Back to Journals » Clinical Ophthalmology » Volume 18
Interocular Symmetry and Intermachine Reproducibility of Optic Disc and Macular Parameters Measured by Two Different Models of Optical Coherence Tomography
Authors Firdaus S, Pereira LF, Yang G , Huang-Link Y
Received 23 May 2024
Accepted for publication 8 July 2024
Published 26 August 2024 Volume 2024:18 Pages 2397—2406
DOI https://doi.org/10.2147/OPTH.S465360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Salma Firdaus,1,* Lívia Figueiredo Pereira,2,* Ge Yang,3 Yumin Huang-Link4
1Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia; 2Pontifical Catholic University of Minas Gerais, Belo Horizonte, Brazil; 3Huizhou Aier Eye Hospital, Aier Eye Hospital Group, Huizhou, Guangdong Province, People’s Republic of China; 4Division of Neurology, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
*These authors contributed equally to this work
Correspondence: Ge Yang, Huizhou Aier Eye Hospital, Aier Eye Hospital Group, Huizhou, Guangdong Province, People’s Republic of China, Email [email protected]
Purpose: To compare the interocular symmetry and investigate the intermachine reproducibility of optic disc and macular data measured by spectral-domain high-definition optical coherence tomography (HD-OCT) Cirrus HD-OCT 4000 and HD-OCT 5000 from healthy subjects.
Patients and Methods: Forty-three volunteers were examined with both HD-OCT 4000 and HD-OCT 5000 at the same visit. Optic nerve head (ONH) and macular data were acquired using ONH Cube 200× 200 scans and macular volume cube 512× 128 scans, respectively.
Results: The average age of the participants was 33 ± 8.6 years. Interocular OCT parameters of ONH and macula showed a high correlation between the right and left eyes regardless of HD-OCT models, displaying a low coefficient of variation (CV). However, the average retinal nerve fiber layer (RNFL) was thicker (96.67± 11.19μm vs 95.3± 10.89μm, p< 0.01), and the average central subfield thickness (261.51± 17.45μm vs 262.51± 17.39 μm, p< 0.01) and cube average thickness (283.91± 13.59μm vs 286.55± 13.09μm, p< 0.05) were thinner when measured by Cirrus 4000 compared to 5000. Intermachine reproducibility and reliability of RNFL and macular parameters exhibited a high intraclass correlation coefficient (ICC) (0.985) and low CV (2.4%). Ganglion cell-inner plexiform layer (GCIPL) measured by two OCT models showed similar values with an average thickness of 85 μm and had high intermachine reproducibility with high ICC (0.993) and low CV (1.2%).
Conclusion: High interocular symmetry was observed across both HD-OCT models. Intermachine reproducibility for RNFL and all macular parameters was also high. GCIPL showed minimal intermachine differences with high reproducibility and reliability. Thus, the results imply that GCIPL values measured by two Cirrus OCT models may be used interchangeably.
Keywords: neuro imaging, optical coherence tomography, interocular symmetry, intermachine reproducibility, retina, ganglion cell-inner plexiform layer, interchangeability
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Galvan has been published for this article.
