Back to Journals » Journal of Inflammation Research » Volume 18
Identification of Cardiometabolic Protein Biomarkers for Acute Myocardial Infarction Using Olink Proteomics
Authors Tan X, Wang X, Xu S, Zeng Y, Zhang G, Xu A , Jiang Y , Jiang H, Song Y, Fan J, Fu Y, Bo X, Fan H , Zhou Y
Received 9 October 2024
Accepted for publication 13 February 2025
Published 22 February 2025 Volume 2025:18 Pages 2629—2646
DOI https://doi.org/10.2147/JIR.S495784
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xin Tan,1,2 Xiangyu Wang,1,2 Shuai Xu,1,2 Yiyao Zeng,1,2 Ge Zhang,3 Anchen Xu,1,2 Yufeng Jiang,1,2 Hezi Jiang,1,2 Yahui Song,4 Jili Fan,5 Yangjun Fu,6 Xiaohong Bo,5 Huimin Fan,1,4 Yafeng Zhou1,2
1Department of Cardiology, the Fourth Affiliated Hospital of Soochow University, Suzhou Dushu Lake Hospital, Medical Center of Soochow University, Suzhou, 215000, People’s Republic of China; 2Department of Hypertension, the Fourth Affiliated Hospital of Soochow University, Institute for Hypertension, Soochow University, Suzhou, 215000, People’s Republic of China; 3Department of Cardiology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China; 4Center of Translational Medicine and Clinical Laboratory, the Fourth Affiliated Hospital to Soochow University, Suzhou Dushu Lake Hospital, Suzhou, 215028, People’s Republic of China; 5Department of Cardiovascular Disease, Taihe County People’s Hospital, Fuyang, 236600, People’s Republic of China; 6Department of Neurology, the Third People’s Hospital of Hefei, Hefei, 230041, People’s Republic of China
Correspondence: Huimin Fan, Email [email protected] Yafeng Zhou, Email [email protected]
Background: Acute myocardial infarction (AMI) is a critical cardiovascular event characterized by sudden coronary blood flow interruption, leading to myocardial ischemia and necrosis. Despite advances in acute therapeutic measures, understanding the metabolic damage related to AMI, particularly through specific protein expressions, remains limited. This study utilized Olink cardiovascular metabolomics technology to explore cardiovascular metabolism-related protein biomarkers associated with AMI, aiming to address the clinical need for early diagnosis and targeted therapy.
Methods: This study utilized Olink cardiovascular metabolomics technology to analyze 92 cardiovascular metabolism-related proteins in coronary blood samples from 20 AMI patients and 10 healthy controls. Differentially expressed proteins were identified using statistical t-tests, followed by functional enrichment analysis (GO and KEGG) and protein-protein interaction network construction. Five core proteins were validated in plasma samples from an additional 125 AMI patients and 120 healthy controls via enzyme-linked immunosorbent assay. To evaluate diagnostic performance, receiver operating characteristic curves were generated using GEO-related datasets, and Mendelian randomization analysis was employed to investigate the causal relationship between core proteins and AMI risk.
Results: The study identified 32 proteins with significantly altered expression levels between AMI patients and healthy controls. Among these, five core proteins—PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1—were significantly associated with key biological processes such as metabolism, collagen formation, and the PI3K/AKT signaling pathway. These proteins showed strong correlations with clinical indicators, including BMI, LVEF, NT-proBNP, CK-MB, and cTnT. FCN2 and DEFA1 were further validated as having a causal relationship with AMI risk, indicating their potential as diagnostic biomarkers.
Conclusion: The identified core proteins PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 are potential biomarkers for the early diagnosis and risk assessment of AMI. These findings suggest that these proteins could serve as targets for future therapeutic interventions aimed at mitigating cardiovascular metabolic damage in AMI.
Keywords: acute myocardial infarction, cardiovascular metabolomics, Olink proteomics, biomarkers
Introduction
Acute myocardial infarction (AMI) is a life-threatening acute disease, which is caused by a sudden obstruction of coronary artery, leading to myocardial ischemia and necrosis. Its main pathological mechanism is thrombosis formation after the rupture of atherosclerotic plaques, resulting in complete or partial occlusion of the coronary arteries. Clinical symptoms usually include chest pain, shortness of breath, sweating, and nausea. AMI is one of the leading causes of death and disability worldwide.1 Timely reperfusion therapy, such as thrombolytic therapy or percutaneous coronary intervention, is critical to improving prognosis. However, despite improvements in acute therapeutic measures, many patients still face challenges of myocardial dysfunction and long-term complications.
During myocardial infarction, ischemia-stimulated cardiomyocytes cause energy metabolism disorders, oxidative stress, cell death, and inflammation. Among these, glucose and lipid metabolism disorders play a crucial role in the development of diabetes and cardiac remodeling.2,3 Changes in glucose and lipid metabolism in the heart can induce myocardial cell apoptosis, myocardial hypertrophy, and cardiac fibrosis.3,4 Additionally, inflammation also participates in repairing and remodeling the infarcted heart.5,6 Research indicated that metabolic damage was significantly correlated with the incidence and mortality of coronary artery disease. In patients with acute myocardial infarction, the presence of metabolic damage may result in more severe myocardial injury and a poorer prognosis.7,8 Multiple studies have shown that IL-18 and CRP activity levels are significantly elevated in AMI patients,9,10 and metabolic markers, such as aldosterone and cortisol are associated with ventricular remodeling in the early to mid-stages of AMI,11 indicating that inflammation and metabolic disorders play important roles in AMI-induced damage. However, current research mostly focuses on a single metabolic marker, lacking comprehensiveness and specificity regarding cardiovascular metabolic damage. This fails to fully reveal the complex relationship between cardiovascular metabolic damage proteins and AMI injury.
