Back to Journals » Patient Preference and Adherence » Volume 18
Improving Adherence of Young Male Patients with HBV Infection to the Regular Follow-Up via Mobile Healthcare Platform Might Be Cost-Effective to Decrease the Morbidity of Advanced Liver Cancer
Authors Liang H, Yang M, Luo D, Wu YK
Received 25 September 2024
Accepted for publication 13 December 2024
Published 19 December 2024 Volume 2024:18 Pages 2581—2595
DOI https://doi.org/10.2147/PPA.S497831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Ortiz
Hao Liang,* Min Yang,* Dan Luo, Ya-Kun Wu
Department of Hepatobiliary Surgery, Suining Central Hospital, Suining, Sichuan Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ya-Kun Wu, Department of Hepatobiliary Surgery, Suining Central Hospital, Suining Dongping North Road No. 27, Suining, 629000, People’s Republic of China, Email [email protected]
Background: Young adults contribute substantially to the social economy. However, the number of young adults with liver cancer has increased recently. In addition, the mortality rate of these patients is high.
Methods: This retrospective study investigated the risk factors of young patients diagnosed with liver cancer over the past 12 years.
Results: The risk factors of liver cancer, including male, HBV infection, and family history of diseases, were more common in young patients. Nearly 80% of young patients (198/253) were tested as positive HBsAg. However, most of these patients did not visit doctors regularly, as recommended. Thus, 55.7% of young patients were diagnosed with advanced liver cancer. The aspartate aminotransferase (AST) levels were independently associated with advanced liver cancer (OR = 4.262, 95% CI = 1.559– 11.65, P = 0.005) in the multivariable logistic regression. The 1-year survival rate of these patients was 19.4%.
Conclusion: The high-risk factors of liver cancer are common in young patients. The poor adherence to regularly visited doctors in young patients might contribute to the high ratio of advanced liver cancer. The 1-year survival rate of these patients is low. Improving patient’s adherence via mobile healthcare platform and monitoring serum AST levels might decrease the incidence and mortality of liver cancer in young adults.
Keywords: young adults, advanced liver cancer, adherence, risk factors, aspartate aminotransferase, tumor staging, history of family, mobile healthcare platform
Background
In many previous studies, older adults have become the focus of early cancer diagnosis and treatment of cancer.1 Compared to older adults, clinical and translational research on cancer in the younger adult group, 18–45 years, is usually lacking, let alone the rapid progress in early diagnosis and treatment.2 However, young adults with a longer lifespan continue to play a critical role in caring for families and, more importantly, have the potential to contribute substantially to the social economy.3 Therefore, young adults with cancer should not be a neglected group of patients. It is worth learning about the current state and analyzing some special features of this group.
As for liver cancer, many controllable high-risk factors are currently known, such as infection by the Hepatitis B virus (HBV), obesity, and alcohol use.4 In addition, an official recommended surveillance program for liver cancer has been conducted for two decades.5 Therefore, the incidence of liver cancer in the older population has decreased.6 However, recent epidemiological data suggest that the incidence of liver cancer in young adults is increasing, particularly in males.7 The 1-year survival rate of these patients is lower than that of the older patients.8
Existing policies for vaccination and early screening programs for young adults may fail to be highly effective in reducing the burden of liver cancer. The potential reasons may be that there is a lack of appreciation of the clinical features of young adults with liver cancer, not to mention the disease history of their family.2 The distribution features of liver cancer in young adults may change over time. In addition, the fact is that regular follow-up every 3–6 months was significantly related to reducing liver cancer mortality.9 Yet, the adherence of younger adults to regular follow-up and HCC surveillance was only suboptimal,10 and the male patients had lower adherence to the surveillance.11 Therefore, new strategies to reduce the incidence and mortality of liver cancer in young adults should be explored.
In this study, we collected data from the past 12 years to investigate the previous and current distributive features and potential risk factors of young adults diagnosed with liver cancer. Furthermore, the clinical prognosis of young adults with liver cancer was analyzed.
Patients and Methods
This retrospective observational study was conducted in a tertiary hospital in China. The study design was reviewed and approved by the Institutional Review Board of Suining Central Hospital.