Introduction
Optical coherence tomography (OCT), a non-invasive cross-sectional retinal imaging technique, was introduced in the 1990s.1 Currently, OCT is a key tool in ophthalmology for diagnosing maculopathies, and glaucoma, as well as determining effective therapies.2,3 Following the first report of OCT findings in multiple sclerosis (MS) in 1999,4 OCT has been rapidly applied in neurology to diagnose and monitor neuroinflammation and neurodegeneration conditions via quantitative measurements of ganglion cell-inner plexiform layer (GCIPL) around the macula and retinal nerve fiber layer (RNFL) around the optic disc.5–7 Furthermore, the application of OCT in measuring RNFL thickness at micrometer precision enables us to precisely and promptly evaluate therapeutic effects on intracranial hypertension and optic neuritis (ON).8,9
The Cirrus spectral domain high definition (HD)-OCT 4000 has been used since 2007 as a diagnostic device in the macula and RNFL.10,11 The model was upgraded to Cirrus 5000 in 2012 to take advantage of fast scan speed to enhance workflow efficiency and a fast tracker to compensate for blinking and eye movements.12,13 In 2019, a more advanced Cirrus HD-OCT 6000 was released. The average speed of acquiring the complete set of six scans was 94 seconds using Cirrus 6000, 152 seconds using Cirrus 5000, and 161 seconds using Cirrus 4000.14 The rapid emergence of these upgraded models has markedly improved practical processing, acquisition quality, and reduced speckle noises, thereby bringing significant progress to the retinal-neuron pathophysiology. Indeed, upgraded OCT enables the observation of ten different retinal microstructures, including GCIPL and RNFL, which are extracranial extensions of the brain, both in vivo and in real-time at the histological level. As known previously, the algorithms used in available machines differ and result in different results for retinal thickness measurement. Measures obtained from different OCT devices are in controversies regarding interchangeability.15–18 To our knowledge, there is limited data available on the interchangeability of different models of Cirrus HD-OCT.5,14,19 Given the high resolution and high sensibility of OCT measures at 1–7 micrometers, a few micrometers difference in examinations may lead to incorrect interpretations and misguide clinical direction.
Furthermore, the significance of interocular symmetry in clinical practice cannot be overstated. Establishing a baseline for comparative analysis between the eyes holds paramount importance, facilitating the detection of deviations or discrepancies indicating pathological conditions or disease progression. Monitoring and evaluating treatment efficacy rely heavily on maintaining symmetry, as deviations between eyes can offer insights into the success or failure of interventions. Therefore, careful consideration of interocular symmetry and intermachine reproducibility is integral to comprehensive clinical assessment, providing clinicians with a robust foundation for accurate diagnosis and effective treatment strategies.
It is necessary to compare the interocular symmetry between both eyes as well as the intermachine reproducibility of Cirrus HD-OCT in order to evaluate the interchangeability of Cirrus-OCT models. In this study, we aim to compare interocular symmetry of the optic disc and macular data measured by Cirrus HD-OCT 4000 and 5000 and evaluate the intermachine reproducibility in healthy subjects examined by both OCT models during the same visit.
Materials and Methods
Participants
Participants were randomly recruited for this study at the Department of Neurology, Linköping University Hospital, Sweden. Seventy-one persons were examined concurrently with both Cirrus HD-OCT 4000 and HD-OCT 5000 between February and July of 2022. The inclusion criteria of participants in this study were individuals aged 20 to 50 years old who provided consent for examination with two OCT models during the same visit. Exclusion criteria, considering interocular symmetry, were as follows: 1) abnormal fundus manifestations like papilledema, swelling, or atrophy of the optic disc visualized by ophthalmoscopy; or 2) eye disorders including retinal disease, a history of intraocular surgery, glaucoma, severe cataract, or refractive errors more than ±6 diopter; and 3) a history of diabetes, optic neuritis, or hypertension.
Ethical Approval
The study received approval from the Ethical Committee of Linköping University, Sweden (approval: study number 2022–07027-01). Prior to participating in the study, all participants were provided with detailed information regarding the purpose of the study. A written consent form was obtained before participants’ involvement in this study. This research adhered to ethical guidelines outlined in the World Medical Association Declaration of Helsinki.