With the development of proteomics technology, especially the application of Olink cardiovascular metabolomics technology, we are provided with new perspectives and tools to comprehensively reveal the relationship between AMI and cardiovascular metabolic damage.12 Recent research highlights the potential of Olink technology in identifying biomarkers for ischemic and hypertrophic cardiomyopathy, offering significant insights into disease mechanisms and aiding in the development of improved diagnostic methods.13,14 Olink cardiovascular metabolomics, through high-throughput detection of proteins in coronary plasma samples, can identify a series of biomarkers closely related to cardiovascular metabolism.15,16 These biomarkers can not only serve as indicators for early diagnosis of AMI but also as potential therapeutic targets. Therefore, our study utilized the Olink cardiometabolic panel to identify metabolic damage proteins associated with AMI, providing comprehensive proteomic insights. We identified new biomarkers and explored their roles and mechanisms in human plasma using GO and KEGG analyses. Among the 12 significantly upregulated proteins, 5 core proteins showed significant differences. They were enriched in key biological processes and pathways such as metabolism, collagen formation, and PI3K/AKT signaling, and were further validated through the public GEO database and Mendelian randomization analysis. This study aims to identify metabolism-related markers in AMI and determine new therapeutic targets for cardiovascular metabolic damage in AMI.
Materials and Methods
General Information
A total of 145 AMI patients hospitalized from January 2023 to December 2023 at Taihe County People’s Hospital affiliated with Wannan Medical College were selected as the study subjects. Inclusion criteria: (1) Diagnosed with AMI through clinical manifestations, laboratory tests, and imaging examinations, meeting relevant diagnostic criteria;1,17 (2) Underwent coronary angiography and received percutaneous coronary intervention; (3) Complete clinical case data. Exclusion criteria: (1) Patients with congenital heart disease, malignant tumors, immune/hematological system diseases, or severe liver and kidney dysfunction; (2) Those who had surgery, trauma, acute infection, or taken glucocorticoids/immunosuppressants within the past month; (3) Poor compliance resulting in incomplete clinical case data. All patients and their families voluntarily participated in this study, which was conducted in accordance with the internationally recognized principles of the Declaration of Helsinki and approved by the Ethics Committee of Taihe County People’s Hospital affiliated with Wannan Medical College (Approval No. 2023–09-2022-34) and the Fourth Affiliated Hospital of Soochow University (Approval No. 2022-KT-105, ChiCTR2100051469). General information such as gender, age, body mass index (BMI), history of hypertension, history of hyperlipidemia, history of diabetes, triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and cardiac troponin I (cTnI) were recorded.
Plasma Sample Collection
Patients diagnosed with AMI and healthy volunteers were recruited for this study. Coronary blood was collected before coronary angiography, placed in Na-EDTA tubes, and centrifuged at 3000×g for 15 minutes at 4°C. Each plasma sample was then aliquoted and stored at −80°C to maintain the stability and integrity of plasma biomolecules.
Screening of Cardiometabolic Biomarkers for AMI
The Olink Cardiometabolic panel (Olink Proteomics AB, Uppsala, Sweden), which includes 92 specific proteins, was utilized to examine samples from 20 AMI patients and 10 healthy controls (CON). Each protein target was detected through dual-antibody labeling, paired with a complementary DNA barcode. Quantification was conducted using the Biomark HD, a high-throughput microfluidic real-time PCR system (Biomark HD, Fluidigm). The results are presented as Normalized Protein eXpression (NPX) values, represented in arbitrary units on a log2 scale, where higher NPX values correspond to increased protein expression levels. The validation data, including detection limits and both intra- and inter-assay precision, are available on the manufacturer’s website (www.olink.com).15
Potential Metabolize Biomarker Screening
Differentially expressed proteins (DEPs) between the two groups were identified utilizing the Olink®Analyze R package. The associations between DEPs and clinical characteristics in AMI patients were examined using OmicStudio (https://www.omicstudio.cn). Diagnostic potential was assessed through receiver operating characteristic (ROC) curves produced by the ROC R package, where higher area under the curve (AUC) values signified better diagnostic accuracy. Only DEPs showing high significance in all bioinformatics analyses were deemed potential biomarkers for AMI.
Protein–Protein Interaction (PPI) Network Construction
The STRING database was employed to construct a PPI network, visualizing interactions among all DEPs and potential biomarkers. PPI pairs with a combined score above 0.4 (medium confidence) were selected. The network visualization was performed using Cytoscape software (http://www.cytoscape.org/).18
Function Enrichment Analysis
Gene enrichment analysis was conducted to clarify the main biological functions and signaling pathways involved. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were carried out and visualized using R packages.15 The DEP profiles between the Control and AMI groups highlighted enriched categories of biological processes (BP), molecular functions (MF), and cellular components (CC). KEGG enrichment analysis was utilized to investigate the genomic and systemic functions of significant DEPs.
