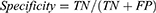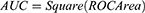Back to Journals » Journal of Pain Research » Volume 17
Identification of Specific Abnormal Brain Functional Activity and Connectivity in Cancer Pain Patients: A Preliminary Resting-State fMRI Study
Authors Hua Y, Geng Y, Liu S, Xia S, Liu Y, Cheng S, Chen C, Pang C, Zhao Z, Peng B, Dai Y, Ji J , Wu D
Received 26 March 2024
Accepted for publication 18 November 2024
Published 22 November 2024 Volume 2024:17 Pages 3959—3971
DOI https://doi.org/10.2147/JPR.S470750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amitabh Gulati
Yingjie Hua,1,* Yongkang Geng,2,* Surui Liu,3 Shuiwei Xia,4 Yan Liu,3 Sufang Cheng,4 Chunmiao Chen,4 Chunying Pang,2 Zhongwei Zhao,1 Bo Peng,3 Yakang Dai,3 Jiansong Ji,4 Dan Wu1
1Department of Pain Medicine, Zhejiang Key Laboratory of Imaging and Interventional Medicine. The Fifth Affiliated Hospital of Wenzhou Medical University, Lishui, Zhejiang Province, People’s Republic of China; 2School of Life Science and Technology, Changchun University of Science and Technology, Changchun, Jilin Province, People’s Republic of China; 3Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou, Jiangsu Province, People’s Republic of China; 4Department of Radiology, Zhejiang Key Laboratory of Imaging and Interventional Medicine. The Fifth Affiliated Hospital of Wenzhou Medical University, Lishui, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiansong Ji; Dan Wu, Email [email protected]; [email protected]
Objective: This study investigates the differences in brain functional activity and connectivity patterns between Cancer Pain (CP) patients and Healthy Controls (HCs) using resting-state functional magnetic resonance imaging (rs-fMRI) to identify potential neuroimaging biomarkers.
Methods: This study collected rs-fMRI data from 25 CP patients and 25 hCs, processed the functional MRI images, and calculated metrics such as amplitude of low-frequency fluctuation (ALFF), Regional Homogeneity (ReHo), and FC. Through statistical analysis, differences in brain functional activity and connectivity between the cancer pain group and the healthy control group were investigated, followed by machine learning classification.
Results: The results showed that compared to the normal group, reductions in the ALFF were primarily observed in the bilateral inferior temporal gyrus; ReHo increased in the right middle temporal gyrus and decreased in the left cerebellum Crus2. Using the statistically different brain areas as seed points to construct FC networks and performing statistical analysis, it was found that the regions with decreased FC connection strength between the cancer pain group and the normal group were mainly in the prefrontal cortex (PFC), the postcentral gyrus of the parietal lobe, and the cerebellum. Statistical results indicated that there was no significant correlation between pain scores (Numeric Rating Scale, NRS) and neuroimaging metrics. According to the machine learning classification, the FC features of the right precentral gyrus achieved higher diagnostic efficacy (AUC = 0.804) compared to ALFF and ReHo in distinguishing between CP patients and HCs.
Conclusion: Brain activity and FC in CP patients show abnormalities in regions such as the inferior temporal gyrus, middle temporal gyrus, prefrontal cortex, parietal lobe, and cerebellum. These areas may be interconnected through neural networks and jointly participate in functions related to pain perception, emotion regulation, cognitive processing, and motor control. However, the precise connections and mechanisms of action require further research.
Keywords: cancer pain, resting-state fMRI, low-frequency fluctuations, regional homogeneity, functional connectivity
Introduction
Cancer pain refers to pain caused by cancer itself or cancer-related factors.1 In recent years, the global incidence of cancer has been steadily increasing.2 Studies indicate that at least 25% of newly diagnosed cancer patients and around 35% of those undergoing treatment experience cancer pain, with the prevalence among late-stage cancer patients reaching up to 75%.3 Managing cancer pain often requires high doses of opioids, which inevitably leads to a series of adverse reactions such as drowsiness, constipation, nausea, and vomiting. These side effects not only disrupt daily life but also contribute to sleep disturbances, psychological issues (such as anxiety, depression, and suicidal thoughts), cognitive impairments, and strained social relationships, all of which significantly reduce patients’ quality of life.4 Previously, cancer pain was viewed as a combination of nociceptive and neuropathic pain, but increasing evidence suggests that cancer pain has unique characteristics and should be recognized as a distinct pain state.1
The generation of pain is a complex process, with the ascending pain pathway being particularly important. When nociceptors are stimulated by pain, they generate action potentials that are transmitted to the second-order nociceptive neurons in the spinal cord. These neurons transmit signals across the contralateral spinothalamic tract anteriorly in the spinal cord and upwards to the thalamus. From there, the third-order nociceptive neurons transmit the signals to the corresponding brain regions, generating the perception of pain. However, no single brain region has been identified as solely responsible for pain processing; rather, it is a complex network of brain regions.5 The specific mechanisms related to cancer pain remain unclear. Current research has mainly focused on peripheral neurons and spinal interneurons,6–9 with the specific brain regions involved in cancer pain information processing still not well defined. Therefore, further understanding the central mechanisms of cancer pain processing and identifying cancer pain-related brain functional areas are of significant clinical importance for improving cancer pain treatment and pain management.