Selection of Patients
The data of liver cancer patients aged between 18 and 45 years were retrieved using automation tools in the Electronic Medical Record from January 2011 to March 2023. After removing duplicate records, 555 patients were included (Figure 1). Then, 24 cases were excluded because of either being under 18 years of age, loss of inpatient number, or automation tool screening. Information was provided for the remaining 531 records.
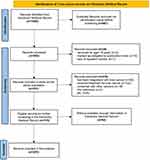 |
Figure 1 The selection flow diagram of young adults with liver cancer at first diagnosis. Abbreviation: HIV, Human Immunodeficiency Virus. |
Furthermore, numerous patients with liver cancer were excluded if they had already undergone any antitumor therapy (eg, surgery, chemotherapy, radiotherapy, or immunotherapy), had other malignancies (except cancer that had been in complete remission for >5 years), had received liver transplantation therapy, had been ever diagnosed with liver cancer, had HIV infection, were pregnant, or lacked sufficient information.
Definitions and Collections of Data
Liver cancer was diagnosed according to the radiological hallmarks of liver cancer, with or without histological confirmation.12 Body mass index (BMI) is a measure of body fat and is the weight (kg) divided by the square of height (m). Obesity is defined as a high body mass index (BMI) (> 30). A positive HBsAg result indicated that the Hepatitis B virus surface antigen had been tested. All clinical symptoms and blood examination results were collected at the time of liver cancer diagnosis at admission. The degree of ascites was defined according to abdominal radiological signs. A history of HBV infection refers to a known history of HBV infection regardless of treatment. The Child-Pugh scoring system included serum bilirubin, serum albumin, prothrombin time, encephalopathy, and ascites. Regarding family history, the estimated population were first-degree relatives of the patients.
Outcomes
Tumor node metastasis (TNM) staging of liver cancer at diagnosis was performed according to the American Joint Committee on Cancer 8th Edition Staging System for Hepatocellular Carcinoma.13 T4 grade indicates multiple tumors, with more than one lobe, and invasion of the major branch of the portal or hepatic vein. The Barcelona Clinic Liver Cancer (BCLC) staging system consists of five stages (0, A, B, C, D). The 1-year survival of the patients was calculated from the start of diagnosis to the last follow-up.
Statistical Analysis
All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Most continuous variables were presented as medians and interquartile ranges (Q1 and Q3). All nominal variables were presented as frequencies and proportions. The χ²-test and Mann–Whitney U-test were used to compare nominal and continuous variables, respectively. Taking the views of statistician and clinician into considerations, variables with statistical significance in χ²-test and Mann–Whitney U-test were included into univariate analysis. Variables that had potential statistical significance in the univariate analysis (P-values <0.1) were included into the multivariate logistic regression analysis. The stepwise multivariate logistic regression analysis was used to validate the statistical significance of variables. Box plots, scatter plots, and forest plots were drawn using SPSS and Adobe Illustrator 2022. Statistical significance was considered as P-values of <0.05.
Results
The Demographic Characteristics of Young Liver Cancer Patients with Different HBsAg States
HBV infection is one of the most common causes of liver cancer in China. Therefore, the traits of 253 patients who were first diagnosed with liver cancer were further analyzed based on the results of HBsAg examinations (Table 1). The number of patients with positive HBsAg results was high, at 198 (78.3%). Moreover, the median age of these patients was 39 years (Table 1), which was lower than that of non-positive HBsAg patients (42 years old, P = 0.028). The proportion of male patients in the HBsAg positive group (Figure 2) was significantly higher than that in the HBsAg negative group (92.4% vs 67.3%, P < 0.001).
 |
Table 1 The Demographic Characteristics of Young Patients at First Diagnosis of Liver Cancer |
Obesity is a potential cause of liver cancers. The median BMI values in the positive HBsAg and non-positive HBsAg groups were similar (21.9 Kg/m2 vs 22.1 Kg/m2), and BMI values of most patients failed to reach the diagnosis of obesity. The differences in the types of medical insurance and occupation of the patients between the two groups were not significant.