Optical Coherence Tomography
Spectral-domain OCT examinations were performed on all participants without pupil dilatation using both Cirrus HD-OCT model 4000 and model 5000 (Carl Zeiss Meditec, Dublin, CA, USA). Two OCT machines were placed in separate dark rooms adjacent to each other. The intervals between HD-OCT 4000 and 5000 examinations were less than 10 minutes. The optic nerve head (ONH) was examined using the ONH Cube 200×200 protocol to obtain measures of RNFL thickness, rim area, disc area, and cup volume. The macula was examined using macular cube 512×128 protocol to obtain measures of central subfield thickness, cube volume, cube average thickness, and GCIPL. Macular GCIPL thickness is the sum of the ganglion cell layer plus the inner plexiform layer thickness. Scans were immediately visually inspected upon acquisition and repeated if necessary to meet acceptance criteria, scans with a signal strength above 7/10.15,20
Statistical Analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 27. The Shapiro–Wilk test was used to analyze data distributions. Interocular symmetry was analyzed through interocular correlation and differences. The interocular correlation of OCT parameters between the right and left eye was examined using Pearson or Spearman tests, depending on the data distribution. Interocular differences were presented using average values between both eyes, coefficient of variation (CV), and 5th and 95th percentile.16,21 Intermachine differences were presented as the mean difference ± standard deviation (SD). A paired t-test or Wilcoxon Matched-Pair Signed Ranked test, based on data distribution, was performed. Intermachine reproducibility was presented as CV, intraclass correlation coefficient (ICC) using two-way mixed methods, and a 95% confidence interval (CI). A CV close to 0 and an ICC close to 1.0 were regarded as the optimal values.22 Scatter plots were used to visualize the correlation of both models. The reproducibility of intermachine was examined using the Bland-Altman plot.22,23 Reproducibility coefficients were calculated as ±2 SDs of the differences between two OCT machines against the means of OCT parameters. A p-value equal to or less than 0.05 was considered statistically significant.
Results
Demographic Characteristics
Forty-three of 71 participants fulfilled the inclusion criteria after screening (Figure 1). The mean age was 33 ± 8.6 years, ranging from 20 to 50 years. Among the participants, twenty-eight of the participants (65%) were females.
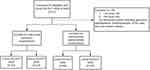 |
Figure 1 Participant eligibility assessment for interocular symmetry and intermachine reproducibility. |
Interocular Symmetry Measured by HD-OCT 4000 versus HD-OCT 5000
All ONH and macular parameters between the right and left eyes were significantly correlated regardless of measures from HD-OCT 4000 or HD-OCT 5000 (Table 1). The Spearman correlation coefficient for interocular symmetry was high for central subfield thickness (0.956 and 0.967), cube average thickness (0.951 and 0.942), cube volume (0.942 and 0.945), GCIPL (0.936 and 0.879) and RNFL (0.932 and 0.942) from HD-OCT 4000 and HD-OCT 5000, respectively. However, the two HD-OCT models demonstrated relatively low symmetry between the right and left eyes for the disc area, rim area, and cup volume.
 |
Table 1 Interocular Symmetry of Optic Disc and Macular Data from HD-OCT 4000 and HD-OCT 5000 |
OCT parameters from the right and left eyes were used to calculate CV for absolute agreement from each HD-OCT model and were used to assess interocular differences. Similar to the results of interocular symmetry, an interocular CV value below 10% was observed in the five aforementioned OCT parameters. From both HD-OCT models (HD-OCT 4000 vs 5000), the interocular difference and CV was found lowest in cube volume (mean difference 0.14 vs 0.13 mm3; CV 1.6%) and cube average thickness (mean difference 3.58 vs 3.74 µm; CV 1.6%), followed by central subfield thickness (mean difference 4.09 vs 3.40 µm; CV 2.0% vs 1.7%), GCIPL (mean difference 1.40 vs 1.53 µm, CV 2.4% vs 2.9%) and RNFL (mean difference 3.58 vs 3.02 µm, CV 4.3% vs 4.1%) (Table 2).
 |
Table 2 Intermachine Reproducibility of Optic Disc and Macular Data from HD-OCT 4000 and HD-OCT 5000 |
The interocular difference and CV were relatively high for rim area (mean difference 0.13 vs 0.10 mm2; CV 10.3% vs 8.5%) and disc area (mean difference 0.14 vs 0.17 mm2; CV 9.8% vs.18.1%). Cup volume had the highest interocular difference and CV (mean difference 0.040 vs 0.048 mm3; CV 207.4% vs 157.6%).