Neuroimaging technologies have empowered the study on the brain’s role in the maintenance and development of pain non-invasively. Techniques such as magnetic resonance imaging (MRI), Positron Emission Computed Tomography (PET), Electroencephalogram (EEG), and Magnetoencephalography (MEG) have been used to investigate abnormal brain functions and structures in pain patients.10 Among these, rs-fMRI measures the brain’s local Blood Oxygenation Level Dependent (BOLD) signals when subjects are at rest, reflecting neural activity.11–13 The application of rs-fMRI in the field of pain has primarily focused on neuropathic pain conditions, such as postherpetic neuralgia (PHN),14 trigeminal neuralgia,15 and chronic musculoskeletal pain,16–18 which can be used to identify brain regions involved in pain processing. For example, the activity in the orbitofrontal cortex, PFC, insula, and cerebellum may be related to pain intensity.5 Activation in the PFC is related to the intensity of chronic musculoskeletal pain,16 increased activity in the amygdala (AMY) enhances fear factors,19 and the medial nucleus of the thalamus, anterior insula, anterior cingulate cortex (ACC), and PFC are related to the emotional state of pain.5
However, the application of rs-fMRI in the field of cancer pain has been limited. Recent studies by Liu et al20 explored ALFF, ReHo, and FC in patients with bone metastasis from lung cancer, finding decreased ALFF in the PFC and ACC, along with increased ReHo in the bilateral thalamus. Additionally, the combination of ALFF and ReHo demonstrated satisfactory classification performance between patients and HCs. Zhou et al investigated FC in patients with bone metastasis from lung cancer, revealing increased FC between the left ACC and AMY, and a positive correlation between FC in the right PFC and ACC with the duration of cancer pain. These findings suggest abnormal activity in the pain-processing pathways of cancer pain (CP) patients. However, current rs-fMRI research on cancer pain has primarily focused on patients with bone metastasis from lung cancer, which represents only a subset of cancer pain and cannot fully represent all CP patients. Whether other, more important brain regions are associated with cancer pain remains to be further explored.
Therefore, this study hypothesizes that CP patients exhibit abnormal FC in specific neural networks of the brain. This research aims to explore brain regions associated with cancer pain using rs-fMRI, evaluates the correlation between neuroimaging metrics and clinical characteristics, and discusses the potential roles of these regions in the perception and regulation of cancer pain. The findings may help identify brain regions related to cancer pain, offering new therapeutic approaches for the treatment of persistent and chronic cancer pain, such as Transcranial Magnetic Stimulation (TMS) and transcranial direct current stimulation (tDCS).
Materials and Methods
Participants
This study was approved by the Research Ethics Committee of Lishui Central Hospital (the Fifth Hospital Affiliated with Wenzhou Medical University) and adheres to the Declaration of Helsinki. All participants signed an informed consent form before undergoing the MRI examination. From November 4, 2022, to June 1, 2023, 25 CP patients were consecutively recruited from hospitalized patients, and 25 hCs matched in age and gender were recruited from the community. Among the initial 25 CP patients recruited, 4 were found to have brain metastases and were excluded. Consequently, 21 CP patients and 25 healthy volunteers were ultimately included in this study. Inclusion criteria for the CP group: ①Age ≥ 18 years; ②CP patients with confirmed pathological results or clinical diagnosis; ③Numeric Rating Scale (NRS)≥1; ④Patients able to tolerate MRI examination; ⑤Right-handed patients.
Exclusion criteria for the CP group: ①Abnormal brain structure or intracranial metastasis; ②Contraindications to MRI examination; ③Pain caused by diseases other than cancer; ④Intellectual disability or severe neurological or psychiatric disorders.
Inclusion and exclusion criteria for the HCs group: ①Gender, age, and educational level matched with the cancer pain group, right-handed; ②No intellectual disabilities or other serious neurological, psychiatric symptoms, and lesions; ③No contraindications to MRI examination.