The Clinical Features of Young Patients Firstly Diagnosed with Liver Cancer
The most common symptom in young patients with liver cancer was abdominal pain (49.8%, 126/253), followed by abdominal distention (40.7%, 103/253). The main complaints of patients in the positive HBsAg and non-positive HBsAg groups were similar (Table 2). Only two (0.7%) patients presented with fever at the time of admission. Patients in the positive HBsAg group had a higher hemoglobin level (131 g/L) than those in the non-positive HBsAg group (119 g/L, P = 0.019). The median level of platelets in the positive HBsAg group was 152.5 × 109/L, lower than that of the non-positive HBsAg group (189 × 109/L), though the difference was not significant (P = 0.067).
 |
Table 2 The Clinical Features of Young Patients at First Diagnosis of Liver Cancer |
Abnormal changes in liver function in young patients were as expected. The median levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the HBsAg positive group were 57 U/L and 115 U/L, respectively, which were higher than those in the HBsAg negative group (39 U/L and 74 U/L). The P-values were 0.001 and 0.012, respectively (Figure 3a and 3b). In addition, the number of young patients with cirrhosis in the positive HBsAg group was 114 (57.6%), which was higher than that in the non-positive HBsAg group (23; 41.8%) (P = 0.038). Moreover, the median serum albumin and total bilirubin levels were not within the reference ranges (Table 2). For patients in the positive HBsAg and non-positive HBsAg groups, the serum levels of albumin and total bilirubin and the degree of ascites were not significantly different (all P > 0.05).
A few (13.8%) patients presented with massive ascites in the hospital, but nearly 35% (87/253) of the patients had mild ascites on CT examinations. Interestingly, the median serum α-fetoprotein (AFP) in the two groups reached the upper limit of detection (1,210 ng/mL). In this special circumstance (Table 2), the difference between the two groups could not be described directly (P = 0.600).
The Child-Pugh Classification of Liver Function and Staging of Liver Cancer in Young Patients Firstly Diagnosed with Liver Cancer
In addition to the analysis of routine examinations, comprehensive indices of live cancer were also investigated. The proportion of patients with liver cancer was 37.5% for Child-Pugh class A and 41.9% for Child-Pugh class B. Only 11.5% of the patients were graded as Child-Pugh class C. The distributive difference in the three types of Child-Pugh class in the non-positive HBsAg and positive HBsAg groups was not significant (Table 3).
 |
Table 3 The Staging of Liver Cancer in the Young Adults at First Diagnosis |
Most young patients with known high-risk factors for liver cancer do not regularly visit doctors, let alone participate in screening programs. Therefore, patients with BCLC stage C disease were the most common group (164, 64.8%). Only 10 and 11 cases had BCLC A and BCLC D staging, respectively. The distributive difference in the BCLC staging of patients in the two groups was not significant (Table 3).
Regarding TNM classification, only 13 patients were classified as having stage II disease. The numbers of patients with IIIA, IIIB, IVA, and IVB staging were 50, 78, 45, and 52, respectively (Figure 4). The distribution difference in the TNM staging of patients in the positive and non-positive HBsAg groups was not significant. Patients with N1 and M1 grades comprised 24.5% and 21.3% of cases, respectively. The distribution of N1 grade or M1 grade cases in the positive and non-positive HBsAg groups was similar.
 |
Figure 4 Heatmap for number of patients with different TNM grades and different Child-Pugh classifications. |
Although the tumor staging between the different HBsAg groups was not different, the local tumor state (T grade) showed some differences: few patients were diagnosed with T2 grade (14, 5.5%), but over half of the patients were diagnosed with T4 grade (141, 55.7%). The number of T4 grade patients was much higher in the positive HBsAg group than in the non-positive HBsAg group (118 vs 23, P = 0.019).
Analysis of the Risk Factors Related to T4 Grade in the Young Adults Firstly Diagnosed with Liver Cancer
In the above staging analysis, T4 grade was common in young patients with liver cancer, and HBV infection may be related to T4 grade (invasion of the major hepatic vein). Therefore, the relationships between the staging of liver cancer and age, sex, BMI, alcohol use, smoking, positive HBsAg, history of HBV, cirrhosis, hemoglobin, ALT > 40 U/L, AST > 50 U/L, and platelet count were analyzed using univariate analysis (Table 4). The relationships between HBsAg positivity, cirrhosis, ALT level >40 U/L, AST level >50 U/L, and T4 grade were quite close (P = 0.012, P = 0.099, P = 0.004, and P < 0.001). The examined variance inflation factor values of HBsAg and AST were 1.078 and 1.161, respectively. Therefore, these factors were included in multivariate logistic regression analysis (Figure 5). Only AST level >50 U/L was independently associated with T4 grade (OR = 4.262, 95% CI = 1.559–11.65, P = 0.005).
 |
Table 4 Univariable and Multivariable Logistic Regression Analysis of the Risk Factors of the T4 Grade |
History Traits of Personal and Family Factors of Patients
Considering that some of the HBsAg-positive patients did not seem to have a history of HBV infection at admission, the history of personal and family factors of patients was investigated further.
