Back to Journals » Journal of Inflammation Research » Volume 18
Intermedin1-53 Improves Atherosclerosis by Reducing Local Endothelial Damage via AMPK Signaling Pathway in Obese apoE-Deficient Mice
Authors Zhu HX, Ren JL, Cao WJ, Wang R, Chen LL, Gao Q, Zhou YB
Received 13 December 2024
Accepted for publication 1 May 2025
Published 22 May 2025 Volume 2025:18 Pages 6583—6596
DOI https://doi.org/10.2147/JIR.S505695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Han-Xu Zhu,1,* Jin-Ling Ren,2,* Wen-Juan Cao,1 Rui Wang,3 Lei-Lei Chen,1,4 Qing Gao,1 Ye-Bo Zhou1
1Department of Physiology, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China; 2Department of Pathology and Pathophysiology, Henan University of Chinese Medicine, Zhengzhou, Henan, People’s Republic of China; 3Laboratory of Cardiovascular Bioactive Molecule, Peking University, Beijing, People’s Republic of China; 4Department of Cardiology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei-Lei Chen, Email [email protected] Qing Gao, Email [email protected]
Background: Atherosclerotic cardiovascular diseases (CVD) are commonly found in obesity. Endothelial inflammation accompanied by oxidative stress is a crucial risk factor and a key initiating step for the pathogenesis of atherosclerosis (AS). In the present study, the role and mechanism of intermedin (IMD), a potent active peptide, in endothelial damage in AS in obese apolipoprotein E-deficient (apoE−/−) mice were investigated.
Methods and Results: In vivo, IMD1-53 was infused via Alzet mini-osmotic pump in apoE−/− mice with high-fat diet (HFD) for 4 weeks. In vitro, palmitic acid (PA) and oxidized low density lipoprotein (Ox-LDL) were used to stimulate human umbilical vein endothelial cells (HUVECs) for exploring the potential mechanism of IMD1-53 action on endothelial damage. We found that IMD1-53 application remarkably improved plasma lipid profiles, hepatic lipid accumulation and its cholesterol levels, and vascular lipid accumulation and lesion sizes. Moreover, IMD1-53 markedly increased eNOS expression and decreased the levels of vascular inflammatory factors and ROS. In vitro, the combination of PA and Ox-LDL caused more severe inflammatory and oxidative damages and lower expression of eNOS, which were significantly inhibited by IMD1-53. IMD1-53 notably induced AMPK phosphorylation, and the inhibition of AMPK activation markedly reversed the anti-inflammatory and antioxidant effects of IMD1-53 on PA and Ox-LDL-treated HUVECs.
Conclusion: IMD1-53 improves AS partially by reducing endothelial inflammatory and oxidative damage via AMPK signaling pathway and decreasing vascular lipid accumulation involving the improvement of lipid profiles in blood and in liver in a state of obesity.
Keywords: intermedin, atherosclerosis, endothelium, inflammation, oxidative stress
Graphical Abstract:

Introduction
Cardiovascular diseases (CVD) have become one of the main causes of mortality worldwide, and atherosclerosis (AS) is a crucial underlying pathology of cardiovascular events such as stroke and myocardial infarction in obese people.1,2 It is well known that metabolic dysregulation and a chronic inflammatory state exist in obesity. Proinflammatory factors, adipokines and fatty acids are involved in the regulation of inflammatory responses, creating an inflammatory condition and promoting the initiation and progression of AS.3 Moreover, hyperlipidemia causes endothelial inflammatory and oxidative damage that is a well-defined risk factor of AS,4 especially apoB-containing low-density lipoproteins (LDL), which can drive the development of AS even in the absence of other risk factors.5 As a long chain saturated fatty acid, palmitic acid (PA) is a major component of free fatty acids in plasma that also promotes atherosclerotic progression and endothelial damage and dysfunction in people with obesity.6,7 Clinical trials for lipid-lowering drugs such as statins which can reduce atherosclerotic damage, but due to statins resistance or intolerance in some patients, as well as side effects in long-term use, the application of statins are limited.8 Furthermore, statins treatment alone decreased the risk of main vascular diseases by only about 40%-50%,9 suggesting that residual cardiovascular risks remain. Therefore, developing effective non-statin lipid-lowering therapies with fewer side effects are required for many patients who cannot be treated with statins alone. Meanwhile, in addition to lipid-lowering therapies, novel therapeutic approaches are needed to be identified.
