Back to Journals » Journal of Inflammation Research » Volume 17
Interrelationships Between Inflammatory Score, Delayed Cerebral Ischemia and Unfavorable Outcome in Patients with aSAH: A Four-Way Decomposition
Authors Zhang P , Zhu H , Li X, Qian Y, Zhu Y, Zhang W , Yan Z, Ni H, Lin Z, Lin X , Li Z, Zhuge Q, Zeng B
Received 2 June 2024
Accepted for publication 8 December 2024
Published 14 December 2024 Volume 2024:17 Pages 11073—11085
DOI https://doi.org/10.2147/JIR.S481066
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam Bachstetter
Peng Zhang,1,2 Haiyang Zhu,1,2 Xinbo Li,1,2 Yiwei Qian,1,2 Yehao Zhu,1,2 Weizhong Zhang,1,2 Zhiyuan Yan,1,2 Haoqi Ni,1,2 Zhongxiao Lin,2,3 Xiao Lin,2,4 Zequn Li,1,2 Qichuan Zhuge,1,2 Bo Zeng1,2
1Department of Neurosurgery, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 2Zhejiang Provincial Key Laboratory of Aging and Neurological Disorder Research, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 3Department of Thoracic Surgery, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China; 4Department of Breast Surgery, the First Affiliated Hospital, Wenzhou Medical University, Wenzhou, 325035, People’s Republic of China
Correspondence: Bo Zeng, Email [email protected]
Background: To identify biomarkers and develop an inflammatory score based on proper integration to improve risk prediction of delayed cerebral ischemia (DCI) and poor outcome in patients with aneurysmal subarachnoid hemorrhage (aSAH). We also further explore the mediation and interaction of DCI within the chain of events using the four-way effect decomposition.
Methods: Machine learning algorithms are used for biomarker selection and constructed the inflammatory score. Multivariate logistic regression was performed to identify the association of inflammatory score with DCI and poor outcome. Next, we employed a four‐way decomposition to assess the extent to which the inflammation effect on the risk of poor outcome is mediated by or interacts with DCI. Finally, the additive value of inflammatory score was measured using the area under the curve (AUC), net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
Results: In total, 368 aSAH patients were included. The inflammatory score was calculated with the combination of lymphocyte, pan-immune-inflammation value (PIV), red blood cell distribution width (RDW), and lactate dehydrogenase (LDH). Multivariate analysis identified that inflammatory score was independently associated with DCI and poor outcome. The effect of high inflammatory score on poor outcome may be partly explained by DCI, where there is both pure mediation and mediated interaction. With DCI as a potential mediator, the excess relative risk could be decomposed into 30.86% controlled direct effect, 3.60% mediation only, 26.64% interaction only, and 38.89% mediated interaction. Adding the inflammatory score to the predictive model improved the AUC from 0.772 to 0.822, with an NRI of 5.3% and IDI of 6.9%.
Conclusion: The inflammatory score was significantly associated with DCI and poor outcome in patients with aSAH. Not only may be a potential synergistic interaction between high inflammatory score and DCI on the risk of poor outcome but also where DCI is an important mediating mechanism.
Keywords: aneurysmal subarachnoid hemorrhage, inflammatory score, delayed cerebral ischemia, poor outcomes, interaction analysis, mediation effect, four-way decomposition
Graphical Abstract:

Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease characterized by high mortality and severe disability.1 Delayed cerebral ischemia (DCI) occurs usually between 4 and 10 days following aSAH and is an important predictor of unfavorable functional outcome.2 As with many other diseases, aSAH has been demonstrated as a state of systemic inflammation and immunosuppression. Previous studies showed that inflammation infiltration is the crucial pathological characteristic of alterations in the biomechanics of intracranial aneurysm wall and contributes to aneurysm growth and rupture.3–5 Moreover, the inflammatory response after bleeding plays an important role in mediating intracranial and extracranial tissue damage as well.6,7 Correspondingly, peripheral blood inflammatory biomarkers may effectively reflect changes in the characteristics of inflammation response. Our previous study has revealed that the systemic inflammation response index on admission predicts poor functional outcome in patients with aSAH.8 However, the prognostic value of inflammatory biomarkers in aSAH patients and which parameters are the most predictive remain largely unknown. In addition, the accuracy of a single biomarker is not good enough. Another key clinical question is whether inflammation may cause DCI during hospitalization (as a mediator) which in turn causes poor outcome, or as an interaction, that is, patients with high inflammation level and DCI experience increased risk of poor outcome.