A total of 153 patients had a known history of HBV infection, and the remaining 100 patients (39.5%) had no history of HBV infection (Table 5). In patients without a known history of HBV infection, 67.0% of the patients still showed HBsAg positivity at admission, which was lower than that in the patients with a history of HBV infection (85.6%) (P < 0.001). The proportion of patients with cirrhosis with and without a history of HBV infection was 65.4% and 37.0%, respectively (P < 0.001).
 |
Table 5 The Features of Patients and Their Families According to History of HBV Infection |
Among 253 patients, 38.3% had a history of alcohol use, and the difference in alcohol use in patients with or without a history of HBV infection was not significant (P = 0.377). The proportion of patients with a history of smoking with and without a history of HBV infection was 45.8% and 32.0%, respectively (P = 0.029). None of the patients had used aspirin or a history of coronary heart disease. The 1-year survival rates of patients with and without a history of HBV infection were 19.4% and 45.0%, respectively (P = 0.043).
The history of family members (Table 5), including regular check-ups, alcohol use, smoking, coffee use, and obesity, was not significantly different between the two groups (all P < 0.05). The family history of diseases, including diabetes mellitus, HBV infection, cirrhosis, liver cancer, and other cancers, was not significantly different between the two groups (all P < 0.05).
Discussions
Although the incidence was quite low and neglected, the number of liver cancers in young adults has increased.7 Recent studies also noted these drastic changes and suggested that researching liver cancer in young adults could be cost-effective for reducing liver cancer burden14 and quickly accumulating social wealth.3 However, few studies have described the clinical features, risk factors, and prognosis of young adults with liver cancer. Importantly, the characteristics of young adults with liver cancer might differ from those of patients with liver cancer. This retrospective observational study with a large sample size revealed the features of young patients firstly diagnosed with liver cancer, including distributive features, clinical characteristics, risk factors, staging of cancer clinical prognosis, and disease history.
The absolute number of young patients with liver cancer has increased over the past 12 years. Young male patients with HBsAg positivity were fairly vulnerable, compared to obese people or drinkers. Amazingly, many patients without a reliable history of HBV infection tested positive for HBsAg, and most of them did not visit doctors regularly, let alone undergo the standard screening program for liver cancer. Liver function of the young patient was satisfactory. However, most patients are initially diagnosed with advanced liver cancers. The proportion of patients with T4 grade tumors (tumors invading the major branch of the portal or hepatic veins) was significantly higher. Elevated AST levels were an independent factor that correlated with T4 grade. Finally, we found that the 1-year survival rates of young patients with liver cancer were low, especially those with a known history of HBV infection.
To prevent liver cancer in younger adults, young male patients and their relatives with HBV infection, rather than obese men or drinkers, deserve more attention. A large number of patients with a reliable family history of HBV infection are diagnosed with liver cancer. Therefore, it is critical for doctors to improve patients' adherence and conduct more interventions for health education, including timely follow-up.
A study noted that the incidence of liver cancer in young adults has not decreased in recent years.15 Additionally, our study showed an increase in the number of young patients with liver cancer. Compared to the ratio of sex in whole liver cancer patients in a previous study,16 the ratio of male patients was much higher than that of female patients in younger patients. In the older patient group, the incidence of liver cancer in male and females may be similar. Therefore, taking care of younger male patients could improve the sex ratio of all patients with liver cancer. Meanwhile, the results of this study showed that the number of young patients in rural areas was higher, similar to the results of a previous study, although the P-value was not shown.6
The strong synergies between androgen and HBV infection in the occurrence of liver cancer should not be neglected. The HBV infection is an important risk-factor of liver cancer.17 Therefore, the clinical features of patients with and without HBV infection were analyzed. Compared with the non-positive HBsAg group, patients in the positive HBsAg group were younger, and male patients were the most common. This may be because androgen levels in younger males are very high.18 Numerous studies have revealed that the active androgen pathway regulates the host immune response, increases replication of HBV,19 and enhances HBV chromosomal integration.20 High levels of androgen and HBV infections could be sufficient to trigger the occurrence of liver cancer in younger patients.