As an independent atherosclerotic risk factor, obesity involves the enhanced systemic inflammation in obese individuals.10 AS is a lipid-driven, chronic inflammatory disease involving endothelial cells (ECs), smooth muscle cells and macrophages.11 Lipid accumulation and blood saturated fatty acids cause inflammatory and oxidative damages that are confirmed to be a key mechanism of AS, so it also provides a complementary therapeutic approach, namely lipid-lowering, anti-inflammatory and antioxidant therapies.12–14 At the innermost layer of aorta, endothelial cells serve as gatekeepers of aortic homeostasis by mediating vascular tone, leukocyte adhesion/extravasation, vascular permeability and hemostasis. Inflammatory or oxidative damages of ECs and endothelial dysfunction are crucial events in the initial step of AS, so during the initiation and progression of AS, endothelial cells are constantly subjected to inflammatory and oxidative damages.14,15 Endothelial dysfunction is an important risk factor for the progression of CVD, and ECs play multiple important roles throughout the whole process of AS, so protecting endothelial cells from inflammation and oxidative stress is pivotal for the therapeutic strategies.14–16
As an endogenous active peptide, intermedin (IMD) belongs to the calcitonin/calcitonin gene-related peptide (CGRP) family and involves cardiovascular homeostasis. It can exert multiple protective roles in some cardiovascular diseases.17 After proteolytic cleavage, IMD1-53 is produced from the prepro-peptide of IMD at Arg94-His95.18 IMD can exert various protective effects on endothelial cells under different harmful stimuli.19–22 For instance, IMD in vitro protects human umbilical vein endothelial cells (HUVECs) against amiodarone-induced damage or ischemia reperfusion injury,19,20 and it in vivo can relieve sepsis-caused inflammation in the endothelial barrier,21 and protect endothelial cell monolayers against thrombin-induced barrier failure.22 Previous studies have reported that IMD1-53 in apoE−/− mice could reduce atherosclerotic lesions by attenuating the macrophage foam-cell formation23,24 and modifying serum lipid profiles.25 Moreover, IMD1-53 can also attenuate atherosclerotic plaque vulnerability by alleviating endoplasmic reticulum stress regulated macrophage apoptosis, and subsequent NLRP3-caused inflammation.26 However, this study mainly explored the direct roles of IMD in endothelial inflammatory and oxidative damages and function, which could further complement the mechanism research of IMD in inhibiting AS. After all, obesity and endothelial dysfunction are important risk factors for the development of AS. Moreover, our previous studies have not demonstrated the direct role and mechanism of IMD in endothelial cells in the environment of high lipid metabolism.
As is well known, blood saturated fatty acids cause inflammatory and oxidative damages, which is a key factor of AS. Therefore, we want to focus on the role and mechanism of IMD under the condition of the combination of PA and Ox-LDL in endothelial cells. Mechanistically, we will clarify the exact signaling pathway of IMD, namely PKA, Akt or AMPK signaling, by which IMD exerts protective effects on endothelial cells treated with PA and Ox-LDL.
It is worth studying and elucidating the effect on endothelium and the related mechanism of IMD in AS in a state of obesity. So in this study, we aim to demonstrate the role and the related mechanism of IMD1-53 in endothelial damage in AS in apoE−/− mice with high-fat diet (HFD) feeding, and in vitro by using Ox-LDL together with PA in endothelial cells to simulate AS in obesity.
Materials and Methods
Materials
Human IMD1-53 was synthesized in Phoenix Pharmaceuticals Corp (Burlingame, CA, USA). Alzet Mini-osmotic Pumps (model 2004) were purchased from DURECT Corp (Cupertino, CA, USA). PA was purchased from (Kunchuang, Xian, China), Ox-LDL was from (Yeasen, Shanghai, China), and Compound C (AMPK activation inhibitor) was purchased from (Aladdin, Shanghai, China). Fetal bovine serum, streptomycin/penicillin, 0.25% trypsin-EDTA, DMEM and trypsin were bought from Thermo Fisher Scientific (Pudong New District, Shanghai, China). Primary antibodies for Mac-3, intercellular cell adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein 1 (MCP-1) were obtained from Abcam PLC (Cambridge, UK); and NOX2, NOX4 and GAPDH were purchased from Proteintech (SANYING, Wuhan, China); and CRLR, RAMP2, RAMP3, vascular cell adhesion molecule-1 (VCAM-1), TNF-α, T-Akt, P-Akt, Akt, P-Akt (ser473), AMPK, P-AMPKα (Thr172), PKA, P-PKA (Thr198), P-eNOS and eNOS were bought from Affinity Biosciences (Pottstown, PA, USA). The horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa C, CA, USA). The oil red O was bought from Sigma Aldrich (St. Louis, MO, USA). Other reagents and chemicals reached the analytical standards.
Animals and Treatment
Twenty-week-old male apoE−/− mice were obtained by Beijing HFK Bioscience Co., LTD, and they were randomly divided into three groups (n = 10 per group): (1) apoE−/− group: mice with normal diet for 4 weeks; (2) apoE−/− plus high-fat diet (HFD) group (apoE−/−+HFD group): mice with a HFD (fat provided 60% kilocalories) for 4 weeks; (3) apoE−/− plus HFD plus IMD1-53 group (apoE−/−+HFD+IMD1-53 group). At the same time as the HFD treatment, IMD1-53 (300 ng/kg/h) was dissolved in sterile saline and subcutaneously applied to the mice for 4 weeks by using Alzet mini-osmotic pumps (model 2004, Cupertino, CA, USA). All mice were fed at the Animal Center of Peking University Health Science Center (Beijing, China). The Animal Care Committee of Peking University Health Science Center (Beijing, China) approved the animal experimental protocols and care, and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011) were complied in this study.