Hence, in this study, we aim to use machine learning (ML) methods to investigate and identify the most predictive biomarkers and develop an inflammatory score that incorporates these valuable indexes. We hypothesized that the inflammatory score might provide more accurate risk stratifications and prognostic values and is more meaningful than individual indicators. On the other hand, we further demonstrate the association between inflammatory score and DCI as well as functional outcome after aSAH and its potential mechanism within the chain of events.
Methods
Patient Population
We retrospectively analyzed patients with spontaneous SAH who were admitted to hospital between January 2019 and December 2021. This retrospective study was approved by the Ethics Committee in Clinical Research of First Affiliated Hospital of Wenzhou Medical University (KY2024-R162). To preserve patient confidentiality, all data were anonymized and de-identified. The detailed inclusion and exclusion criteria are shown in Appendix E1.
Patient Management and Data Collection
All patients were treated homogeneously according to the (inter)national guidelines.9,10 A detailed description is available in Appendix E2. The following data were recorded for each patient, including age, gender, smoking, alcohol drinking, and past medical history (hypertension, diabetes mellitus, heart disease, previous stroke, or hyperlipidemia), and clinical and radiological status on admission (Glasgow Coma Scale [GCS] score, World Federation of Neurosurgical Societies [WFNS] grade, Hunt Hess [HH] grade, modified Fisher Scale [mFS] grade, acute hydrocephalus). Moreover, morphological parameters of aneurysms were analyzed including aneurysm size, location, neck width, maximum height, body length, maximum width, aneurysm maximum height to neck diameter (AR) ratio, and aneurysm shape. Further, timing of treatment, procedural record, and final angiographic results were also recorded.11
Biomarkers Selection and Inflammatory Score Construction
The inflammatory biomarkers analyzed in this study were comprehensively evaluated using both single and derived parameters. Table S1 lists the 22 markers considered in this study, along with their mathematical definitions and references. The process of inflammatory risk score was described in Appendix E3. Additionally, we also built the hybrid model using a combination of patient clinical data.
Outcome Ascertainment
The primary outcome measure was the modified Rankin Scale (mRS) score at 6 months after discharge, and an mRS score ≥3 was considered as a poor outcome. The secondary outcome was the occurrence of DCI during hospitalization, defined in accordance with the criteria used by Vergouwen et al.12 In patients with DCI, the day of DCI onset after ictus was collected. DCI definition was described in Appendix E4.
Statistical Analysis
Data are presented as mean ± standard deviation (SD), median [interquartile range (IQR)], or number (percentage) as appropriate. The differences between groups were determined with t test, Mann–Whitney U-test, χ2 test or Fisher’s exact test wherever appropriate. To minimize selection bias and balance the baseline characteristics, two approaches, propensity score matching (PSM) and inverse probability of treatment weighting (IPTW), were used.13 Multivariate logistic regression analysis was performed to determine independent predictors of the poor outcome and DCI, adjusted by confounding variables according to the results of the univariate analysis (p ≤ 0.10). The collinearity of variable combinations entered into the multivariate logistic regression analysis was assessed using variance inflation factors (VIF) (WFNS grade, GCS score, HH grade, and mFS grade had VIFs ≥5, and hence they were removed).14
Interaction and stratified analyses were conducted to examine further the association between inflammatory score and poor outcome among different subgroups, as previously described.15–17 To evaluate the trends among different exposures, we performed the Cochran-Armitage and Jonckheere-Terpstra tests.18,19 We hypothesized that high inflammatory score has an interaction effect with DCI on the risk of poor outcomes and multiplicative and additive models were used by cross-analysis to calculate the interaction. The following interactive indicators were calculated, including the relative excess risk due to interaction (RERI), the attributable proportion due to interaction (AP), and the synergy index (SI).20 To assess the mediation effect of DCI, causal mediation analysis (CMA) was performed under a counterfactual framework, providing a general framework that offers clear definitions of causal mediation and related effects. We utilized the med4way command to obtain appropriate estimates of the four components, facilitating the estimation of the proportion of interaction or mediation.21 The details of constructing a four‐way decomposition analysis were presented in Appendix E5.