Clinical Assessments
On the day of the MRI scan, under the guidance of an experienced physician, each participant completed demographic information, clinical pain, and depression assessments. The Numeric Rating Scale (NRS) was used to assess pain intensity, where 0 indicates “no pain” and 10 represents “the worst imaginable pain.” Disease duration, duration of cancer pain, and use of opioid medications were recorded based on clinical records for the CP group. The depression status was assessed using the Patient Health Questionnaire-9 (PHQ-9). Demographic information for all participants was obtained through self-reported questionnaires.
MRI Image Acquisition
Imaging was performed on a 3.0T MRI scanner (Philips, Ingenia, the Netherlands) using a 64-channel head-neck coil. During the scanning, all patients were instructed to remain still, close their eyes, and avoid any specific thoughts, while using earplugs to reduce noise and pads to minimize head movement. T1-weighted structural MRIs were acquired using a three-dimensional magnetization-prepared rapid gradient echo imaging sequence with the following parameters: repetition time (TR) = 7.599 ms, echo time (TE) = 3.501 ms, flip angle (FA) = 8°, field of view (FOV) = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm, slice gap = 0 mm, and 192 sagittal slices. Resting-state fMRIs were obtained using an echo planar imaging (EPI) sequence with the following parameters: TR = 2000 ms, TE = 30 ms, FA = 90°, FOV = 220 × 220 mm, matrix = 64 × 54, slice thickness = 4 mm, slice gap = 0.6 mm, and 240 volumes acquired over 8 minutes and 8 seconds. All MRI images were reviewed by two radiologists with at least five years of experience.
Image Preprocessing
Preprocessing of resting-state fMRIs were conducted using Data Processing Assistant for Resting-State fMRI (DPARSF; http://rfmri.org/dparsf) software based on statistical parametric mapping (SPM12; http://www.fil.ion.ucl.ac.uk/spm) on the MatLab platform (MathWorks, Natick, MA, USA),21 including the following steps: (1) converting the data from DICOM to NIfTI format, (2) removing the first 10 time points of the resting-state functional images, (3) slice timing to adjust time offsets between slices, (4) head motion correction to ensure the brain is in the same position in every image, (5) spatial normalization to realign the functional images into the Montreal Neurological Institute (MNI) space using co-registered and segmented T1 images.
ALFF and ReHo Analysis
The low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) were analyzed from the normalized images. For ALFF analysis, DPARSF was used to further remove linear trends and reduce low- and high-frequency physiological noise using a band-pass filter (0.01–0.1 hz). The filtered time series of each voxel were transformed into the frequency domain using a Fast Fourier Transform, and the power spectrum was calculated. For each subject, ALFF was measured by calculating the square root of the signal across the 0.01–0.1 hz band and then dividing it by the global mean ALFF value. For ReHo analysis, Kendall’s coefficient of concordance (KCC) was calculated to measure the similarity of the time series of a seed voxel and its 26 neighboring voxels.22 Similar to the ALFF standardization procedure, the ReHo of each voxel was divided by the global mean ReHo value within the whole-brain mask. Smoothing was performed before ALFF calculation and after ReHo calculation using a Gaussian kernel with a full-width at half-maximum (FWHM) value of 6 mm.
Seed-Based FC Analysis
The voxels exceeding the established statistical threshold in the ALFF analysis and ReHo analysis were selected as the seeds. We first extracted the averaged time series of the seeds and calculated the Pearson correlation coefficient values between the seeds and the remaining whole-brain voxels. Then, we normalized the distribution correlation coefficient using the Fisher r-to-z transformation.
Machine Learning Model
The statistically significant ALFF, ReHo, and FC features were used to train the classification model. We utilized the open-source AutoGluon platform to classify cancer pain patients and healthy controls. The AutoGluon model employs a novel form of multilayer stack ensembling. In the first layer, AutoGluon uses *n* types of base learners, including extremely randomized trees, k-nearest neighbors, gradient boosting machines (eg, XGBoost, CatBoost), random forests, and neural networks. The outputs of these base learners are then passed into the next layer, which computes a weighted sum of these models, where the weights are determined through training. AutoGluon uses random search for hyperparameter tuning and model selection. The flowchart of the image processing and classification based on resting-state fMRIs is shown in Figure 1.
 |
Figure 1 The flowchart of the image processing and classification based on resting-state fMRIs. |
Statistical Analysis
This study analyzed demographic and neuropsychological data using SPSS statistical software (version 25.0; IBM Corp., Armonk, NY, USA). Quantitative data were presented as mean ± standard deviation (x ± s). Group comparisons were performed using two-sample t-tests, while gender differences were assessed using chi-square tests. A p-value of <0.05 was considered statistically significant.