Interestingly, none of the young patients was diagnosed through a surveillance program for liver cancer. The limitations in the implementation of the existing surveillance for liver cancer in younger patients may be one of the reasons.21 Some patients with HBV infection irregularly received anti-HBV therapy and were diagnosed with liver cancer. Second, younger patients at diagnosis present with atypical clinical symptoms, including abdominal pain and distention, in the non-positive or positive HBsAg groups.4 It is difficult to identify younger patients diagnosed with liver cancer during surveillance.
However, we analyzed the medical history of the patients and their relatives. A method could be used to address these issues. A part of patients without a known history of HBV infection are diagnosed with HBV infection (HBsAg positive) and cirrhosis after admission. However, the relatives of many young patients have an HBV infection, or their relatives were diagnosed with liver cancer. Therefore, paying more attention to surveillance of patients with HBV infection or liver cancer and to educate their young male relatives may be effective.
Existing surveillance programs for liver cancer in the target HBV population22 (men over 40-year or women over 50-year) have been performed for some years.4 It was difficult to conduct new surveillance programs for liver cancer in young men with HBV infection in rural areas, but conducting comprehensive education liver cancer for adolescents in school could reduce parts of the liver cancer burden in the future.23 Currently, no age-specific screening tests for liver cancer are available in young adults.24 Therefore, improving the adherence of the existing target HBV population in the surveillance program could protect some of the target young patients from liver cancer. Further, paying extra attention to the health education of young relatives of the target HBV population would help reduce the occurrence of liver cancer.
Disturbingly, good adherence to therapeutic regimens and regular follow-up were much more problematic in young adults than in other groups of patients,25 either due to the lack of spousal oversight or impediments to compliance due to conflicts with their life or study.1 However, the emerging mobile healthcare platform with immense potential to deliver behavior change techniques might solve this problem smoothly. Young population had attempted to use mobile healthcare platforms to get some health services.26 Previous studies have reported that the use of mobile healthcare platform could significantly improve patients’ adherence behaviors,27,28 including young adults with HIV,29 and patients with type 1 diabetes mellitus.30 The use of mobile healthcare platform had advantages of using less time and fewer resources.31 Meanwhile, the use of mobile healthcare platform did not increase hospital workload.32 In the future, research undertaking cost–benefit analyses of using mobile healthcare platform to improve patients’ adherence is needed, and the good results of the research should inform policymakers and advocates.
Another concern of this study was the body condition of the young adults and the staging of liver cancer. Although their body condition was satisfactory, young patients were diagnosed with advanced liver cancer, and their 1-year mortality was high. Surely, early diagnosis and treatments could improve prognosis. Improvement of the adherence of patients is the foundation, and an increase in the frequency of screening for liver cancer, such as examination of serum AFP levels and conducting liver ultrasonography, might make early diagnosis come true.
HBV infection does not appear to lead to severe liver dysfunction in young patients with liver cancer. Young patients have a higher potential for residual liver function. Most of the patients were not diagnosed with other chronic diseases. While there were elevated levels of ALT and AST, especially in the HBsAg-positive groups, the liver functions of most young patients were satisfactory, Child-Pugh class A, or Child-Pugh class B. The difference in Child-Pugh scoring scores between the non-positive and positive HBsAg groups was not significant. In contrast to the high ratio of cirrhosis in all liver cancer patients in previous articles,4 few young patients were diagnosed with cirrhosis in the positive HBsAg groups and fewer patients in the non-positive HBsAg groups. Good physiological functions of organs are the basis for good reactions to surgery, systemic chemotherapy, immunotherapy, or locoregional therapies for liver cancer.
Therefore, for young patients with liver cancer, opportunities to improve their mortality rates may be high. In the previous study, the overall survival was significantly better in younger patients than in older patients with BCLC stage 0-B.33 However, numerous patients are diagnosed with BCLC C stage in the disease. Many of these patients lost the opportunity for surgical treatment according to the common recommendation of the guidelines.34
Also, in TNM staging, many patients are diagnosed with advanced cancer (stage IIIB, IVA, or IVB). However, this study found that many patients were diagnosed with T4 tumors, and only a few patients had positive N or M grades. In the HBsAg-positive group, the proportion of patients with T4 grade disease was significantly higher. Further, our study found that it was the AST levels that were independently correlated with the T4 grade. There were two reasons leading to this finding.