Intermachine Difference Measured by HD-OCT 4000 versus HD-OCT 5000
The average RNFL thickness was thicker (96.67±11.19 µm vs 95.3±10.89 µm, p<0.01) (Table 2), while the average central subfield thickness (261.51±17.45 µm vs 262.51±17.39 µm, p<0.01) and cube average thickness (283.91± 13.59 µm vs 286.55±13.09 µm, p<0.05) were thinner measured by HD-OCT 4000 compared to HD-OCT 5000. Cube volume (10.26± 0.48 mm3 vs 10.31± 0.47 mm3, p=0.052) was smaller measured by HD-OCT 4000 than by HD-OCT 5000 without statistical significance. However, average rim area, disc area, cup volume, and GCIPL did not differ significantly between the two models.
Intraclass correlation coefficient (ICC) and CV for an absolute agreement were calculated to assess intermachine reproducibility and reliability (Table 2). ICC values between 0 and 0.2 suggest low reliability; between 0.21 and 0.4 indicate fair reliability; between 0.41 and 0.6 imply moderate reliability; those between 0.61 and 0.8 imply high reliability, while values above 0.81 suggest almost perfect reliability. A CV of less than 10% was considered an acceptable reproducibility and less than 6% was considered as high reproducibility.
High intermachine reproducibility was found in central subfield thickness (CV 0.9%), GCIPL (CV 1.2%), cube volume (CV 1.5%), cube average thickness (CV 2.3%), RNFL (CV 2.4%), and rim area (CV 5.1%). Intermachine reliability expressed as ICC was greater than 0.9 for all OCT parameters, except cup volume (ICC 0.359). The highest intermachine reliability was observed in central subfield thickness (ICC 0.995), followed by GCIPL (ICC 0.993), RNFL (ICC 0.985), and cube volume (ICC 0.975). The disc area demonstrated acceptable reproducibility and reliability (CV 8.1%, ICC 0.953). However, cup volume exhibited low intermachine reproducibility (CV 23.7%) with relatively low reliability (ICC 0.359).
Scatter plots (Figure 2) showed the intermachine correlation of ONH and macular parameters measured by HD-OCT 4000 vs HD-OCT 5000. In line with the interocular symmetry results, central subfield thickness has the highest correlation between the two machines (R2 =0.983), followed by GCIPL (R2 = 0.972), RNFL (R2= 0.956), and cube volume (R2 = 0.910).
The Bland and Altman plots (Figure 3) showed the highest intermachine reproducibility for GCIPL with more than 97% of the average values falling within ±2 SDs of the differences between the two OCT models. RNFL, central subfield thickness, and cube volume had similar intermachine reproducibility of 95.3% of the average values falling within ±2 SDs of the differences between the two OCT models.
Discussion
In this prospective study, we evaluated the data for interocular symmetry of ONH and macular parameters in healthy subjects using both Cirrus HD-OCT 4000 and 5000 during the same visit. Furthermore, we compared the intermachine reproducibility of ONH and macular parameters. The correlations between right and left eyes were high, and differences between the eyes were small regardless of whether HD-OCT 4000 or 5000 was used. These results aligned with previous studies.17,21,22 Intermachine reproducibility was generally high for all parameters in the macula and RNFL in the ONH. Minimal intermachine differences were observed in macular parameters and RNFL. This suggests that the interchangeability of macular parameters, particularly for GCIPL, and RNFL measured with Cirrus HD-OCT models is feasible to some extent,16 but with certain considerations.10,17,24–26
To our knowledge, there exists a lack of data comparing extensive OCT parameters from both the ONH and macula across different HD-OCT models.16,19,27–29 OCT has increasingly been featured in longitudinal studies within ophthalmology and neurology.13,30,31 The results derived from our study offer valuable insights into both possibilities and limitations regarding the interchangeability of two models in clinical practice, research, and clinical trials.
Our findings highlight the high symmetry between right and left healthy eyes as detected by both HD-OCT models. Parameters related to the ONH, such as retinal nerve fiber layer (RNFL), rim and disc area, cup volume, and macular parameters, such as central subfield thickness, cube volume, cube average thickness, and ganglion cell-inner plexiform layer (GCIPL), exhibited high correlations between the left and right eyes, demonstrating minimal interocular differences. Notably, RNFL appeared as the most symmetric parameter within the ONH, while all macular parameters displayed high interocular symmetry.