Lipid Assay
At the end of experiment, the plasma from blood samples was collected by centrifugation. Separation of plasma lipoproteins including high density lipoprotein (HDL), very low density lipoprotein (VLDL) and low density lipoprotein (LDL) was performed using fast performance liquid chromatography (FPLC) as previously described.27 Plasma triglycerides (TG) and total cholesterol (TC) were tested by using colorimetric methods with kits from Zhong Sheng Biotechnology (Beijing, China).
Oil Red O Staining
Lipid accumulation in aorta, arterial roots or liver was shown by Oil-red O staining. Aortas from the heart to the iliac arteries were collected for en face analysis and opened longitudinally; kept the aortas for 2 hours in the working solution including Oil-red O following 70% ethanol for 5 min and washed with Deionized water. For liver or arterial roots samples analysis, the optimal cutting temperature (OCT) compound was used to embed the liver tissues and arterial roots with heart for obtaining frozen sections. 10 μm cross sections were prepared, and Oil red O was used for staining. As previously described,22 vascular lipid accumulation was assessed and quantified.
Hematoxylin/Eosin (HE) Staining
4% paraformaldehyde was used to fix the liver tissues for 24 hours, and the liver tissues were embedded in paraffin. As previously described,24 7-μm cross sections were cut for HE staining for morphological analysis.
Immunohistochemistry Staining
7-µm paraffin sections of arterial roots with the heart were used for Immunohistochemical staining. After rehydrated and antigen-retrieved, the sections underwent the incubation process with primary antibodies for Mac-3 (1:100 dilution), MCP-1 (1:100 dilution) and ICAM-1 (1:100 dilution) at 4°C overnight, then underwent another incubation process with HRP-conjugated secondary antibodies for 1 hour. They were stained with chromogen diaminobenzidine (Zhongshan Golden Bridge Biotechnology, Beijing, China). A Leica fluorescence microscopy (Leica Imaging Systems, Cambridge, UK) was used to acquire the images.
Cell Culture and Treatment
HUVECs were obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). Briefly, Endothelial Cells Medium (ECM, cat. #1001, Sciencell, USA) containing 100 U/mL penicillin and 100 μM/mL streptomycin and 10% fetal bovine serum (FBS) was used to culture HUVECs in an incubator with 5% CO2 at 37°C. 100 nM IMD1-53 was applied to cells for 30 min, then the cells were treated with PA combined with Ox-LDL for 24 h. For inhibition of signaling, Compound C (CC, 10 μM), namely AMPK activation inhibitor, was applied before IMD1-53 administration.
Measurements of ROS Level and NADPH Oxidase Activity
In HUVECs, the ROS level and the activity determination of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase were examined with the enhanced lucigenin-derived chemiluminescence method. To trigger the photon emission, both 100 µM NADPH and dark-adapted 5 µM lucigenin were added into the protein suspension, and the background chemiluminescence was examined using a luminometer (Turner, CA, USA). The data were obtained according to the average of ten measurements in 10 minutes and presented as the mean of light unit (MLU)/min/mg protein.
DHE Staining
DHE staining was used to detect reactive oxygen species (ROS) generation in HUVECs and arterial roots. 10 µM DHE in PBS was used to incubate HUVECs in six-well plates and paraffin-embedded tissue sections at 37°C in a dark environment for 30 min. Then, they were washed with PBS three times and observed by using a fluorescence microscopy (DP70, Olympus Optical, Tokyo, Japan).
Western Blotting
According to the manufacturer’s protocol, total proteins were extracted from cells or aorta with RIPA buffer (Epizyme, Shanghai, China) and quantified with a BCA protein assay kit (Beyotime, Shanghai, China). After electrophoresis and electrotransfer, the membranes were blocked with 5% non-fat milk for 3 hours at room temperature and incubated with CRLR (1:1000), RAMP1/2/3 (1:1000), ICAM-1 (1:2000), VCAM-1 (1:2000), NOX2 (1:2000), NOX4 (1:1000), eNOS (1:1000), TNF-α (1:1000), Akt (1:2000), p-Akt (Ser473, 1:2000), AMPK (1:2000), P-AMPKα (Thr172, 1:2000), PKA (1:2000), P-PKA (Thr198, (1:2000), P-eNOS (Ser1177, 1:2000) and GAPDH (1:3000) antibodies at 4°C overnight and then incubated with the corresponding HRP-conjugated secondary antibodies. Protein band intensities were normalized with nonphosphorylated total‐PKA, total‐Akt, total-AMPK or GAPDH levels. Odyssey Imaging System (LI‐COR Biosciences, Lincoln, Nebraska) was used to quantify the signals, and the protein expression level was assessed by Image J software.
Statistical Analysis
The GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA, USA) was used for Statistical analysis. All data are expressed as mean ± SEM. The unpaired Student’s t-test was used to identify significant differences between the two groups. Differences among the different animal groups were evaluated using one-way ANOVA followed by the Newman-Keuls test for more than 2 groups. Statistical significance was accepted at p < 0.05.