Enzyme-Linked Immunosorbent Assay (ELISA) Validation in Plasma
Plasma samples from AMI patients (n=125) and healthy normal controls (n=120) were further validated using ELISA. The top five up-regulated key genes among the DEPs were confirmed, focusing on DEPs with at least a 0.5-fold change (FC) and significant roles in KEGG pathways. Plasma levels of core genes were quantified using REG1A ELISA Kits (MBS1751638, MyBioSource, USA), Human Ficolin-2 ELISA Kit (EH192RB, Thermo Fisher Scientific, USA), Human PCPE-1 ELISA Kit (EH362RB, Thermo Fisher Scientific, USA), DEFA1 (CSB-E14155h, CUSABIO, China), CRTAC1 (CSB-EL005997HU, CUSABIO, China) according to the manufacturer’s instructions. To clarify the accuracy of key genes and their impact on chronic ischemic myocardial injury, validation was performed using the public GEO database. The key genes were further validated in external datasets - AMI (GSE66360, GSE48060) and ischemic cardiomyopathy (ICM) (GSE5406, GSE57388, GSE57345) (Supplementary Table 1).
Mendelian Randomization
The dataset originates from the FinnGen project, a large-scale genomic research initiative aimed at exploring disease mechanisms by analyzing the relationship between the genomes and health data of over 218,000 participants of European ancestry. Specifically, the GWAS dataset used in this study includes 26,060 cases and 343,079 controls, with multiple sensitivity analyses performed to ensure the reliability of causal inference.19 The causal relationships between the five selected proteins and AMI were evaluated using two-sample Mendelian randomization (MR). SNPs were chosen as instrumental variables based on genome-wide significance (p < 5 × 10−8) and linkage disequilibrium (LD, r² < 0.01). The inverse variance weighting (IVW) method was used as the primary analysis, with significance set at p < 0.05.20 Sensitivity analyses, including MR Egger and weighted median methods, were conducted to ensure robustness and reduce potential biases. Data sources included genetic instruments from https://www.decode.com/summarydata/ and outcome data from https://www.finngen.fi/en/access_results.
Statistical Analysis
Statistical analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA). Differences between the AMI and Con groups were evaluated using unpaired Student’s t-tests or Chi-square tests, where appropriate. To control for multiple testing in bioinformatics analyses, the Benjamini-Hochberg procedure was applied to adjust p-values, with an adjusted false discovery rate (FDR) threshold set at < 0.05 to determine statistical significance. This adjustment was specifically applied to the identification of differentially expressed proteins (DEPs) and GO/KEGG enrichment analyses, ensuring the reliability of the results. ROC curve analysis was used to assess the diagnostic performance of the models. Sensitivity and specificity were calculated using the maximum Youden index (sensitivity + specificity - 1). Pearson correlation analysis examined the relationship between DEP levels and clinical indicators such as age, WBC, RBC, Cr, BUN, Hb, PLT, ALT, AST, CRP, eGFR, TG, BMI, NT-proBNP, HDL-C, TG, TC, LDL-C, cTnT, and CK-MB. A p-value < 0.05 was deemed statistically significant. Significance levels were indicated as *p < 0.05, **p < 0.01, and ns (non-significant).
Result
Clinical Characteristics of the Participants
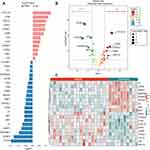
The methodology of the study is illustrated in a comprehensive flowchart (Figure 1A). A total of 275 participants were enrolled, comprising 145 AMI patients and 130 healthy volunteers. The general clinical characteristics of all subjects are presented in Table 1. No significant differences were observed between the two groups regarding gender, age, WBC, RBC, Cr, BUN, Hb, PLT, ALT, AST, CRP, eGFR, TG, diabetes mellitus, and hypertension values (p > 0.05). Conversely, significant differences were noted in LVEF, LVFS, number of vascular lesions, BMI, NT-proBNP, HDL-C, TG, TC, LDL-C, cTnT, and CK-MB between the groups (p < 0.01) (Table 1 and Figure 1B).
 |
Table 1 Clinical Features of the Olink Cohort and the ELISA Cohort |
Identification of Cardiometabolic-Related Biomarkers Using Olink
We evaluated and compared the expression levels of 92 cardiometabolic-related proteins between the AMI and the Control group. Quality control box plot of the control and AMI groups is presented in Figure 1C. The heatmap demonstrated the landscape of protein profiles (Figure 1D). At a threshold of p < 0.05, a total of 32 proteins showed a significant differential expression pattern between the two groups. Among them, PCOLCE, FCN2, REG1A, DEFA1, CRTAC1, LCN2, COMP, PRSS2, CA3, GNLY, EFEMP1, and CA4 were up-regulated, while ENG, ST6GAL1, PROC, TIE1, OSMR, SERPINA5, PAM, C1QTNF1, APOM, CNDP1, MET, TIMP1, TGFBI, MEGF9, F7, NID1, C2, SERPINA7, FETUB, and IGFBP3 were downregulated (Figures 2A and B). The heatmap of these differentially expressed cardiometabolic-related proteins is shown in Figure 2C.
GO, KEGG Enrichment and PPI Network Analysis of DEPs in AMI
The enrichment analysis results indicated that the DEPs were linked to 91 GO terms and 52 KEGG pathways. The top 30 GO term annotations revealed that the intersecting genes were mainly involved in processes such as collagen-containing extracellular matrix, collagen binding, extracellular matrix structural constituent, vascular-associated smooth muscle cell development, and humoral immune response (Figure 3A). KEGG enrichment results highlighted pathways including nitrogen metabolism, PI3K-AKT signaling pathway, histidine metabolism, cytoskeleton in muscle cells, and arginine and proline metabolism and other pathways (Figure 3B).
 |
Figure 3 GO, KEGG, and PPI Analysis. (A) Bar chart of the top 30 GO differential analyses of DEPs; (B) Bar chart of DEPs KEGG analysis; (C) PPI analysis of the connections between DEPs. |
In addition, we conducted a PPI network analysis on the 32 DEPs in AMI using the STRING database. After excluding isolated target genes without interactions, 22 target proteins were incorporated into the PPI network with a confidence score threshold of 900. The gene interactions were visualized using Cytoscape software. In the PPI analysis, collagen binding, humoral immune response, and collagen-containing extracellular matrix were notably activated and strongly interconnected. A PPI network of key DEPs was created to further investigate the connections among these candidate proteins. This network included the top five proteins with the highest fold changes and the previously mentioned candidate biomarkers. PCOLCE, FCN2, DEFA1, COMP, FETUB, IGFBP3, SERPINA7, and C2 were all highly interconnected in the candidate biomarker PPI analysis (Figure 3C).