Interocular symmetry of RNFL and cup volume was found among healthy children aged 5–17 years, whereas a comparable study by Song and Hwang noted an interocular asymmetry in the rim and disc area, as well as in GCIPL.21 In a Swedish population-based study, high interocular correlation was observed for RNFL, rim and disc area, and cup volume in healthy children aged 6–15 years.22 Among healthy adults, high interocular symmetry was noted for RNFL and GICPL.17 Understanding interocular differences in OCT parameters holds significance in evaluating neuro-ophthalmic conditions.24 Peripapillary RNFL and macular GCIPL are the most commonly used OCT parameters to diagnose and monitor demyelinating optic neuritis, idiopathic intracranial hypertension, papilledema, and ischemic optic neuropathies, and for evaluating therapeutic effects.5,17,24 Notably, interocular differences of 5 micrometers for RNFL and 4 micrometers for GCIPL are considered robust thresholds for identifying optic nerve lesions.17 Our data from two HD-OCT models support the premise that healthy eyes exhibit high interocular symmetry with minimal differences between the right and left eyes in both the ONH and the macula. Consideration of pathophysiological conditions should be considered when OCT reveals significant interocular asymmetry.
High intra- and inter-operator, as well as inter-visit reproducibility of spectral-domain OCT were reported.28,31,32 When considering intermachine reproducibility for Cirrus HD-OCT 4000 and 5000, the best reproducibility of OCT parameters was found in GCIPL. The average value of GCIPL thickness detected by the two OCT models was the same (85 µm) exhibiting a very minimal intermachine difference of 0.047 µm. This thickness closely resembled previous results from other studies conducted with Cirrus HD-OCT 4000.5,19 The coefficient of variation between the two OCT models was as small as 1.2% against the average value of GCIPL, indicating excellent reproducibility.22,23,25 The intraclass correlation coefficient of GCIPL thickness between Cirrus HD-OCT 4000 and 5000 was significantly high (ICC 0.993) suggesting perfect reliability. In addition, the Bland and Altman plot displayed the highest intermachine reproducibility with more than 97% of all the GCIPL values falling within the limit of the agreement of the two HD-OCT models. Taken together, GCIPL thickness showed high reproducibility with perfect reliability and low differences measured by two HD-OCT models, implying that GCIPL values measured by different OCT models may be used interchangeably.10,26,32
The intermachine correlation of RNFL, central subfield thickness, cube volume, and cube average thickness was significantly high when measured by the two OCT models. These parameters had identical reproducibility with 95.3% of the average values falling within ±2 SDs of the average values from the two OCT models. Furthermore, these four parameters also had high ICC, suggesting excellent reliability. However, significant intermachine differences were observed in these four parameters. Consequently, the interchangeability of RNFL, central subfield thickness, cube volume, and cube average thickness should be considered with caution, particularly in a longitudinal study that aims at determining therapeutic effects. In our study, rim and disc area, as well as cup volume showed low reproducibility and low reliability, diminishing their interchangeability.
This study has several limitations. It exclusively included the healthy eyes to investigate interocular symmetry, but intermachine reproducibility may require further investigation in both normal and abnormal eyes. The sample size was modest, and the study population was not based on age and gender. It predominantly comprised of female and subjects’ ages ranging from 20 to 50 years. A retrospective study involving 225 healthy eyes revealed no discernible difference between males and female concerning GCIPL and RNFL thickness.19 Nevertheless, it indicated thinner GCIPL and RNFL in older age. Subsequent studies encompassing larger and diversified populations across various age groups and equal gender distribution are essential to corroborate these findings. Moreover, the inclusion of more advanced Cirrus OCT models, such as the 6000 series, in such an investigation is crucial. Insights from such studies can guide clinicians and researchers in implementing data interchangeability across different OCT models.