Results
IMD1-53 Alleviated Vascular Lipid Accumulation in apoE−/− Mice Fed with HFD
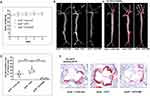
Intimal lipoprotein accumulation is the initial process in AS and drives the development of AS.13,14,28,29 To explore the effect of IMD1-53 on lipid accumulation in vascular wall, twenty-week-old male apoE−/− mice by feeding a HFD for 4 weeks were used to induce AS. Firstly, body weight (BW) was markedly increased in the apoE−/−+HFD group when compared to the apoE−/− group. However, there was no significant alteration in BW after IMD1-53 application (Figure 1A), which is consistent with previous study.22 After isolating aortas and opening longitudinally, we found much more white fatty streaks or fibrous plaque in the intima of apoE−/− mice with HFD, which were obviously improved by IMD1-53 application (Figure 1B). Oil Red O staining further confirmed a marked reduction of vascular lipid accumulation in IMD1-53-treated obese apoE−/− mice (Figure 1C). Moreover, IMD1-53 treatment resulted in an 84% reduction of lipid content in the aortic root (Figure 1D and E). Collectively, these results demonstrated that IMD1-53 decreased vascular lipid accumulation and atherosclerotic lesion size in obese apoE−/− mice.
IMD1-53 Improved Lipid Profiles in Plasma in apoE−/− Mice with HFD Feeding
Hyperlipidemia is a well-known risk factor of AS, and could induce endothelial dysfunction, which makes intimal retention of lipoprotein easily.29,30 Therefore, to explore the mechanism of IMD1-53 alleviating vascular lipid accumulation, we evaluated the circulatory lipid concentrations at different time points after HFD feeding and IMD1-53 treatment. As shown in Figure 2A, we could visually observe the notable improvement of lipids in plasma after IMD1-53 treatment. Indeed, IMD1-53 significantly improved the plasma lipid profiles at different time points including lowering the levels of TG, TC, LDL-C and VLDL-C compared with apoE−/− mice with HFD feeding (Figure 2B–F). Meanwhile, IMD1-53 markedly increased HDL-C levels (Figure 2G). These results suggest that IMD1-53 can reduce vascular lipid accumulation partly via improving lipid profiles in blood.
IMD1-53 Ameliorated Liver Lipid Accumulation in apoE−/− Mice with HFD Feeding
As well known, the liver is the main organ for cholesterol metabolism. Surprisingly, a significantly increased visceral adipose accompanied by fatty liver was visually observed in apoE−/− mice with HFD feeding, which was significantly reversed by IMD1-53 application (Figure 3A). Moreover, IMD1-53 treatment also markedly decreased the liver weight to BW ratio, hepatic TG and TC levels (Figure 3B–D). HE and Oil Red O staining also showed that apoE−/− mice with HFD feeding had a higher degree of steatosis, which was effectively relieved by IMD1-53 treatment (Figure 3E and F). These data indicate that IMD1-53 could ameliorate liver lipid accumulation in apoE−/− mice.
IMD1-53 Improved Endothelial Dysfunction by Inhibiting Inflammatory and Oxidative Damage in apoE−/− Mice with HFD Feeding
Vascular endothelium is an important barrier that is essential for the integrity and function of blood vessels. Hyperlipidemia can induce endothelial dysfunction which upregulates inflammation-related proteins or chemokines such as ICAM-1, MCP-1, and Mac-3.30 Indeed, our immunostaining results revealed that ICAM1, MCP-1 and Mac-3 were notably increased in apoE−/− mice with HFD feeding, and they were significantly reduced with IMD1-53 treatment (Figure 4A–C). Hyperlipidemia can also induce oxidative stress and decrease the expression of eNOS (being responsible for NO production which relaxes blood vessels and has antithrombotic and anti-inflammatory effects) in endothelium.31,32 In this study, we found that aortic superoxide anion levels were remarkably increased (Figure 4D and E), but eNOS expression was dramatically decreased in apoE−/− mice with HFD feeding, which were also markedly reversed by IMD1-53 treatment (Figure 4F). These results indicate that IMD1-53 improves endothelial dysfunction partially by reducing inflammatory response and oxidative damage and increasing eNOS expression.
IMD1-53 Inhibited Inflammatory and Oxidative Damage and Increased eNOS Activation via AMPK Signaling Pathway in HUVECs Stimulated by PA and Ox-LDL
PA and Ox-LDL-caused endothelial dysfunction is mainly involved in inflammation and oxidative stress.6,28 In the present study, PA was adopted to mimic the high-saturated fatty acids state of obesity, and Ox-LDL simulated dyslipidemia in AS. To verify whether IMD1-53 could suppress PA and Ox-LDL-evoked inflammatory and oxidative damage in HUVECs, we examined the protein levels of inflammation-related cytokines including VCAM-1, ICAM-1 and TNF-α, and oxidative stress-related indicators including the protein levels of NOX2 and NOX4 which are the essential catalytic subunits of NADPH oxidase, superoxide anion levels and NADPH oxidase activity. We found that they were further significantly increased in HUVECs-incubated by PA and Ox-LDL compared with PA treatment alone (Figure 5A–H), and which were effectively inhibited by the application of IMD1-53 (Figure 6A–H), suggesting that IMD1-53 has anti-inflammatory and antioxidant capacities in endothelium in AS state. The activation of PKA, Akt or AMPK signaling pathways involves IMD’s effects on inflammation and oxidative stress in previous studies.33–35 So the phosphorylation of PKA, Akt and AMPK was determined by Western blotting method in HUVECs stimulated by PA and Ox-LDL, respectively. As shown in Figure 7, IMD1-53 administration notably enhanced AMPK phosphorylation, rather than PKA and Akt phosphorylation (Figure 7A–C). Moreover, AMPK activation inhibitor significantly inhibited AMPK phosphorylation caused by IMD1-53 (Figure 7D), indicating that AMPK activation in endothelial cells may be associated with the protective mechanism of IMD1-53 on endothelial dysfunction. Indeed, the pretreatment of CC (an inhibitor of AMPK activation) effectively blocked the roles of IMD1-53 in inflammatory and oxidative damage in HUVECs stimulated by PA and Ox-LDL (Figure 8A–H). In addition, eNOS activation was determined by examining its phosphorylation level. The significant reduction of eNOS phosphorylation in HUVECs stimulated by PA and Ox-LDL was improved by IMD1-53 application, which was also inhibited by the inhibition of AMPK activation (Figure 8I). These results suggest that IMD1-53 via AMPK pathway can effectively inhibit PA and Ox-LDL-induced inflammatory and oxidative damage in HUVECs, and it may perform a protective role in endothelial dysfunction in AS in a state of obesity.