Firstly, T4 grading refers to a large tumor size. The large size of the tumor implies that the growth patterns of liver cancer in young patients might be rapid growth. The doubling time of liver tumors with rapid growth patterns might be less than 3 months.35 A subsequent study showed that, in Asian hepatitis B-predominant populations, the tumor volume doubling time of liver cancer was short, about 4 months.36 The rapid growth of tumor may have great demands of oxygen. Secondly, some thrombosis in the portal vein or hepatic vein were also regarded as T4 grading. The oxygen tensions in liver cancer tissues were ten times lower than that in the periportal region of normal liver.37 There was a relatively anoxic condition in liver cancer tissues, where the portal vein or hepatic vein was thrombosed. Tumor with huge demands of oxygen as well as relatively anoxic conditions in the liver were enough to trigger the changes of AST level.
AST is highly concentrated in liver tissues,38 and its half-life in the blood is approximately 17 hours.39 The sensitivity of AST to reveal dynamic damage to the hepatic acinus is high, especially in ischemic circumstances.38 Therefore, a close correlation between the T4 grade and abnormal AST levels may be rational. The anoxic conditions of the liver probably existed in the early stages of liver cancer. Minor changes in AST levels were noted and investigated in detail.
AST levels may have the potential to predict prognosis. In clinical practice, transarterial chemoembolization (TACE) has been used for several years to treat advanced liver cancer. This could create tumor cells in an ischemic environment. A previous study showed that elevated AST levels are common in patients who received TACE treatment.40 Abnormal AST levels would have the strongest influence on patient prognosis of patients.41 Young adults are usually in good condition without chronic diseases. Therefore, the AST levels remained steady. The dynamic changes in AST levels would have shown some potential value for rating the effort of TACE treatment and the subsequent clinical outcomes of patients. In the future, a prospective study on liver cancer in young adults is needed.
In addition, we noted that the AFP level was quite high in young patients, and it reached the upper limit of detection, although the half-life of AFP was quite short, approximately 4–5 days. Interestingly, the extremely high levels of AFP in young patients with liver cancer may also imply rapid tumor growth patterns. Therefore, an increase in the frequency of liver cancer screening may improve the early diagnosis. In our study, delay in diagnosis caused worse outcomes in young patients with liver cancer, especially in patients with a history of HBV infection. Other studies have revealed the poor prognosis and short survival time of liver cancer in the young adults.42
Currently, examination of serum AFP levels and liver ultrasonography are recommended per 3–6 months for target patients. The frequency of screening for HBV infection in young male patients and their younger male relatives could be increased to once every 3 months. There is no solid evidence from the economics of health studies on this screening method. In this study, we only report this phenomenon and propose a screening method. In future studies, related studies exploring target patients to receive more frequent screening will be conducted.
Young adults are among the most financially productive members of all societies. From a long-term perspective, it is clear that enhancing the strength of the diagnosis of liver cancer in young adults will not only generate large societal benefits but will also be cost-effective: approximately 40 years of life expectancy remain.43 Hepatectomy may be curative in young adults with early-stage liver cancer.44 However, a delay in the diagnosis of liver cancer in young adults is common.1 Therefore, devoting more effort to the development of better diagnostic plans for early liver cancer may improve the overall survival of young adults with good body condition.44
This study had several limitations. First, many patients failed to undergo surgery or biopsy, let alone histological subtype analysis and regular follow-up. However, we attempted to collect data from the outpatient information system for survival analysis. Second, confounding factors for AST levels were identified. Hence, we analyzed and excluded the main factors (coronary heart disease and drugs). Third, some patients may have received anti-HBV therapy either irregularly or regularly. This might have led to different times of onset of liver cancer in the HBsAg-positive population. In the future, subgroup analyses based on prospective data may yield more accurate conclusions. Fourth, only limited family history data were available.
Conclusion
The absolute amounts of young adults with liver cancer have increased. Male, HBV infection, and a family history of related diseases are common high-risk factors for liver cancer in young adults. Most young patients fail to visit doctors regularly as recommended and are diagnosed with advanced liver cancer. Serum AST levels were independently associated with advanced liver cancer. The 1-year survival rate of young patients with liver cancer was low. For the young male patients with HBV infection, the use of mobile healthcare platform might improve their adherence to screening and treatments and might further decrease the incidence and mortality of liver cancer.