Relationship Between DEPs and AMI
In the Olink expression matrix, differential expression of up-regulated core proteins in DEPs is shown in Figure 4A, indicating that these 12 proteins are significantly elevated in AMI. We performed a correlation analysis between the up-regulated core proteins in and clinical indicators of AMI to evaluate the relationship between the up-regulated core proteins and AMI (Table 2, Figure 4B). Although the p-values consistently indicated significance, the R-values varied, suggesting different strengths of these correlations. These findings highlighted the significant and meaningful correlations between up-regulated core proteins and various clinical characteristics, with varying strengths of association, underscoring their potential roles in the pathophysiology and clinical assessment of AMI.
 |
Table 2 Correlation Between the DEPs and Clinical Features |
ELISA Validation of Differentially Expressed Proteins in AMI Plasma
Based on the highest fold changes and bioinformatics analysis, we selected PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 for further validation using ELISA assay. We found that the plasma levels of PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 were significantly elevated in AMI patients compared to the control group (P<0.01), consistent with the trends observed in the Olink data (Figure 5A). Validation of core protein expression in external datasets GEO-AMI (GSE66360, GSE48060) and GEO-ICM (GSE5406, GSE57388, GSE57345, DEFA1 was not included in the three datasets) revealed that PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 exhibited differential expression pattern in different datasets. The predictive diagnostic ROC of the core proteins indicated that the AUCs were 0.782 (GSE66360), 0.78 (GSE48060), 0.825 (GSE5406), 0.678 (GSE57388), and 0.693 (GSE57345), demonstrating diagnostic specificity in both AMI and ICM (Figures 5B-F).
Causal Association Between the Identified Five Core Markers and AMI
In order to further explore the causal relationship between the five key proteins and AMI, Mendelian analysis was conducted. The results suggested a causal relationship between FCN2, DEFA1 levels and AMI risk, while PCOLCE, REG1A, and CRTAC1 did not show a significant causal relationship with AMI in the existing data. Next, we conducted further analysis on FCN2 and DEFA1 to determine the accuracy of the results. All SNPs were not weak instruments. Using the IVW method, we found that FCN2 and DEFA1 were associated with AMI risk. DEFA1 had an OR of 1.179 (95% CI = 1.024–1.358, P = 0.022) and FCN2 had an OR of 1.514 (95% CI = 1.095–2.095, P = 0.012) (Supplementary Table 2, Figures 6A-E). The funnel plots of causal effects were approximately symmetrical (Figures 6F and G), and the intercept of MR Egger regression did not indicate horizontal pleiotropy, further confirming the absence of bias in the causal effects. As shown in Figures 6H and I, we systematically performed MR analysis on the remaining SNPs after removing each SNP, and the results remained consistent, indicating that all SNPs were significant in the causal relationships. This also suggested that there were no dominant SNPs in FCN2 and DEFA1 levels and AMI, validating the previous MR results. In summary, FCN2 and DEFA1 levels have a causal relationship with increased AMI risk and can serve as diagnostic predictive biomarkers for AMI.
Discussion
Cardiovascular metabolic damage refers to damage to the cardiovascular system caused by metabolic disorders, including obesity, diabetes, hypertension, and hyperlipidemia. Therefore, exploring the expression of cardiovascular metabolism-related proteins in AMI injury helps us understand this pathological process. In recent years, high-throughput proteomics technologies, particularly the Olink cardiovascular panel, have demonstrated significant value in identifying novel biomarkers for cardiovascular diseases. Several studies have highlighted the application of Olink proteomics in identifying biomarkers with diagnostic and prognostic significance, providing a powerful approach for understanding complex disease mechanisms.13,21,22 In this study, we used Olink cardiovascular metabolomics to analyze coronary blood samples from the control and AMI groups, identifying 32 DEPs and determining that PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 are core proteins with biomarker significance among the 12 up-regulated DEPs. Finally, we validated these findings using ELISA assay, external GEO datasets, and Mendelian randomization studies, finding that the core proteins exhibited good diagnostic performance.
PCOLCE encodes a glycoprotein that enhances the activity of procollagen C-proteinase, an enzyme that cleaves procollagen peptides during collagen fibril assembly.23 PCOLCE knockout mice exhibit collagen fibril morphological disturbances and reduced diet-induced liver fibrosis but do not affect liver injury. PCOLCE may serve as a specific marker for liver fibrosis and chronic kidney disease.24,25 Obesity can increase PCOLCE expression, leading to cardiac fibrosis by promoting collagen synthesis. Therefore, drug treatment which reduces PCOLCE expression can alleviate myocardial fibrosis.26 In ischemic diseases such as myocardial infarction and stroke, PCOLCE plays an important role in extracellular matrix (ECM) remodeling. Ischemic injury leads to changes in ECM components, including collagen fiber proliferation and rearrangement. PCOLCE can contribute to tissue repair and remodeling by accelerating collagen fiber maturation. However, excessive ECM remodeling can exacerbate fibrosis, thereby impairing tissue function.27,28 In the context of AMI, ECM remodeling driven by PCOLCE may lead to pathological myocardial fibrosis, impacting ventricular compliance and contributing to heart failure. This highlights PCOLCE as a potential target for therapeutic intervention aimed at reducing myocardial fibrosis and improving cardiac recovery following ischemic injury.