Conclusion
In conclusion, this study highlights the high interocular symmetry and intermachine reproducibility of OCT parameters measured by both Cirrus HD-OCT 4000 and 5000. GCIPL has minimal intermachine differences and high reproducibility with exceptional reliability for both Cirrus HD-OCT 4000 and 5000. These results imply that GCIPL values obtained from both HD-OCT models may be used interchangeably.
Acknowledgments
The authors gratefully acknowledge the support of the Faculty of Medicine and Health Sciences at Linköping University and Linköping University Hospital. Special thanks for the support from the Henry and Ella Margareta Ståhl Foundation. This study was supported by the County Council of Östergötland and Linköping University Hospital, project numbers: LIO-799111, LIO-940688, LIO 941169. This paper has been uploaded to ResearchSquare as a preprint: https://www.researchsquare.com/article/rs-1970109/v1.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi:10.1126/science.1957169
2. Gabriele ML, Wollstein G, Ishikawa H, et al. Optical coherence tomography: history, current status, and laboratory work. Invest Ophthalmol Vis Sci. 2011;52(5):2425–2436. doi:10.1167/iovs.10-6312
3. Shin JW, Sung KR, Park SW. Patterns of progressive ganglion cell-inner plexiform layer thinning in glaucoma detected by OCT. Ophthalmology. 2018;125(10):1515–1525. doi:10.1016/j.ophtha.2018.03.052
4. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40(11):2520–2527.
5. Petzold A, Balcer LJ, Calabresi PA, et al.; ERN-EYE IMSVISUAL. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797–812. doi:10.1016/S1474-4422(17)30278-8
6. Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One. 2012;7(5):1–7. doi:10.1371/journal.pone.0036847
7. Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(2):521–533. doi:10.1093/brain/awr264
8. Albrecht P, Blasberg C, Ringelstein M, et al. Optical coherence tomography for the diagnosis and monitoring of idiopathic intracranial hypertension. J Neurol. 2017;264(7):1370–1380. doi:10.1007/s00415-017-8532-x
9. Huang-Link Y, Eleftheriou A, Yang G, et al. Optical coherence tomography represents a sensitive and reliable tool for routine monitoring of idiopathic intracranial hypertension with and without papilledema. Eur J Neurol. 2019;26(5):808–857. doi:10.1111/ene.13893
10. Syc SB, Warner CV, Hiremath GS, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 2010;16(7):829–839. doi:10.1177/1352458510371640
11. Stoor K, Karvonen E, Leiviskä I, Liinamaa J, Saarela V. Comparison of imaging parameters between OCT, GDx and HRT in the northern Finland birth cohort eye study. Acta Ophthalmol. 2022;100(5):1103–1111. doi:10.1111/aos.15046
12. Callaway NF, Park JH, Maya-Silva J, Leng T. THINKING LEAN: improving vitreoretinal clinic efficiency by decentralizing optical coherence tomography. Retina. 2016;36(2):335–341. doi:10.1097/IAE.0000000000000712
13. Han J, Byun MK, Lee J, Han SY, Lee JB, Han SH. Longitudinal analysis of retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in ethambutol-induced optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(12):2293–2299. doi:10.1007/s00417-015-3150-8
14. Makedonsky K, Fischer J, Durbin M. Workflow Efficiency of CIRRUS 6000 Compared to CIRRUS 5000 and CIRRUS 4000. Carl Zeiss Meditec Inc; 2020.
15. Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al.; IMSVISUAL consortium. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303–2309. doi:10.1212/WNL.0000000000002774
16. Yamashita T, Yamashita T, Shirasawa M, Arimura N, Terasaki H, Sakamoto T. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012;53(3):1102–1107. doi:10.1167/iovs.11-8836
17. Nolan-Kenney RC, Liu M, Akhand O, et al. International multiple sclerosis visual system consortium. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: an international study. Ann Neurol. 2019;85(5):618–629. doi:10.1002/ana.25462
18. Mahmoudinezhad G, Mohammadzadeh V, Amini N, et al. Detection of longitudinal ganglion cell/inner plexiform layer change: comparison of two spectral-domain optical coherence tomography devices. Am J Ophthalmol. 2021;231:1–10. doi:10.1016/j.ajo.2021.05.016
19. Xu X, Xiao H, Lai K, Guo X, Luo J, Liu X. Determinants of macular ganglion cell-inner plexiform layer thickness in normal Chinese adults. BMC Ophthalmol. 2021;21(1):267. doi:10.1186/s12886-021-02023-0
20. Huang-Link YM, Al-Hawasi A, Oberwahrenbrock T, Jin YP. OCT measurements of optic nerve head changes in idiopathic intracranial hypertension. Clin Neurol Neurosurg. 2015;130:122–127. doi:10.1016/j.clineuro.2014.12.021
21. Song MY, Hwang YH. Interocular symmetry of optical coherence tomography parameters in healthy children and adolescents. Sci Rep. 2022;12:653. doi:10.1038/s41598-021-04563-3
22. Larsson E, Molnar A, Holmström G. Repeatability, reproducibility and interocular difference in the assessments of optic nerve OCT in children- a Swedish population-based study. BMC Ophthalmol. 2018;18(1):270. doi:10.1186/s12886-018-0940-x
23. Napoli PE, Nioi M, Gabiati L, et al. Repeatability and reproducibility of post-mortem central corneal thickness measurements using a portable optical coherence tomography system in humans: a prospective multicenter study. Sci Rep. 2020;10(1):14508. doi:10.1038/s41598-020-71546-1
24. Minakaran N, de Carvalho ER, Petzold A, Wong SH. Optical coherence tomography (OCT) in neuro-ophthalmology. Eye. 2021;35(1):17–32. doi:10.1038/s41433-020-01288-x
25. Francoz M, Fenolland JR, Giraud JM, et al. Reproducibility of macular ganglion cell-inner plexiform layer thickness measurement with cirrus HD-OCT in normal, hypertensive and glaucomatous eyes. Br J Ophthalmol. 2014;98(3):322–328. doi:10.1136/bjophthalmol-2012-302242
26. Kumar KK, Prakash AA, Neeraja TG, Adappa KT, Prabha TS, Gangasagara SB. To compare central corneal thickness measurements obtained by Pentacam with those obtained by IOLMaster 700, Cirrus anterior segment optical coherence tomography and Tomey specular microscopy in normal healthy eyes. Indian J Ophthalmol. 2021;69(7):1713–1717. doi:10.4103/ijo.IJO_3364_20
27. Yamashita T, Miki A, Iguchi Y, Kimura K, Maeda F, Kiryu J. Reduced retinal ganglion cell complex thickness in patients with posterior cerebral artery infarction detected using spectral-domain optical coherence tomography. Jpn J Ophthalmol. 2012;56(5):502–510. doi:10.1007/s10384-012-0146-3
28. Pérez-García P, Morales-Fernández L, Fernández-Vigo JI, et al. Repeatability of macular and optic nerve head measurements by optical coherence tomography angiography in healthy children. Curr Eye Res. 2021;46(10):1574–1580. doi:10.1080/02713683.2021.1908568
29. Garcia-Martin E, Ara JR, Martin J, et al. Retinal and optic nerve degeneration in patients with multiple sclerosis followed up for 5 years. Ophthalmology. 2017;124(5):688–696. doi:10.1016/j.ophtha.2017.01.005
30. Tur C, Goodkin O, Altmann DR, et al. Longitudinal evidence for anterograde trans-synaptic degeneration after optic neuritis. Brain. 2016;139:816–828. doi:10.1093/brain/awv396
31. Mutlu U, Colijn JM, Ikram MA, et al. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol. 2018;75(10):1256–1263. doi:10.1001/jamaneurol.2018.1563
32. Garcia-Martin E, Pinilla I, Idoipe M, Fuertes I, Pueyo V. Intra and interoperator reproducibility of retinal nerve fibre and macular thickness measurements using Cirrus Fourier-domain OCT. Acta Ophthalmol. 2011;89(1):23–29. doi:10.1111/j.1755-3768.2010.02045.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