We evaluated the additive value of inflammatory score for poor outcome comparing predictive models with and without it. The discrimination performance of the models for predicting poor outcome was investigated with the area under the receiver operating characteristic curve (AUC), and DeLong test was performed to compare model performance. Calibration plots were used to compare the predicted values with the observed values, and decision curve analyses (DCA) were analyzed to assess the clinical utility and net benefits. We also used net reclassification improvement (NRI) with a category-free option and integrated discrimination improvement (IDI) calculations to quantify the improvement in actual reclassification and sensitivity resulting from the addition of inflammatory score.22 At last, the rms package was used to construct a nomogram model, and a web calculation was created based on the results of the study (https://zhangpeng.shinyapps.io/DynNomapp/). An overview of the study design is shown in Figure 1.
 |
Figure 1 Study flow diagram in the present study. |
We performed four‐way decomposition analysis using Stata 16.0, and other analyses were conducted with SPSS, version 25.0, software (SPSS, Inc.) and R language, version 4.2.0 (Feather Spray). All tests were two-sided, and a significant difference was indicated by p < 0.05.
Results
Patients’ Characteristics
A total of 368 consecutive patients with aSAH were included (Figure S1). The median age of patients was 55 years, and 40.8% were men. At admission, the median of GCS score was 14 (IQR 13–15), WFNS grade was 2.5 (IQR 1–3), HH grade was 2 (IQR 1–3), and mFS grade was 3 (IQR 2–4). DCI was detected in 20.7% (76/368) of all patients and occurred at median day 8 (range 4–15 days) after ictus (Figure S2). Based on the clinical outcome, 96 patients (26.1%) exhibited unfavorable outcome. The detailed characteristics of patients were presented in Table 1.
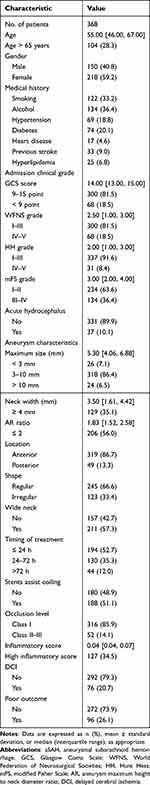 |
Table 1 Baseline Characteristics and Outcomes of 368 Patients with aSAH |
Development and Derivation of Inflammatory Score
Based on our pre-defined biomarker selection strategy, 5 features with non-zero coefficients including lymphocyte, pan-immune-inflammation value (PIV), red blood cell distribution width (RDW), and lactate dehydrogenase (LDH) were selected as important predictors (Figures S3 and S4). From the single models, support vector machine (SVM) showed the best discrimination capacity with the highest area under the receiver operating characteristic curve (AUC) of 0.747 [95% confidence interval (CI): 0.686–0.809] (Table S2).