A Jarque–Bera goodness-of-fit test was performed to ensure the normal distribution of ALFF, ReHo, and FC data before conducting t-tests. The DPABI software was then used to analyze ALFF, ReHo, and FC following these steps: (1) performing one-sample t-tests on the functional activity and connectivity patterns for each group; (2) adjusting for covariates and analyzing t-statistics to assess group effects; (3) correcting for multiple comparisons using Gaussian random-field theory (GRF) with a voxel-level threshold of p < 0.001 and a cluster-level threshold of p < 0.05; (4) extracting ALFF, ReHo, and FC features from significantly altered brain regions; and (5) conducting Spearman correlation analyses between the extracted features and pain scores in the CP group, also corrected using GRF (voxel-level p < 0.001, cluster-level p < 0.05). These extracted features were then used in classification experiments to evaluate their performance in distinguishing cancer pain patients from healthy controls.
All subjects were divided into a training dataset and a testing dataset in a 9:1 ratio. To prevent potential overestimation in the training dataset, leave-one-out cross-validation was employed. The splitting of the training and testing datasets was repeated three times, and the average values from the three testing datasets were calculated as the final classification results. Accuracy, sensitivity, specificity, and the area under the receiver operating characteristic curve (AUC) were used as evaluation metrics to assess the classification performance in distinguishing cancer pain patients from healthy controls.
Result
Demographic and Clinical Information
CP and HC patients showed no significant differences in age, gender, or education level (p>0.05). The CP group had higher PHQ-9 depression scores than the HC group, with a significant difference (p=0.00), where three individuals showed no depression, and the remaining 18 exhibited mild to severe depression. The average duration of illness for CP patients was 679 days, with the average duration of cancer pain being 271 days. The Oral Morphine Equivalent average was 121mg, 61.9% of patients experienced Breakthrough cancer pain, and 95.24% of CP had mild-to-moderate pain, with NRS scores ranging from 2 to 8. See Table 1 for details.
 |
Table 1 Sociodemographic and Clinical Characteristics |
Functional Activity and Connectivity Analysis
Based on the one-sample t-test, the ALFF and ReHo in both CP and HC groups were mainly higher in the temporal lobes and lower in the parietal and occipital lobes. According to the two-sample t-test, the CP patients group showed a decrease in ALFF mainly in the bilateral inferior temporal gyrus, an increase in ReHo in the right middle temporal gyrus, and a decrease in Left Cerebellum Crus2.
According to the two-sample t-test, brain regions with abnormal activity were used as seed regions for calculating FC. In the CP group, FC was higher in the parietal and occipital lobes than in the temporal lobe; compared to HCs, the CP group had lower FC in the right precentral gyrus, right superior frontal gyrus, middle frontal gyrus, middle orbitofrontal cortex of the frontal lobe, left postcentral gyrus of the parietal lobe, and left cerebellum Crus1 of the cerebellum. Details can be found in Table 2 and Figures 2 and 3.
 |
Table 2 Brain Regions with Aberrant ALFF, ReHo, and FC in Cancer Pain Patients |
Correlation and ROC Curve Analysis
ALFF and ReHo values extracted from individual brain regions showed moderate discriminative ability, whereas FC Z-scores demonstrated better discriminative ability compared to ALFF or ReHo values from individual regions, with the FC of the right precentral gyrus yielding better classification results (AUC = 0.804) as shown in Figure 4 and Table 3. There was no significant correlation between the neuroimaging indices of the CP group and pain scores (P > 0.05) as shown in Table 4.
 |
Table 3 Classification Performance of Neuroimaging Indices in Discriminating Between Cancer Pain Patients and Healthy Controls |
 |
Table 4 Results of Spearman Correlation Between Resting-State fMRI Features and Pain Scores in Cancer Pain Patients |
Discussion
The perception of cancer pain is complex as it involves multiple brain region networks. Through rs-MRI, this research explored the differences in brain functional activity and connectivity between HCs and the CP group, preliminarily identifying brain regions related to cancer pain. Compared to HCs, our results found a reduction in ALFF primarily in the bilateral inferior temporal gyrus; a decrease in ReHo in the left cerebellum, and an increase in the right middle temporal gyrus. The CP group showed decreased FC in the frontal, parietal, and cerebellar cortices. Among these neuroimaging indices, the FC in the right precentral gyrus demonstrated better discriminatory performance between the two groups, offering new treatment options for improving persistent and chronic cancer pain through the modulation of brain functional areas, such as TMS and tDCS.