The FCN2 gene encodes protein of L-ficolin, which plays a key role in the innate immune system. Studies have shown that L-ficolin levels are significantly elevated in patients with ischemic stroke, suggesting its crucial role in the inflammatory responses following ischemic injury.29 L-ficolin promotes inflammation by activating the complement system, helping to clear damaged cells and tissue debris, but it can also lead to excessive inflammatory responses and tissue damage.30 FCN2 is one of the risk factors for cardiac metabolic disturbances in AMI.31 Additionally, polymorphisms in the FCN2 gene are linked to susceptibility to ischemic diseases. For instance, studies in the Chinese Han population have shown that certain single nucleotide polymorphisms (SNPs) in the FCN2 gene are associated with the risk of type 2 diabetes and ischemic stroke.32 In AMI pathophysiology, the overexpression of FCN2 is indicative of its dual role in inflammatory regulation and tissue repair. While activation of the complement cascade via FCN2 facilitates clearance of necrotic myocardial debris, excessive activation may exacerbate inflammatory injury, worsening myocardial ischemia. Additionally, its genetic variants may provide insights into patient-specific inflammatory responses, offering a potential pathway for precision medicine approaches in managing AMI.
The detailed biological functions of PCOLCE and FCN2 have been elucidated, and their roles in the pathophysiology of AMI further highlight the clinical significance of these proteins. For example, therapeutic inhibition of PCOLCE-mediated excessive ECM remodeling could provide a novel approach to mitigating myocardial fibrosis and enhancing long-term prognosis in AMI patients. Similarly, precision therapies targeting FCN2 could modulate complement cascade activation, reduce inflammatory damage, and offer new intervention avenues for AMI management.
The REG1A gene encodes a regenerating protein that plays a crucial role in tissue regeneration and repair in the pancreas and gastrointestinal tract. The protein encoded by this gene consists of 166 amino acids and primarily functions to promote cell proliferation, anti-apoptosis, and tissue repair.33 In ischemic bowel disease, the expression level of REG1A in damaged intestinal tissues are significantly elevated. REG1A can also accelerate the repair of damaged tissues by promoting cell proliferation and inhibiting apoptosis, suggesting its important role in tissue repair process.34,35 Additionally, the role of REG1A in the cardiovascular system has also garnered attention. After myocardial infarction, the regeneration and repair of cardiomyocytes are crucial for restoring heart function. REG1A may play a role in myocardial repair after myocardial infarction through similar mechanisms. Although specific studies on REG1A in the cardiovascular system are currently limited, findings of its role in other ischemic tissues provide clues to its potential function in the cardiovascular system.
The DEFA1 gene encodes an antimicrobial peptide called human alpha-defensin 1 (HNP-1), which belongs to the defensin family. In ischemic diseases, the role of the DEFA1 gene and its encoded HNP-1 protein in inflammatory responses and tissue damage has attracted widespread attention.36 The expression levels of HNP-1 are significantly elevated in patients with coronary heart disease (CHD), suggesting its important role in atherosclerosis and vascular endothelial dysfunction.37 HNP-1 accelerates the process of atherosclerosis by activating neutrophils and monocytes, promoting the release of inflammatory factors, and exacerbating vascular endothelial damage.38 Furthermore, the expressions of DEFA1 and DEFA3 genes are also significantly up-regulated in patients with hyperlipidemia and coronary heart disease. Polymorphisms in the DEFA1 gene are closely associated with susceptibility to hyperlipidemia and coronary heart disease. These variations may influence the progression of atherosclerosis and the occurrence of coronary heart disease by regulating the expression levels of HNP-1.38
The CRTAC1 gene encodes a carboxylic acid-rich protein. The encoded protein mainly participates in cell-cell and cell-matrix interactions, as well as calcium ion binding. These functions suggest that CRTAC1 potentially participates in cell signaling and tissue repair. The CRTAC1 gene and its encoded protein play important roles in the formation and stability of atherosclerotic plaques. Studies have shown that CRTAC1 expression level is significantly elevated in coronary atherosclerotic plaques. It can influence the stability and vulnerability of atherosclerotic plaques by regulating extracellular matrix remodeling and cell-cell interactions.39,40 Though existing studies have provided evidence, the specific mechanisms of CRTAC1 in the cardiovascular system are not yet fully understood. The expression levels of CRTAC1 may be related to the severity and prognosis of heart failure.41
Among the DEPs, up-regulated core proteins PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 were re-validated by ELISA, showing significantly elevated expression levels in AMI patients compared to the healthy people, consistent with previous studies. Additionally, up-regulated core proteins demonstrated good diagnostic accuracy for both acute and chronic ischemia, and Mendelian analysis revealed a causal relationship between FCN2, DEFA1 SNPs and AMI risk. Correlation analysis showed strong correlations with clinical indicators such as BMI, LVEF, NT-pro BNP, CK-MB, and cTnT. Through the enrichment analysis of DEPs, we identified several important biological processes and pathways. The PI3K−AKT signaling pathway is crucial for maintaining the integrity and function of heart tissue, and disruption of this pathway leads to impaired heart function and increased myocardial fibrosis.42 Studies have shown that the extracellular matrix plays an important role in myocardial fibrosis in AMI.43 Vascular smooth muscle cells (VSMCs) switch from a contractile phenotype to a synthetic phenotype, promoting vascular remodeling and the development of atherosclerosis. Mitochondrial dynamics, involving processes such as mitophagy and the fusion-fission balance, are crucial for regulating the proliferation and survival of VSMCs under ischemic conditions, thereby affecting vascular injury and repair mechanisms during AMI.44,45 Similarly, our study also indicates that these biological processes and pathways play important roles in AMI. Therefore, PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 have potential diagnostic and prognostic value in AMI, offering new insights for the diagnosis and treatment of ischemic metabolic damage in AMI.