Discussion
Endothelial damage is associated with inflammation and oxidative stress, which promotes the progression of AS and predicts the future of cardiovascular events.14,36,37 We explored the role and the related mechanism of IMD1-53, a cardiovascular active peptide, in the endothelial damage of AS. ApoE-deficient dyslipidemic mice fed with an HFD were used in vivo study, and HUVECs-stimulated by PA and Ox-LDL were used in vitro research. The results showed that the coexistence of dyslipidemia and obesity indeed remarkably augmented AS involving the deterioration of lipid profiles in blood, increased vascular and hepatic lipid accumulation and hepatic cholesterol levels, and aggravated endothelial inflammation and oxidative stress, which effectively inhibited by IMD1-53 administration. In this study, PA and Ox-LDL further enhanced inflammatory and oxidative responses in HUVECs that were also significantly suppressed by IMD1-53 application. Moreover, IMD1-53 increased vascular eNOS expression and promoted AMPK activation, and AMPK inhibitor significantly reversed the roles of IMD1-53 in endothelial cells in vitro.
IMD, a novel active peptide of CGRP family, has important physiological and pathophysiological effects on the cardiovascular system.18,38 For instance, IMD exerts vasodilator effects to lower blood pressure, enhances myocardial contractility and increases the perfusion of heart.38 IMD application also shows protective effects in some CVD models, including ischemia-reperfusion injury,39,40 vascular calcification,41,42 abdominal aortic aneurysm,43 cardiac hypertrophy44 and cardiac fibrosis.33 Previous studies have found that IMD alleviated atherosclerotic lesions by suppressing the macrophage foam-cell formation in apoE−/− mice,19,20 increasing cholesterol efflux45 and modifying serum lipid profiles.21 Previous studies found that IMD1-53 could attenuate atherosclerotic plaque vulnerability via the inhibition of apoptosis triggered by endoplasmic reticulum stress, and subsequent NLRP3-caused inflammation in macrophages.22 Endothelium suffers from inflammatory and oxidative damages that serves interrelated roles in the progression of AS and other vascular diseases.12,14,37 However, the previous studies have not explored the exact role and the related mechanism of IMD1-53 in the endothelium, especially in AS under obesity condition. In this study, we not only discovered that IMD1-53 effectively inhibited endothelial inflammation and oxidative stress, but also clarified the possible mechanism that AMPK signaling pathway by which IMD1-53 plays a protective role in endothelial cells.
ApoE deficiency exacerbates AS. However, the coexistence of dyslipidemia and obesity synergistically exacerbates the severity of AS. It has been reported that obesity induced by HFD can intensify AS.22 The synergistic roles of dyslipidemia and obesity in endothelial damage are closely associated with a mass of metabolic factors, such as low high-density lipoprotein cholesterol levels, high triglyceride and glucose levels, and so on. In this study, apoE deficient mice with HFD diet resulted in a more severe lipid disorder in blood and more pronounced endothelial inflammatory response, oxidative stress and dysfunction correlated with decreased expression of eNOS, increased inflammatory factors VCAM-1, MCP and Mac-3, and elevated ROS levels. However, IMD1-53 administration not only significantly attenuated vascular and hepatic lipid accumulation, but also improved plasma lipid profiles. More importantly, IMD1-53 application effectively relieved endothelial inflammatory and oxidative damages, and increased eNOS expression. Therefore, our results suggest that IMD1-53 exerting a protective effect on endothelial damage may partially involve the suppression of endothelial inflammation and oxidative stress, and the improvement of lipid profiles in blood and lipid metabolism in liver. Previous studies indicate that metabolic dysfunction-associated steatotic liver disease is closely related to AS and cardiovascular disease.46,47 However, the precise effects and molecular mechanisms of IMD1-53 in improving lipid metabolism require further exploration.