Previous research has linked cancer pain with frontal brain regions. For example, studies by Liu et al found decreased ALFF in the right superior frontal gyrus, inferior frontal gyrus, and left anterior cingulate cortex in patients with bone metastases from lung cancer. Besides, they found increased ReHo in the bilateral thalamus and left fusiform gyrus,20 as well as decreased FC in the frontal cortex.23 Our results identified a decrease in ALFF in the bilateral inferior temporal gyrus and an increase in ReHo in the right middle temporal gyrus in CP patients, indicating the involvement of the temporal lobe in cancer pain processing, consistent with previous studies.
Studies have shown that the temporal lobe is related to pain, playing an important role in the perception and processing of pain, especially in the regulation of cognition, emotion, and memory related to pain.24 The inferior temporal gyrus and middle temporal gyrus may be involved in cancer pain processing, particularly in terms of emotional regulation and cognitive processing. These regions, closely related to the perception of pain in the frontal cortex and postcentral gyrus of the parietal lobe, may be interconnected and participate in the perception and regulation of cancer pain.25 Other temporal lobe regions have also been shown to be related to pain. For example, research by BU et al, found that increased activity in the right inferior temporal gyrus could alleviate neuropathic pain.26 Foteini Christidi et al, observed the gray matter density and brain FC in patients with chronic pain from medication-overuse headache (MOH),27 finding that higher pain scores were associated with decreased gray matter density in the inferior temporal gyrus. This study found a reduction in resting-state activity in the bilateral inferior temporal gyrus of CP patients, consistent with previous findings. Additionally, researchers have found that increased ReHo in the middle temporal gyrus can reduce intermittent pain. This study also observed an increase in ReHo in the middle temporal gyrus, consistent with previous research results.28 However, more studies are needed to explain the relationship between increased ReHo in the middle temporal gyrus and intermittent pain from the perspective of pain mechanisms.
The cerebellum is an essential motor control center related to maintaining body balance, controlling posture, and gait. Increasing research finds the cerebellum responds to both acute and chronic pain, suggesting its involvement in pain regulation and sensory adaptation.29–31 Particularly, the posterior part of the cerebellum is involved in motor control and pain processing, guiding pain-related movements.32 For example, Liu et al, found that changes in cerebellar gray matter volume in PHN patients were positively correlated with pain scores,29 and Guo et al found decreased ALFF in the left cerebellum of knee osteoarthritis (KOA) pain patients compared to healthy subjects.33 This study found decreased ReHo and FC in the left cerebellum of CP patients, hypothesizing that chronic pain and occurrences of breakthrough cancer pain lead to long-term posture adjustments or forced body positions, thus causing reduced activity and connectivity in the cerebellum. Additionally, studies have shown that the posterior hemispheric lobules, including Crus 1 and 2, are involved in emotional34 and cognitive processing. We also found decreased brain functional activity and connectivity in the Left Cerebellum Crus 1 and Left Cerebellum Crus 2 in CP patients, which might be related to the prevalence of depressive states among most of these patients. With an average Oral Morphine Equivalent of 121mg in the CP group, literature35 suggests that high-dose opioid consumption is related to cognitive decline. We speculate that cognitive function in CP patients may be secondarily impaired due to high-dose opioid use, leading to cerebellar dysfunction. Therefore, cancer pain might affect motor functions, and CP patients may experience problems with motor control. As mentioned, the cerebellum may be connected with other brain areas (such as the inferior temporal gyrus and middle temporal gyrus) to regulate motor control and coordination, emotional processing, and cognition in CP patients.
We also discovered abnormal brain functional activity in the postcentral gyrus of the parietal lobe. The postcentral gyrus, as a higher center for pain sensation processing, plays a crucial role in the adaptation to pain stimuli and the balance of pain regulation circuits. In patients with fibromyalgia, decreased activity in the prefrontal cortex is related to a reduction in pain intensity following Cognitive Behavioral Therapy, associated with increased PFC activity.36,37 An rs-MRI study on PHN showed that FC between the PFC, parietal cortex, and postcentral gyrus is negatively correlated with pain intensity,38 and other studies have found the PFC in chronic pain patients is also related to negative emotions and cognition.39,40 Similar to our results, we found decreased FC between the prefrontal cortex and the postcentral gyrus of the parietal lobe, suggesting the prefrontal cortex and postcentral gyrus play roles in the perception and regulation of pain, as well as emotional regulation and cognitive processing, which is particularly important in CP patients, as pain often accompanies emotional issues and cognitive impairments.