We acknowledge that the relatively small sample size in the initial Olink panel group may limit the generalizability of our findings. This limitation arises primarily from the high cost of high-throughput proteomics and the exploratory nature of this study. To address this, we employed rigorous statistical methods, including FDR correction, to control for biases and ensure the reliability of our results. Additionally, we conducted large-scale ELISA validation, supplemented by external GEO datasets and Mendelian randomization analyses, which collectively enhanced the robustness of our findings. Despite these efforts, future studies with larger, multicenter cohorts are warranted to validate and extend these findings, particularly in diverse populations and clinical settings. Moreover, incorporating longitudinal analyses may provide insights into the temporal dynamics of these biomarkers during AMI progression. Meanwhile, developing specific small-molecule inhibitors or antibody drugs to target these proteins could enable personalized therapeutic strategies. Notably, integrating multi-omics analysis of these biomarkers could aid in accurately predicting both short-term and long-term risks in AMI patients, advancing the translation from basic research to clinical practice.
Conclusion
In this study, we used Olink technology to detect 92 cardiovascular metabolism proteins in normal people and AMI patients, identifying 32 DEPs between the two groups. Additionally, PCOLCE, FCN2, REG1A, DEFA1, and CRTAC1 as core proteins were validated by ELISA assay and further validated in external AMI and ICM datasets from the GEO database, constructing diagnostic models that showed good predictive performance. These proteins are mainly involved in collagen−containing extracellular matrix, vascular-associated smooth muscle cell development, and PI3K−AKT-related biological processes and signaling pathways. Our findings highlight the potential of these proteins as biomarkers for the diagnosis and risk stratification of cardiovascular metabolic damage in AMI. Moreover, their involvement in key pathophysiological mechanisms suggests their utility as therapeutic targets for mitigating ischemic metabolic damage and improving patient outcomes. Future studies, including longitudinal analyses, multicenter cohorts, and clinical trials, are warranted to validate these results and explore their practical applications in clinical settings.
Abbreviations
AMI, acute myocardial infarction; ICM, ischemic cardiomyopathy; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, GeneOntology; GEO, Gene Expression Omnibus; ELISA, enzyme-linked immunosorbent assay; ROC, receiver operating characteristic; DEPs, Differentially expressed proteins; PPI, protein-protein interaction; AUC, area under the curve; WBC, white blood cell; Hb, hemoglobin; PLT, Platelet; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive proteine; eGFR, estimated Glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVEF, left ventricular ejection fractions; LVFS, Left ventricular fractional shortening; PCOLCE, Procollagen C-endopeptidase enhancer 1; FCN2, Ficolin-2; REG1A, Lithostathine-1-alpha; DEFA1, Neutrophil defensin 1; CRTAC1, Cartilage acidic protein 1; BP, Biological processes; MF, molecular functions; CC, cellular components; SNPs, single nucleotide polymorphisms.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Informed written consent was obtained from all participants, which was conducted in accordance with the internationally recognized principles of the Declaration of Helsinki and approved by the Ethics Committee of Taihe County People’s Hospital affiliated with Wannan Medical College (Approval No. 2023-09-2022-34) and the Fourth Affiliated Hospital of Soochow University (Approval No. 2022-KT-105, ChiCTR2100051469).
Acknowledgments
The authors would like to thank the participants and investigators of the FinnGen study, as well as the Olink Target platform for providing valuable data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from National Natural Science Foundation of China (81873486, 82403624), the Science and Technology Development Program of Jiangsu Province-Clinical Frontier Technology (BE2022754), Clinical Medicine Expert Team (Class A) of Jinji Lake Health Talents Program of Suzhou Industrial Park (SZYQTD202102), Suzhou Key Discipline for Medicine (SZXK202129), Demonstration of Scientific and Technological Innovation Project (SKY2021002), Suzhou Dedicated Project on Diagnosis and Treatment Technology of Major Diseases (LCZX202132), Research on Collaborative Innovation of medical engineering combination (SZM2021014), Research on Collaborative Innovation of medical engineering combination (SZM2022003), Suzhou Key Laboratory of Diagnosis and Treatment of Panvascular Diseases (SZS2023021), Natural Science Foundation of Jiangsu Province (BK20240637), Suzhou Science and Education Youth Science and Technology Project (KJXW2023086), Anhui clinical medical research transformation project (202304295107020117), Anhui provincial health research project (AHWJ2022c008), Research project of Fuyang Health Commission in 2021(fy2021-110), Anhui Province University Research Project (2024AH051883), Fuyang key research and development program project clinical medical research transformation project (FK20245525).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. B RA, Xavier R, C JJ, et al. ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38): 55–161. doi:10.1093/eurheartj/ehad191
2. Mehmet G, Hakan P. A review of myocardial ischaemia/reperfusion injury: pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem Funct. 2020;39: 190–217.