As a saturated fatty acid, PA involves the inflammatory process in CVD.36 Under obesity condition, the increased PA can trigger a pro-inflammatory response in endothelium by activating immune cells such as macrophages.48 Ox-LDL is widely confirmed to involve inflammation and oxidative stress in endothelial cells.28 In this study, the results indicated that PA and Ox-LDL promoted marked endothelial damage which manifested by an obvious decrease in eNOS activation and notable increases in proinflammatory related factors and superoxide anion levels in HUVECs as well as the increased activity and protein expression (essential catalytic subunits NOX2 and NOX4) of NADPH oxidase. However, IMD1-53 application promoted the improvement of endothelial damage, including increasing eNOS activation and diminishing inflammatory factors levels, superoxide production and NADPH oxidase activity. So these data indicate IMD1-53 is a potent peptide in improving endothelial dysfunction in AS in obesity state.
AMP-activated protein kinase (AMPK) is a crucial enzyme in cellular metabolism and energy balance. It has been shown to exert protective roles in CVD involving its anti-inflammatory and antioxidant properties.49,50 The activation of the AMPK pathway can inhibit lipid accumulation and improve intracellular lipid metabolism.51 Moreover, AMPK activation can perform multiple anti-atherosclerotic roles to promote vascular health.50 A number of cholesterol-lowering drugs activate AMPK that facilitate their beneficial roles in the cardiovascular system.51,52 The downstream signals are activated by the interaction of IMD with its receptor, and the main signals identified include cAMP/PKA, PI3K/Akt and AMPK.18,38 It was reported that IMD can activate the phosphorylation of AMPK to exert its protective roles in some studies.35 In the present study, PA and Ox-LDL treatment caused the reduced AMPK activation in HUVECs, which was associated with decreased levels of AMPK phosphorylation, whereas IMD1-53 significantly upregulated AMPK phosphorylation but not Akt or PKA phosphorylation. Moreover, the inhibition of AMPK activation effectively prevented the anti-inflammatory and antioxidant roles of IMD1-53, which may account for the finding that IMD1-53 activating AMPK signaling pathway could improve endothelial damage via the suppression of inflammation and oxidative stress.
An intact endothelium is a major factor for the conservation of vascular homeostasis, and endothelial nitric oxide synthase (eNOS)-derived NO can relax vessels and exert antithrombotic and anti-inflammatory roles in the vasculature.53,54 The reduced NO bioavailability will lead to endothelial dysfunction involving the progression of hypertension and AS.54 AMPK activation can directly lead to eNOS phosphorylation which increases NO bioavailability in endothelial cells. However, AMPK inhibition reduces eNOS phosphorylation, leading to the impaired NO generation. In this study, IMD1-53 increased eNOS activation in PA and Ox-LDL-treated HUVECs, which was reversed by the inhibition of AMPK activation, suggesting that IMD1-53 increased eNOS activity to protect endothelial function against inflammatory and oxidative damages in AS in obese status.
Conclusions
In conclusion, obesity and dyslipidemia-associated risk factors, palmitic acid and Ox-LDL synergistically exacerbate endothelial inflammation, oxidative stress and dysfunction. IMD1-53 is a potent anti-atherosclerotic peptide by improving lipid profiles in blood and in liver and activating AMPK pathway to inhibit inflammatory and oxidative damages for relieving endothelial dysfunction. Thus, the ability of IMD1-53 for improving lipid profiles and endothelial dysfunction makes it a promising lead compound for the prevention and treatment of AS in people with obesity.
Data Sharing Statement
The data of this study will be available from the authors according to reasonable requests.
Consent for Publication
This study is comprised of animal data without any human data.
Acknowledgments
All the authors thank the support of the Collaborative Innovation Center of Cardiovascular Disease Translational Medicine.
Funding
This work was funded by the National Nature Science Foundation of China (81970356), the Foundation from the Department of Health of Jiangsu Province, China (LKM2023029), China Postdoctoral Science Foundation (2023M741089) and the Scientific and Technological Project of Henan Province (242102311253).
Disclosure
There are no competing interests.
References
1. Ozbeyaz NB, Gokalp G, Algul E, et al. Could systemic inflammation in healthy individuals with obesity indicate subclinical atherosclerosis? Angiology. 2023;74(1):62–69. doi:10.1177/00033197221089375
2. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circul Res. 2016;118(11):1723–1735. doi:10.1161/CIRCRESAHA.115.306825
3. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. doi:10.1172/JCI92035
4. Gallo G, Savoia C. New insights into endothelial dysfunction in cardiometabolic diseases: potential mechanisms and clinical implications. Int J Mol Sci. 2024;25(5):2973. doi:10.3390/ijms25052973
5. Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi:10.1161/CIRCULATIONAHA.106.676890
6. Choroszy M, Środa-Pomianek K, Wawrzyńska M, Chmielarz M, Bożemska E, Sobieszczańska B. The role of palmitic acid in the co-toxicity of bacterial metabolites to endothelial cells. Vasc Health Risk Manage. 2023;19:399–409. doi:10.2147/VHRM.S408897
7. Shrestha C, Ito T, Kawahara K, et al. Saturated fatty acid palmitate induces extracellular release of histone H3: a possible mechanistic basis for high-fat diet-induced inflammation and thrombosis. Biochem Biophys Res Commun. 2013;437(4):573–578. doi:10.1016/j.bbrc.2013.06.117
8. Reiner Z. Resistance and intolerance to statins. Nutrit Metabol Cardiovasc Dis. 2014;24(10):1057–1066. doi:10.1016/j.numecd.2014.05.009
9. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi:10.1016/S0140-6736(10)61350-5
10. Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol. 2015;31(2):177–183. doi:10.1016/j.cjca.2014.11.031
11. Liu M, Samant S, Vasa CH, et al. Co-culture models of endothelial cells, macrophages, and vascular smooth muscle cells for the study of the natural history of atherosclerosis. PLoS One. 2023;18:e0280385.
12. Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. doi:10.3390/ijms12053117
13. Smirnov AN. Lipid signaling in the atherogenesis context. Biochem Biokhimiia. 2010;75(7):793–810. doi:10.1134/S0006297910070011
14. Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. 2021;73(3):924–967. doi:10.1124/pharmrev.120.000096
15. Poredos P, Poredos AV, Gregoric I. Endothelial dysfunction and its clinical implications. Angiology. 2021;72(7):604–615. doi:10.1177/0003319720987752
16. Liao M, He X, Zhou Y, Peng W, Zhao XM, Jiang M. Coenzyme Q10 in atherosclerosis. Eur J Pharmacol. 2024;970:176481. doi:10.1016/j.ejphar.2024.176481
17. Ni X, Zhang J, Tang C, Qi Y. Intermedin/adrenomedullin2: an autocrine/paracrine factor in vascular homeostasis and disease. Sci China Life Sci. 2014;57(8):781–789. doi:10.1007/s11427-014-4701-7
18. Zhang SY, Xu MJ, Wang X. Adrenomedullin 2/intermedin: a putative drug candidate for treatment of cardiometabolic diseases. Br J Pharmacol. 2018;175(8):1230–1240. doi:10.1111/bph.13814
19. Wang Y, Yang R, Chen X, et al. Intermedin inhibits uptake of oxidized LDL via CD36 pathway in RAW264.7 cells. Die Pharmazie. 2014;69(6):473–476.
20. Dai XY, Cai Y, Sun W, et al. Intermedin inhibits macrophage foam-cell formation via tristetraprolin-mediated decay of CD36 mRNA. Cardiovasc Res. 2014;101(2):297–305. doi:10.1093/cvr/cvt254
21. Zhang X, Gu L, Chen X, et al. Intermedin ameliorates atherosclerosis in ApoE null mice by modifying lipid profiles. Peptides. 2012;37(2):189–193. doi:10.1016/j.peptides.2012.07.011
22. Ren JL, Chen Y, Zhang LS, et al. Intermedin(1-53) attenuates atherosclerotic plaque vulnerability by inhibiting CHOP-mediated apoptosis and inflammasome in macrophages. Cell Death Dis. 2021;12(5):436. doi:10.1038/s41419-021-03712-w
23. Wang Y, Wu Z, Tian J, et al. Intermedin protects HUVECs from ischemia reperfusion injury via Wnt/β-catenin signaling pathway. Renal Failure. 2019;41(1):159–166. doi:10.1080/0886022X.2019.1587468
24. Wang Y, Wang J, Yang J, et al. Study on protection of human umbilical vein endothelial cells from amiodarone-induced damage by intermedin through activation of wnt/ β -catenin signaling pathway. Oxid Med Cell Longev. 2021;2021(1):8889408. doi:10.1155/2021/8889408
25. Xiao F, Wang D, Kong L, et al. Intermedin protects against sepsis by concurrently re-establishing the endothelial barrier and alleviating inflammatory responses. Nat Commun. 2018;9(1):2644. doi:10.1038/s41467-018-05062-2
26. Aslam M, Pfeil U, Gündüz D, et al. Intermedin (adrenomedullin2) stabilizes the endothelial barrier and antagonizes thrombin-induced barrier failure in endothelial cell monolayers. Br J Pharmacol. 2012;165(1):208–222. doi:10.1111/j.1476-5381.2011.01540.x
27. Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50(3):574–585. doi:10.1194/jlr.D800028-JLR200
28. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Media Inflamm. 2013;2013:152786. doi:10.1155/2013/152786
29. Higashi Y. Endothelial function in dyslipidemia: roles of LDL-cholesterol, HDL-cholesterol and triglycerides. Cells. 2023;12(9):1293. doi:10.3390/cells12091293
30. Gimbrone MA Jr, García-Cardeña G. and García-Cardeña G: endothelial cell dysfunction and the pathobiology of atherosclerosis. Circulation Research. 2016;118(4):620–636. doi:10.1161/CIRCRESAHA.115.306301
31. Ponnuswamy P, Schröttle A, Ostermeier E, et al. eNOS protects from atherosclerosis despite relevant superoxide production by the enzyme in apoE mice. PLoS One. 2012;7:e30193.
32. Cheng LC, Guo BC, Chen CH, Chang CJ, Yeh TS, Lee TS. Endothelial nitric oxide mediates the anti-atherosclerotic action of torenia concolor Lindley var Formosama Yamazaki. Inter J Molecular Sci. 2020;21.