Recently, neuroimaging indices have been used as biomarkers to distinguish between pain patients and healthy control groups. For example, Liu et al,20 found that a combination of ALFF and ReHo from rs-MRI showed satisfactory classification performance (AUC = 0.963) between patients with bone metastasis from lung cancer and healthy individuals, and Liu et al29 found the left lentiform nucleus (AUC = 0.826) could distinguish between herpes zoster (HZ) and postherpetic neuralgia (PHN) patients. Our study found that FC Z-scores demonstrated better discriminative ability compared to ALFF or ReHo values extracted from individual brain regions, with the FC of the right precentral gyrus yielding better classification results (AUC = 0.804), indicating significant diagnostic relevance when the AUC value is >0.6.41 The precentral gyrus has been reported in numerous chronic pain fMRI studies for its reduced gray matter volume and abnormal FC.42–44 The analgesic effect of high-frequency (>5Hz) repeated TMS (rTMS) on the motor cortex of the precentral gyrus (area M1) for neuropathic pain has been established, with an evidence level of A. This was already included in the French guidelines for repetitive TMS as early as 2011.45 In the context of cancer pain, Julien Nizard et al, reported in 201546 the first cases of refractory CP patients who underwent high-frequency rTMS targeting the M1 area for five sessions, resulting in significant reductions in NRS scores and opioid consumption, with improvements in anxiety and depression. This suggests that the precentral gyrus not only plays a crucial role in distinguishing between CP patients and healthy individuals but may also be involved in pain perception and regulation, and emotional processing, becoming a potential target for future cancer pain treatments. However, not all cancer pain patients can benefit from appropriate screening and management methods. In addition to neuroimaging biomarkers, there may be other new markers, such as hematological indicators, genetic susceptibility, and composite biomarkers.47 Thus, the treatment and management of cancer pain remain a global challenge.
Our study has several limitations: First, the sample size is relatively small, and future studies should validate our findings in larger datasets. Second, there are many influencing factors in the CP group, making it difficult to exclude the confounding effects of painkillers, cancer itself, anti-tumor treatments, and social factors. Third, we did not analyze in detail the impact of the psychological state (such as anxiety, depression, fear, suicidal thoughts, etc) and cognitive functions (such as attention, executive function, etc) of CP patients on different brain regions. Besides changes in brain network connectivity, cancer pain might also cause changes in brain structure. We did not find a correlation between brain activity or FC and pain intensity in CP patients, which could be due to the subjective nature of NRS scoring, influenced by the patient’s negative emotions, education level, language, etc.,48,49 and sometimes may not accurately reflect the true intensity of the patient’s pain. Additionally, the number of CP patients included in this study is small, and mainly involves mild-to-moderate pain (95.24%), which may limit the ability to discriminate between different levels of pain intensity. In the future, we can conduct more detailed classifications of CP patients, improve the detection of cognitive functions and neuropsychological scales, and explore the exact connections and mechanisms of the central nervous system more deeply through neuroimaging technology.
Conclusion
Our study preliminarily identified abnormalities in brain activity and FC in areas such as the inferior temporal gyrus, middle temporal gyrus, prefrontal cortex, parietal lobe, and cerebellum in patients with cancer pain. These brain regions may be interconnected through neural networks, jointly participating in functions related to pain perception, emotional regulation, cognitive processing, and motor control. Machine learning research found that the FC of the right precentral gyrus demonstrated better discriminatory performance between CP patients and healthy individuals. The cancer pain-related brain regions identified in this study offer new treatment options for improving persistent and chronic cancer pain through the modulation of brain functional areas, such as TMS and tDCS.
Data Sharing Statement
The datasets and codes generated for this study are available on request to the corresponding author.