3. Zhen S, Lili Z, Lihua L, et al. Galectin-3 mediates cardiac remodeling caused by impaired glucose and lipid metabolism through inhibiting two pathways of activating Akt. Am J Physiol Heart Circ Physiol. 2020;320: H364–80.
4. Harikrishnan V, Anis H, Claudio H, Nikolaos GF, Lorusso L, Ribatti D. Properties and functions of fibroblasts and myofibroblasts in myocardial infarction. Cells. 2022;12(1):11. doi:10.3390/cells12010011
5. Yaping W, Liangliang J, Jian S, et al. Cathepsin B aggravates coxsackievirus B3-induced myocarditis through activating the inflammasome and promoting pyroptosis. PLoS Pathog. 2018;14: e1006872.
6. Yang Y, Li J, Rao T, Fang Z, Zhang J. The role and mechanism of hyperoside against myocardial infarction in mice by regulating autophagy via NLRP1 inflammation pathway. J Ethnopharmacol. 2021;276:114187. doi:10.1016/j.jep.2021.114187
7. American Heart Association Atherosclerosis H, Obesity in the Young Committee of the Council on Lifelong Congenital Heart D, Mietus-Snyder M, Perak AM, Cheng S, et al. Next generation, modifiable cardiometabolic biomarkers: mitochondrial adaptation and metabolic resilience: a scientific statement from the American Heart Association. Circulation. 2023;148(22):1827–1845. doi:10.1161/CIR.0000000000001185.
8. Yunhao L, Zhanming Z, Zheming Z, Ningning Z, Xudong D. Empagliflozin, a sodium-glucose cotransporter inhibitor enhancing mitochondrial action and cardioprotection in metabolic syndrome. J Cell Physiol. 2024;239(6).
9. Stefano T, Antonio A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat Rev Cardiol. 2023;21(4): 219–237.
10. Johanne S, Mathieu K, Michel Z, et al. Interleukin-1β and risk of premature death in patients with myocardial infarction. J Am Coll Cardiol. 2020;76(15): 1763–1773.
11. Weir R, Tsorlalis I, Steedman T, et al. Aldosterone and cortisol predict medium-term left ventricular remodelling following myocardial infarction. Eur J Heart Failure. 2011;13(12):1305–1313. doi:10.1093/eurjhf/hfr129
12. Tian Z, Jingjing Z, Ruijie Z, et al. Serum olink proteomics-based identification of protein biomarkers associated with the immune response in ischemic stroke. J Proteome Res. 2024;23: 1118–1128.
13. Mohsen M, Neil W, Pang Y, et al. Risk prediction of ischemic heart disease using plasma proteomics, conventional risk factors and polygenic scores in Chinese and European adults. Eur J Epidemiol. 2024;39(11): 1229–40.
14. Usman AT, Paul K, Raymond YK, et al. Protein Biomarkers of Adverse Clinical Features and Events in Sarcomeric Hypertrophic Cardiomyopathy. Circ Heart Fail. 2024;17(12): e011707.
15. Chanchal C, Tay WT, Jasper T, et al. Sex differences in proteomic correlates of coronary microvascular dysfunction among patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2022;24(4): 739–751.
16. Daan C, Adriaan AV, Jasper T, et al. Pathophysiological pathways related to high plasma growth differentiation factor 15 concentrations in patients with heart failure. Eur J Heart Fail. 2022;24(2): 308–320.
17. McDonagh TA, Metra M, Adamo M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
18. De Bodt S, Hollunder J, Nelissen H, Meulemeester N, Inzé D. CORNET 2.0: integrating plant coexpression, protein-protein interactions, regulatory interactions, gene associations and functional annotations. New Phytol. 2012;195(3):707–720. doi:10.1111/j.1469-8137.2012.04184.x
19. Mitja IK, Juha K, Priit P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:7944.
20. Lin L, Haixin F, Fuzhen L, et al. Regulation mechanisms of disulfidptosis-related genes in ankylosing spondylitis and inflammatory bowel disease. Front Immunol. 2024;15: 1326354.