Data Sharing Statement
Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
This study was conducted in accordance with the principles of the Declaration of Helsinki. The study was reviewed and approved by the Institutional Review Board of Suining Central Hospital. Owing to compliance with the minimum risk exemption criteria of the Institutional Review Board, the requirement to obtain informed consent from each patient was waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Bleyer A. Young adult oncology: the patients and their survival challenges. Ca a Cancer J Clin. 2007;57(4):242–255. doi:10.3322/canjclin.57.4.242
2. Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nature reviews. Cancer. 2008;8(4):288–298. doi:10.1038/nrc2349
3. Magrath I, Epelman S. Cancer in adolescents and young adults in countries with limited resources. Curr Oncol Rep. 2013;15(4):332–346. doi:10.1007/s11912-013-0327-3
4. JM Llovet, J Zucman-Rossi, E Pikarsky. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7(1):7. doi:10.1038/s41572-021-00245-6
5. Yuen MF, Lai CL. Screening for hepatocellular carcinoma: survival benefit and cost-effectiveness. Ann Oncol. 2003;14(10):1463–1467. doi:10.1093/annonc/mdg400
6. An L, Zheng R, Zhang S, et al. Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: estimates based on data from 188 population-based cancer registries. Hepatobiliary Surg Nutr. 2023;12(1):45–55. doi:10.21037/hbsn-21-75
7. Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147–1155. doi:10.1038/bjc.2016.304
8. Chen CH, Chang TT, Cheng KS, et al. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver Int. 2006;26(7):766–773. doi:10.1111/j.1478-3231.2006.01309.x
9. Shim JJ, Kim GA, Oh CH, et al. Reduced liver cancer mortality with regular clinic follow-up among patients with chronic hepatitis B: a nationwide cohort study. Cancer Med. 2020;9(20):7781–7791. doi:10.1002/cam4.3421
10. Zhao C, Jin M, Le RH, et al. Poor adherence to hepatocellular carcinoma surveillance: a systematic review and meta-analysis of a complex issue. Liver Int. 2018;38(3):503–514. doi:10.1111/liv.13555
11. Chen Jr MS, Fang DM, Stewart SL, et al. Increasing hepatitis B screening for hmong adults: results from a randomized controlled community-based study. Cancer Epidemiol Biomarkers Prev. 2013;22(5):782–791. doi:10.1158/1055-9965.Epi-12-1399
12. van der Pol CB, Lim CS, Sirlin CB, et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology. 2019;156(4):976–986. doi:10.1053/j.gastro.2018.11.020
13. Park S, Choi S, Cho YA, et al. Evaluation of the American Joint Committee on Cancer (AJCC) 8th Edition Staging System for Hepatocellular Carcinoma in 1,008 Patients with Curative Resection. Cancer Res Treat. 2020;52(4):1145–1152. doi:10.4143/crt.2020.208
14. Liu Z, Mao X, Jin L, Zhang T, Chen X. Global burden of liver cancer and cirrhosis among children, adolescents, and young adults. Digestive Liver Dis. 2020;52(2):240–243. doi:10.1016/j.dld.2019.11.001
15. Rich NE, Yopp AC, Singal AG, Murphy CC. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clin Gastroenterol Hepatol. 2020;18(1):242–48.e5. doi:10.1016/j.cgh.2019.04.043
16. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol. 2016;34(15):1787–1794. doi:10.1200/jco.2015.64.7412
17. Ganesan P, Kulik LM. Hepatocellular Carcinoma: new Developments. Clin Liver Dis. 2023;27(1):85–102. doi:10.1016/j.cld.2022.08.004
18. Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol. 2020;16(5):284–296. doi:10.1038/s41574-020-0336-x
19. Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology. 2009;50(5):1392–1402. doi:10.1002/hep.23163
20. Li CL, Li CY, Lin YY, et al. Androgen Receptor Enhances Hepatic Telomerase Reverse Transcriptase Gene Transcription After Hepatitis B Virus Integration or Point Mutation in Promoter Region. Hepatology. 2019;69(2):498–512. doi:10.1002/hep.30201
21. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261. doi:10.1016/j.jhep.2019.08.025
22. Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: an evidence-based approach. World J Gastroenterol. 2019;25(13):1550–1559. doi:10.3748/wjg.v25.i13.1550
23. Grant-Alfieri A, Burke K, Zeinomar N, Delgado ML, Terry MB. Cancer Education Interventions in Adolescents: a Systematic Review of Scope and Content. Health Educ Behav. 2022;49(6):993–1003. doi:10.1177/10901981221109142
24. Coccia PF, Altman J, Bhatia S, et al. Adolescent and young adult oncology. Clinical practice guidelines in oncology. JNCCN. 2012;10(9):1112–1150. doi:10.6004/jnccn.2012.0117
25. Festa RS, Tamaroff MH, Chasalow F, Lanzkowsky P. Therapeutic adherence to oral medication regimens by adolescents with cancer. I Laboratory assessment.. J Pediatr. 1992;120(5):807–811. doi:10.1016/s0022-3476(05)80256-2
26. Grimm M, Link E, Albrecht M, Czerwinski F, Baumann E, Suhr R. Exploring Functions and Predictors of Digital Health Engagement Among German Internet Users: survey Study. J Med Internet Res. 2023;25:e44024. doi:10.2196/44024
27. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52. doi:10.2196/jmir.3951
28. Huang CY, Nguyen PA, Clinciu DL, et al. A personalized medication management platform (PMMP) to improve medication adherence: a randomized control trial. Comput Methods Programs Biomed. 2017;140(140):275–281. doi:10.1016/j.cmpb.2016.12.012
29. Stoner MCD, Smith L, Ming K, et al. Results From a Pilot Study of an Automated Directly Observed Therapy Intervention Using Artificial Intelligence With Conditional Economic Incentives Among Young Adults With HIV. J Acquired Immune Deficiency Syndrom. 2024;96(2):136–146. doi:10.1097/qai.0000000000003397
30. Castensøe-Seidenfaden P, Reventlov Husted G, Teilmann G, Hommel E, Olsen BS, Kensing F. Designing a Self-Management App for Young People With Type 1 Diabetes: methodological Challenges, Experiences, and Recommendations. JMIR mHealth and uHealth. 2017;5(10):e124. doi:10.2196/mhealth.8137
31. Jörntén-Karlsson M, Pintat S, Molloy-Bland M, Berg S, Ahlqvist M. Patient-Centered Interventions to Improve Adherence to Statins: a Narrative Synthesis of Systematically Identified Studies. Drugs. 2016;76(15):1447–1465. doi:10.1007/s40265-016-0640-x
32. Absolom K, Warrington L, Hudson E, et al. Phase III Randomized Controlled Trial of eRAPID: eHealth Intervention During Chemotherapy. J Clin Oncol. 2021;39(7):734–747. doi:10.1200/jco.20.02015
33. Yan H, Wang X, Liu X, et al. The survival strength of younger patients in BCLC stage 0-B of hepatocellular carcinoma: basing on competing risk model. BMC Cancer. 2022;22(1):185. doi:10.1186/s12885-022-09293-x
34. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
35. Rich NE, John BV, Parikh ND, et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology. 2020;72(5):1654–1665. doi:10.1002/hep.31159
36. Nathani P, Gopal P, Rich N, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021;70(2):401–407. doi:10.1136/gutjnl-2020-321040
37. Yuen VW, Wong CC. Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Invest. 2020;130(10):5052–5062. doi:10.1172/jci137553
38. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367–379. doi:10.1503/cmaj.1040752
39. Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46(12):2027–2049. doi:10.1093/clinchem/46.12.2027
40. Miksad RA, Ogasawara S, Xia F, Fellous M, Piscaglia F. Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: the LiverT study. BMC Cancer. 2019;19(1):795. doi:10.1186/s12885-019-5989-2
41. Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2261–2273. doi:10.1002/hep.26256
42. Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009–1016. doi:10.1002/cncr.29869
43. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet. 2017;18(12):1579–1589. doi:10.1016/s1470-2045(17)30677-0
44. Liu CY, Chen KF, Chen PJ. Treatment of Liver Cancer. Cold Spring Harbor Perspectives Medicine. 2015;5(9):a021535. doi:10.1101/cshperspect.a021535
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.