Acknowledgments
This study was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant Number: 2024KY564) and Chinese Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant Number: 2024ZL1291). We thank all participating patients.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Raja Srinivasa N, Carr Daniel B, Milton C, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–1982. doi:10.1097/j.pain.0000000000001939
2. Mao JJ, Pillai GG, Andrade CJ, et al. Integrative Oncology: addressing the Global Challenges of Cancer Prevention and Treatment. CA Cancer J Clin. 2022;72(2):144–164. doi:10.3322/caac.21706
3. Neufeld NJ, Elnahal SM, Alvarez RH. Cancer Pain: a Review of Epidemiology, Clinical Quality and Value Impact. Future Oncol. 2017;13(9):833–841. doi:10.2217/fon-2016-0423
4. Marianne Jensen H, Stein K, Augusto C, et al. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support Palliat Care. 2016;6:344–352. doi:10.1136/bmjspcare-2015-000887
5. Morton Debbie L, Sandhu Javin S, Jones Anthony Kp. Brain imaging of pain: state of the art. J Pain Res. 2016;9:613–624
6. Juan Miguel J-A, Mantyh William G, Bloom Aaron P, et al. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181. doi:10.1111/j.1749-6632.2009.05429.x
7. Laird BJ, Walley J, Murray GD, et al. Characterization of cancer-induced bone pain: an exploratory study. Support Care Cancer. 2011;19:1393–1401. doi:10.1007/s00520-010-0961-3
8. Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain. 2013;154:S54–S62. doi:10.1016/j.pain.2013.07.044
9. Park Sun H, Eber Matthew R, Brooke WD, et al. Role of the Bone Microenvironment in the Development of Painful Complications of Skeletal Metastases.Cancers. Basel. 2018;10.
10. Yiheng T, Jin C, Yanzhi B, et al. Magnetic resonance imaging for chronic pain: diagnosis, manipulation, and biomarkers. Sci China Life Sci. 2021;64:879–896. doi:10.1007/s11427-020-1822-4
11. Buchbinder BR. Functional magnetic resonance imaging. Handb Clin Neurol. 2016;135:61–92.
12. Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi:10.1016/j.braindev.2006.07.002
13. Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi:10.1002/mrm.1910340409
14. Song C, Ying L, Wenwen D, et al. Local Brain Activity Differences Between Herpes Zoster and Postherpetic Neuralgia Patients: a Resting-State Functional MRI Study. Pain Physician. 2017;20:E687–E699.
15. DeSouza Danielle D, Hodaie Mojgan, Davis Karen D. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain. 2014;155:37–44
16. Zhang B, Jung M, Tu Y, et al. Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br J Anaesth. 2019;123:e303–11. doi:10.1016/j.bja.2019.02.021
17. Barroso J, Vigotsky AD, Branco P, et al. Brain gray matter abnormalities in osteoarthritis pain: a cross-sectional evaluation. Pain. 2020;161:2167–2178. doi:10.1097/j.pain.0000000000001904
18. Robinson ME, O’Shea AM, Craggs JG, Price DD, Letzen JE, Staud R. Comparison of machine classification algorithms for fibromyalgia: neuroimages versus self-report. J Pain. 2015;16:472–477. doi:10.1016/j.jpain.2015.02.002
19. Andreas F, Johannes B, Mark L, et al. Dopamine and fear memory formation in the human amygdala. Mol Psychiat. 2022;27:1704–1711. doi:10.1038/s41380-021-01400-x
20. Daihong L, Xiaoyu Z, Yong T, et al. Altered brain functional activity and connectivity in bone metastasis pain of lung cancer patients: a preliminary resting-state fMRI study. Front Neurol. 2022;13:936012. doi:10.3389/fneur.2022.936012
21. Yan C, Zang Y. DPARSF: a MATLAB toolbox for pipeline data analysis of resting-state fMRI. Frontiers in Systems Neuroscience. 2010;4:1377.
22. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi:10.1016/j.neuroimage.2003.12.030
23. Xiaoyu Z, Yong T, Jiao C, et al. Altered Functional Connectivity in Pain-Related Brain Regions and Its Correlation with Pain Duration in Bone Metastasis with Cancer Pain. Dis Marke. 2022;2022:3044186. doi:10.1155/2022/3044186
24. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi:10.1073/pnas.98.2.676
25. Argaman Y, Kisler LB, Granovsky Y, et al. The endogenous analgesia signature in the resting brain of healthy adults and migraineurs. J Pain. 2020;21:905–918. doi:10.1016/j.jpain.2019.12.006
26. Chunxiao B, Huan R, Qingqing L, et al. Alteration of static and dynamic intrinsic brain activity induced by short-term spinal cord stimulation in postherpetic neuralgia patients. Front Neurosci. 2023;17:1254514. doi:10.3389/fnins.2023.1254514