21. Ong KL, Chung RWS, Hui N, et al. Usefulness of certain protein biomarkers for prediction of coronary heart disease. Am J Cardiol. 2020;125(4):542–548. doi:10.1016/j.amjcard.2019.11.016
22. Smith JG, Gerszten RE. Emerging affinity-based proteomic technologies for large-scale plasma profiling in cardiovascular disease. Circulation. 2017;135(17):1651–1664. doi:10.1161/CIRCULATIONAHA.116.025446
23. Wong VW, You F, Januszyk M, Gurtner GC, Kuang AA. Transcriptional profiling of rapamycin-treated fibroblasts from hypertrophic and keloid scars. Ann Plast Surg. 2014;72(6):711–719. doi:10.1097/SAP.0b013e31826956f6
24. Liepinsh E, Banyai L, Pintacuda G, Trexler M, Patthy L, Otting G. NMR structure of the netrin-like domain (NTR) of human type I procollagen C-proteinase enhancer defines structural consensus of NTR domains and assesses potential proteinase inhibitory activity and ligand binding. J Biol Chem. 2003;278(28):25982–25989. doi:10.1074/jbc.M302734200
25. Gokce O, Ozenirler S, Atak Yucel A, Oruklu N, Yilmaz Esendagli G, Karahan S. Evaluation of serum procollagen C-proteinase enhancer 1 level as a fibrosis marker in patients with chronic hepatitis B. Eur J Gastroenterol Hepatol. 2018;30(8):918–924. doi:10.1097/MEG.0000000000001123
26. Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol. 2012;303(2):H234–240. doi:10.1152/ajpheart.00227.2012
27. Raz V, Sterrenburg E, Routledge S, et al. Nuclear entrapment and extracellular depletion of PCOLCE is associated with muscle degeneration in oculopharyngeal muscular dystrophy. BMC Neurol. 2013;13(1):70. doi:10.1186/1471-2377-13-70
28. Zhang G, Wang X, Rothermel BA, Lavandero S, Wang ZV. The integrated stress response in ischemic diseases. Cell Death Differ. 2022;29(4):750–757. doi:10.1038/s41418-021-00889-7
29. Tsakanova G, Stepanyan A, Steffensen R, Soghoyan A, Jensenius JC, Arakelyan A. Pattern recognition molecules of lectin complement pathway in ischemic stroke. Pharmgenomics Pers Med. 2021;14:1347–1368. doi:10.2147/PGPM.S326242
30. Osthoff M, Ngian GS, Dean MM, et al. Potential role of the lectin pathway of complement in the pathogenesis and disease manifestations of systemic sclerosis: a case-control and cohort study. Arthritis Res Ther. 2014;16(6):480. doi:10.1186/s13075-014-0480-6
31. Koplev S, Seldin M, Sukhavasi K, et al. A mechanistic framework for cardiometabolic and coronary artery diseases. Nat Cardiovasc Res. 2022;1(1):85–100. doi:10.1038/s44161-021-00009-1
32. Szala-Poździej A, Świerzko AS, Gajek G, et al. Association of the FCN2 gene promoter region polymorphisms with very low birthweight in preterm neonates. Int J mol Sci. 2022;23(23):15336. doi:10.3390/ijms232315336
33. He GW, Lin L, DeMartino J, et al. Optimized human intestinal organoid model reveals interleukin-22-dependency of Paneth cell formation. Cell Stem Cell. 2022;29(9):1333–1345.e1336. doi:10.1016/j.stem.2022.08.002
34. Lu J, Wang Z, Maimaiti M, Hui W, Abudourexiti A, Gao F. Identification of diagnostic signatures in ulcerative colitis patients via bioinformatic analysis integrated with machine learning. Hum Cell. 2022;35(1):179–188. doi:10.1007/s13577-021-00641-w
35. Van beelen granlund A, Østvik AE, Brenna Ø, Torp SH, Gustafsson BI, Sandvik AK. REG gene expression in inflamed and healthy colon mucosa explored by in situ hybridisation. Cell Tissue Res. 2013;352(3):639–646. doi:10.1007/s00441-013-1592-z
36. Mansanguan C, Maneerat Y. PPBP gene as a biomarker for coronary heart disease risk in postmenopausal Thai women. PeerJ. 2022;10:e13615.
37. Maneerat Y, Prasongsukarn K, Benjathummarak S, Dechkhajorn W, Chaisri U. Increased alpha-defensin expression is associated with risk of coronary heart disease: a feasible predictive inflammatory biomarker of coronary heart disease in hyperlipidemia patients. Lipids Health Dis. 2016;15(1):117. doi:10.1186/s12944-016-0285-5
38. Maneerat Y, Prasongsukarn K, Benjathummarak S, Dechkhajorn W. PPBP and DEFA1/DEFA3 genes in hyperlipidaemia as feasible synergistic inflammatory biomarkers for coronary heart disease. Lipids Health Dis. 2017;16(1):80. doi:10.1186/s12944-017-0471-0
39. Mosquera JV, Auguste G, Wong D, et al. Integrative single-cell meta-analysis reveals disease-relevant vascular cell states and markers in human atherosclerosis. Cell Rep. 2023;42(11):113380. doi:10.1016/j.celrep.2023.113380
40. Alsaigh T, Evans D, Frankel D, Torkamani A. Decoding the transcriptome of calcified atherosclerotic plaque at single-cell resolution. Commun Biol. 2022;5(1):1084. doi:10.1038/s42003-022-04056-7
41. Klimentova J, Rehulka P, Stulik J, et al. Proteomic profiling of dilated cardiomyopathy plasma samples ─ searching for biomarkers with potential to predict the outcome of therapy. J Proteome Res. 2024;23(3):971–984. doi:10.1021/acs.jproteome.3c00691
42. Luca L, Yustina MP, Stefano M, et al. JCAD promotes arterial thrombosis through PI3K/Akt modulation: a translational study. Eur Heart J. 2022;44(20): 1818–1833.
43. Xing F, Hadi K, Onur K, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128(5):125–136.
44. Huynh DTN, Heo KS. Role of mitochondrial dynamics and mitophagy of vascular smooth muscle cell proliferation and migration in progression of atherosclerosis. Arch Pharm Res. 2021;44(12):1051–1061. doi:10.1007/s12272-021-01360-4
45. Cao G, Xuan X, Hu J, Zhang R, Jin H, Dong H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun Signal. 2022;20(1):180. doi:10.1186/s12964-022-00993-2
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Prognostic Value of Human Epididymis Protein 4 in Acute Myocardial Infarction
Tang Y, Zhu WY, Peng SL, Huang S, Zhao QN, Tan SY, Yin ZH, Zhang Y, Peng JQ, Pan HW
International Journal of General Medicine 2024, 17:6243-6251
Published Date: 14 December 2024
Using Olink Proteomics to Identify Inflammatory Biomarkers in the Cerebrospinal Fluid in Guillain-Barré Syndrome
Sun S, Li M, Song J, Zhong D
Journal of Inflammation Research 2025, 18:6703-6717
Published Date: 25 May 2025