (the target population of IxaRd). Moreover, as mentioned above, we were not able to include IsaKd, DPd and IsaPd triplets in our analysis because their approval for NHS reimbursement arrived after the end of our inclusion period. Likewise, despite the approval of the RVd regimen (lenalidomide, bortezomib, and dexamethasone) in Italy in 2019, its uptake within the study timeframe was minimal, resulting in an insufficient patient sample for separate analysis. On the other hand, this time-shift that prevented us to have a broader view of all the newly approved combinations is a further proof of the constant efforts by researchers to amplify the portfolio of therapeutic combinations for RRMM. In addition, due to the nature of administrative databases, the clinical rationale for treatment discontinuation, such as disease progression, adverse events, or patient/physician preference, could not be captured, limiting our ability to interpret the underlying reasons behind therapy changes or interruptions. Lastly, although data on ASCT procedures were captured in the dataset, the limitations of administrative databases prevented us from clearly determining whether ASCT occurred before or after the initiation of each triplet regimen. As such, the role of transplantation in influencing treatment outcomes could not be fully analyzed. Therefore, other studies through both randomized clinical trials and RWE are equally necessary to give more hopes to patients affected by diseases previously burdened by a particularity poor prognosis.
Conclusion
This real-world analysis highlights the complex treatment journey of RRMM patients in Italy, who face both clinical challenges and high-cost burdens. The findings confirm that triplet regimens, particularly KRd, DaraRd, and EloRd, are commonly used and differ in terms of treatment duration and cost profiles. Drug expenses remain the predominant cost component, placing a considerable burden on the healthcare system. However, the dominance of drug costs across all treatment lines underscores the need for ongoing pharmacoeconomic evaluations. While generic formulations offer cost savings, continuous real-world evidence is essential to guide future therapeutic strategies and ensure the sustainability of healthcare systems. Ultimately, improving outcomes for this frail population demands both innovative treatments and thoughtful resource allocation.
Data Sharing Statement
All data used for the current study are available upon reasonable request next to CliCon S.R.L. which is the body entitled of data treatment and analysis by the healthcare entities involved in the study.
Acknowledgments
The authors are grateful to all the study group components of the participating Italian LHUs: Fausto Bartolini (Dipartimento Farmaceutico, USL Umbria 2, Terni), Andrea Ciaccia (Servizio Farmaceutico Territoriale, ASL Foggia), Stefania Dell’Orco (UOC Farmaceutica Territoriale, ASL RM 6, Albano Laziale, RM), Cataldo Procacci (Dipartimento Farmaceutico, ASL BAT, Andria); Giuseppe Taurino (Dipartimento del Farmaco, ASL Toscana Nord Ovest, Pisa).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548–567. doi:10.1002/ajh.25791
2. Padala SA, Barsouk A, Barsouk A, et al. Epidemiology, staging, and management of multiple myeloma. Med Sci. 2021;9(1):3.
3. Huang J, Chan SC, Lok V, et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022;9(9):e670–e677.
4. Rajkumar SV, Palumbo A. Management of newly diagnosed myeloma. Hematol Oncol Clin North Am. 2007;21(6):1141–x.
5. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498.
6. Richardson PG, Blood E, Mitsiades CS, et al. A randomized Phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108(10):3458–3464.
7. Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867–874.
8. Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443–2448.
9. Gozzetti A, Ciofini S, Simoncelli M, et al. Anti CD38 monoclonal antibodies for multiple myeloma treatment. Hum Vaccin Immunother. 2022;18(5):2052658.
10. Goldschmidt H, Mai EK, Bertsch U, et al. Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised, active-controlled, Phase 3 trial. Lancet Haematol. 2022;9(11):e810–e821.–0.
11. Boudreault JS, Touzeau C, Moreau P. Triplet combinations in relapsed/refractory myeloma: update on recent phase 3 trials. Expert Rev Hematol. 2017;10(3):207–215.
12. Pantani L, Brioli A, Tacchetti P, et al. Current and emerging triplet combination therapies for relapsed and refractory multiple myeloma. Expert Rev Hematol. 2016;9(3):315–323.
13. Bertamini L, Bertuglia G, Oliva S. Beyond clinical trials in patients with multiple myeloma: a critical review of real-world results. Front Oncol. 2022;12:844779.
14. Chari A, Romanus D, Palumbo A, et al. Randomized clinical trial representativeness and outcomes in real-world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(1):8–17.e16.
15. Italian Medicines Agency (AIFA): determina 24 marzo 2022: regime di rimborsabilità e prezzo, a seguito di nuove indicazioni terapeutiche, del medicinale per uso umano «Sarclisa». Available from: https://www.gazzettaufficiale.it/eli/id/2022/04/04/22A02091/sg.
16. Italian Medicines Agency (AIFA): determina 19 febbraio 2019. Riclassificazione del medicinale per uso umano «Ninlaro». Available from: https://www.aifa.gov.it/documents/20142/847786/NINLARO_GU_n.57_8-3-2019.pdf.
17. Italian Medicines Agency (AIFA): determina 9 settembre 2021. Riclassificazione del medicinale per uso umano «Sarclisa». Available from: https://www.aifa.gov.it/documents/20142/847786/determina_Sarclisa_GUn.226.pdf.
18. Italian Medicines Agency (AIFA): determina 13 gennaio 2023. Regime di rimborsabilità’ e prezzo, a seguito di nuove indicazioni terapeutiche, del medicinale per uso umano «Darzalex». Available from: https://www.aifa.gov.it/documents/20142/961234/Determina_24-2023_Darzalex.pdf.
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
20. Kleber M, Ihorst G, Terhorst M, et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J. 2011;1(9):e35.
21. Chakraborty R, Majhail NS. Treatment and disease-related complications in multiple myeloma: implications for survivorship. Am J Hematol. 2020;95(6):672–690.
22. Engelhardt M, Dold SM, Ihorst G, et al. Geriatric assessment in multiple myeloma patients: validation of the international myeloma working group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101(9):1110–1119.
23. Nourallah A, Alshehri A, Alhejazi A, et al. Real-world registry on the pharmacotherapy of multiple myeloma and associated renal and pulmonary impairments in the greater gulf region: protocol for a retrospective real-world data study. JMIR Res Protoc. 2024:
24. Chari A, Romanus D, Luptakova K, et al. Duration of therapy (DOT) and time to next therapy (TTNT) of bortezomib, carfilzomib and ixazomib combinations with lenalidomide/dexamethasone (VRd, KRd, IRd) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): clinical practice in the United States Vs clinical trial experience. Blood. 2017;130(Supplement 1):1818.
25. Kocaata Z, Wilke T, Fischer F, Welte R, Einsele H. Healthcare resource utilization and cost of patients with multiple myeloma in Germany: a retrospective claims data analysis. Pharmacoecon Open. 2022;6(4):619–628.
26. Jagannath S, Joseph N, He J, et al. Healthcare costs of multiple myeloma patients with four or more prior lines of therapy, including triple-class exposure in the United States. Oncol Ther. 2022;10(2):411–20.
27. Iida S, Nakakoji M, Spanopoulos D, Okazuka K, Parulekar V, Ishida T. Practice patterns and outcomes for triple-class exposed patients with relapsed/refractory multiple myeloma in Japan. Future Oncol. 2022;18(34):3839–52.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