27. Foteini C, Efstratios K, Lars M, et al. Dimensions of pain catastrophising and specific structural and functional alterations in patients with chronic pain: evidence in medication-overuse headache.World. J Biol Psychiatry. 2020;21:726–738. doi:10.1080/15622975.2019.1669822
28. Xiaochong F, Huan R, Chunxiao B, et al. Alterations in local activity and functional connectivity in patients with postherpetic neuralgia after short-term spinal cord stimulation. Front Mol Neurosci. 2022;15:938280. doi:10.3389/fnmol.2022.938280
29. Jiaqi L, Lili G, Qing H, et al. Altered gray matter volume in patients with herpes zoster and postherpetic neuralgia. J Pain Res. 2019;12:605–616. doi:10.2147/JPR.S183561
30. Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev. 2010;65(1):14–27. doi:10.1016/j.brainresrev.2010.05.005
31. Mehnert J, Schulte L, Timmann D, May A. Activity and connectivity of the cerebellum in trigeminal nociception. Neuroimage. 2017;150:112–118. doi:10.1016/j.neuroimage.2017.02.023
32. Chen Y, Xiang CQ, Liu WF, et al. Application of amplitude of low-frequency fluctuation to altered spontaneous neuronal activity in classical trigeminal neuralgia patients: a resting-state functional MRI study. Mol Med Rep. 2019;20(2):1707–1715. doi:10.3892/mmr.2019.10404
33. Hua G, Yuqing W, Lihua Q, et al. Structural and Functional Abnormalities in Knee Osteoarthritis Pain Revealed With Multimodal Magnetic Resonance Imaging.[J]. Front Hum Neurosci. 2021;15:783355. doi:10.3389/fnhum.2021.783355
34. Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. 2011;31:3795–3804. doi:10.1523/JNEUROSCI.6709-10.2011
35. Copenhaver Michael M, Victoria S, Roman S, et al. Developing a cognitive dysfunction risk score for use with opioid-dependent persons in drug treatment. Drug Alcohol Depend. 2021;224:108726. doi:10.1016/j.drugalcdep.2021.108726
36. Schrepf A, Harper DE, Harte SE, et al. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. 2016;157:2217–2225. doi:10.1097/j.pain.0000000000000633
37. Jensen KB, Kosek E, Wicksell R, et al. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153:1495–1503. doi:10.1016/j.pain.2012.04.010
38. Li J, Huang X, Sang K, Bodner M, Ma K, Dong X-W. Modulation of prefrontal connectivity in postherpetic neuralgia patients with chronic pain: a resting-state functional magnetic resonance-imaging study. Journal of Pain Research. 2018;11:2131–2144. doi:10.2147/JPR.S166571
39. Brooke N, Negin H-S, James H M, et al. Reduced Glutamate in the Medial Prefrontal Cortex Is Associated With Emotional and Cognitive Dysregulation in People With Chronic Pain. Front Neurol. 2019;10:1110. doi:10.3389/fneur.2019.01110
40. Martucci KT, Mackey SC. Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology. 2018;128:1241–1254. doi:10.1097/ALN.0000000000002137
41. Zhou F, Gu L, Hong S, et al. Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. J Pain Res. 2018;11:165–176. doi:10.2147/JPR.S151562
42. Yanan W, Jing X, Qing Z, et al. Immediate Analgesic Effect of Acupuncture in Patients With Primary Dysmenorrhea: a fMRI Study. Front Neurosci. 2021;15:647667. doi:10.3389/fnins.2021.647667
43. Azqueta-Gavaldon M, Youssef Andrew M, Storz C, et al. Implications of the putamen in pain and motor deficits in complex regional pain syndrome. Pain. 2020;161(3):595–608. doi:10.1097/j.pain.0000000000001745
44. Yuan-Hsiung T, Rui Y, Dharni P, et al. Altered structure and functional connection in patients with classical trigeminal neuralgia. Hum Brain Mapp. 2018;39:609–621. doi:10.1002/hbm.23696
45. Galhardoni R, Correia GSC, Araujo H, et al. Repetitive Transcranial Magnetic Stimulation (rTMS) in Chronic Pain: a review of the literature. ARCHIV PHYS MEDI REHABILIT. 2014.
46. Julien N, Amélie L, Nathalie D, et al. Interest of repetitive transcranial magnetic stimulation of the motor cortex in the management of refractory cancer pain in palliative care: two case reports. Palliat Med. 2015;29:564–568. doi:10.1177/0269216315574260
47. Ferrari F, Giannini A. Approaches to prevention of gynecological malignancies. BMC Women’s Health. 2024;24(1):1–3. doi:10.1186/s12905-024-03100-4
48. Yoo H, Cho Y, Cho S. Does past/current pain change pain experience? Comparing self-reports and pupillary responses. Front Psychol. 2023;14:1094903. doi:10.3389/fpsyg.2023.1094903
49. Gandhi W, Rosenek NR, Harrison R, Salomons TV. Functional connectivity of the amygdala is linked to individual differences in emotional pain facilitation. Pain. 2020;161:300–307. doi:10.1097/j.pain.0000000000001714
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.