33. Zhang LS, Zhang JS, Hou YL, et al. Intermedin(1-53) inhibits NLRP3 inflammasome activation by targeting IRE1α in cardiac fibrosis. Inflammation. 2022;45(4):1568–1584. doi:10.1007/s10753-022-01642-z
34. Ni XQ, Zhang YR, Jia LX, et al. Inhibition of Notch1-mediated inflammation by intermedin protects against abdominal aortic aneurysm via PI3K/Akt signaling pathway. Aging. 2021;13(4):5164–5184. doi:10.18632/aging.202436
35. Liu SM, Zhang YR, Chen Y, et al. Intermedin alleviates vascular calcification in CKD through sirtuin 3-mediated inhibition of mitochondrial oxidative stress. Pharmaceuticals. 2022;15.
36. Karbasforush S, Nourazarian A, Darabi M, et al. Docosahexaenoic acid reversed atherosclerotic changes in human endothelial cells induced by palmitic acid in vitro. Cell Biochem Funct. 2018;36(4):203–211. doi:10.1002/cbf.3332
37. Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845. doi:10.1155/2019/8563845
38. Hong Y, Hay DL, Quirion R, Poyner DR. The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol. 2012;166(1):110–120. doi:10.1111/j.1476-5381.2011.01530.x
39. Yang SM, Liu J, Li CX. Intermedin protects against myocardial ischemia-reperfusion injury in hyperlipidemia rats. Gene Molecular Res. 2014;13(4):8309–8319. doi:10.4238/2014.October.20.7
40. Li H, Bian Y, Zhang N, et al. Intermedin protects against myocardial ischemia-reperfusion injury in diabetic rats. Cardiovasc Diabetol. 2013;12(1):91. doi:10.1186/1475-2840-12-91
41. Chang JR, Guo J, Wang Y, et al. Intermedin1-53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of α-Klotho. Kidney Int. 2016;89(3):586–600. doi:10.1016/j.kint.2015.12.029
42. Chen Y, Zhang LS, Ren JL, et al. Intermedin(1-53) attenuates aging-associated vascular calcification in rats by upregulating sirtuin 1. Aging. 2020;12(7):5651–5674. doi:10.18632/aging.102934
43. Lu WW, Jia LX, Ni XQ, et al. Intermedin 1− 53 attenuates abdominal aortic aneurysm by inhibiting oxidative stress. Arteriosclerosis Thrombosis Vasc Biol. 2016;36(11):2176–2190. doi:10.1161/ATVBAHA.116.307825
44. Zhang LS, Liu Y, Chen Y, et al. Intermedin alleviates pathological cardiac remodeling by upregulating klotho. Pharmacol Res. 2020;159:104926. doi:10.1016/j.phrs.2020.104926
45. Liao H, Wan S, Zhang X, Shi D, Zhu X, Chen X. Intermedin ameliorates atherosclerosis by increasing cholesterol efflux through the cAMP-PKA pathway in macrophage RAW264.7 cell line. Med Sci Monitor. 2017;23:5462–5471. doi:10.12659/MSM.907298
46. Yanai H, Adachi H, Hakoshima M, Iida S, Katsuyama H. Metabolic-dysfunction-associated steatotic liver disease-its pathophysiology, association with atherosclerosis and cardiovascular disease, and treatments. Int J Mol Sci. 2023;24(20):15473. doi:10.3390/ijms242015473
47. Thompson D, Mahmood S, Morrice N, et al. Fenretinide inhibits obesity and fatty liver disease but induces Smpd3 to increase serum ceramides and worsen atherosclerosis in LDLR(-/-) mice. Sci Rep. 2023;13(1):3937. doi:10.1038/s41598-023-30759-w
48. Zhou H, Urso CJ, Jadeja V. Saturated fatty acids in obesity-associated inflammation. J Inflamm Res. 2020;13:1–14. doi:10.2147/JIR.S229691
49. Qiu F, Yuan Y, Luo W, et al. Asiatic acid alleviates ischemic myocardial injury in mice by modulating mitophagy- and glycophagy-based energy metabolism. Acta Pharmacol Sin. 2022;43(6):1395–1407. doi:10.1038/s41401-021-00763-9
50. Jansen T, Kvandová M, Daiber A, et al. The AMP-activated protein kinase plays a role in antioxidant defense and regulation of vascular inflammation. Antioxidants. 2020;9(6). doi:10.3390/antiox9060525
51. Song YM, Lee YH, Kim JW, et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11(1):46–59. doi:10.4161/15548627.2014.984271
52. Zhang D, Zhang Y, Wang Z, Lei L. Thymoquinone attenuates hepatic lipid accumulation by inducing autophagy via AMPK / mTOR / ULK1 -dependent pathway in nonalcoholic fatty liver disease. Phytotherap Res. 2023;37(3):781–797. doi:10.1002/ptr.7662
53. Xie X, Zhang Z, Wang X, et al. Stachydrine protects eNOS uncoupling and ameliorates endothelial dysfunction induced by homocysteine. Molecular Med 2018;24(1):10. doi:10.1186/s10020-018-0010-0
54. Kim B, Zhao W, Tang SY, et al. Endothelial lipid droplets suppress eNOS to link high fat consumption to blood pressure elevation. J Clin Invest. 2023;133(24). doi:10.1172/JCI173160
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.