Stratification by Inflammatory Score
The optimal cut-off value of inflammatory score was 0.046 for predicting the poor outcome, according to the principle of maximum Youden’s index. Subsequently, 241 patients (65.5%) and 127 patients (34.5%) were stratified into the low and high groups, respectively. The patients with high inflammatory score, who were older, had worse clinical status on admission, a greater amount of acute hydrocephalus and posterior circulation aneurysms, and a higher rate of endovascular treatment within 72 hours. Regarding the endpoints, we observed the DCI and poor outcome occurred more frequently in patients with high inflammatory score (Table S3). To account for confounding bias between patients in high and low groups, PSM and IPW were performed, after which two evenly balanced cohorts were available for the analysis of outcomes (Figure S5). Notably, the proportion of patients with DCI and poor functional outcome was significantly increased in high inflammatory score patients as well (Tables S4 and S5).
Associations of Inflammatory Score with DCI and Poor Outcome
Boxplot analysis revealed a significantly higher inflammatory score in patients with DCI and poor outcome, compared with those without (Figure S6). Subsequently, after adjustment for the confounders including age, hypertension, previous stroke, WFNS grade, acute hydrocephalus, and aneurysm location, high inflammatory score remained significantly and independently associated with DCI and poor outcome in aSAH patients. When included as a continuous variable, the selected inflammatory score also significantly contributed to the DCI and poor functional outcome, consistent with the results of the primary analysis (Tables S6 and S7).
Interaction Analysis Between Inflammatory Score and DCI on Poor Outcome
The patients were then divided into four subgroups according to the exposure definition. The incidence of poor outcome increased with the exposures, which reached 77.8% among patients with the high inflammatory score and DCI. The trend test also demonstrated that a trend in the proportion of poor functional outcome across increasing exposure definition and stratifications (p < 0.001). In the multivariable regression, individuals with high inflammatory score and DCI possessed the highest risk of poor functional outcome as compared with those with low inflammatory score and non-DCI (OR = 27.13, 95% CI: 11.93–61.67, p < 0.001). Meanwhile, the multiplicative interaction model showed that statistical significance between inflammatory score and DCI on the risk of poor outcome in both unadjusted and adjusted models (OR = 15.03, 95% CI: 7.06–32.02, p < 0.001; OR = 13.09, 95% CI: 5.80–29.52, p < 0.001). Additionally, a supra-additive effect did demonstrate in all indicators as well: RERI = 17.84 (95% CI: −1.50–37.17), AP = 0.77 (95% CI: 0.55–0.98), and SI = 5.07 (95% CI: 1.80–14.30). Table 2 presents the interaction effect in both additive and multiplicative scales. Measures quantifying interaction on an additive scale suggested a supra-additive effect of the combination of high inflammatory score and DCI on the risk of poor outcome (Figure 2).
 |
Table 2 The Cochran-Armitage Trend Test in the Prevalence of Poor Outcome, As Well As Multiplicative and Additive Interaction Between Inflammatory Score and DCI on Poor Outcome Risk |
Mediation Effect of Inflammatory Score on Poor Outcome via DCI
We conducted CMA to explore the direct and indirect effects of inflammation on the risk of poor outcome. For the unadjusted model, the mediator DCI explained 10.6% (95% CI: 5.2–16.0%; p < 0.001) of the association between inflammation and poor outcome when other covariates were not considered. After adjustment, the mediation effect was 7.8% and remained statistically significant (95% CI: 3.5–13.0%, p < 0.001; Figure 3).
Four-Way Decomposition of the Association Between Inflammatory Score and DCI as Well as Poor Outcome
Table S8 presents the results of the four-way decomposition analysis. The total effect was 7.26 (95% CI: 1.21–13.31), indicating a correlation between high inflammatory score and a heightened risk of poor outcome. The controlled direct effect (CDE), not involving mediation nor interaction accounted for 30.86% (excess relative risk = 2.24, 95% CI: 0.29–4.19). CDE implies a positive association between high inflammatory score and poor functional outcome even in the absence of DCI. INTref (excess relative risk = 1.93, 95% CI: −0.25–4.12) comprised 26.64% of the total effect estimate. This result, compared to the other three decomposed effects (CDE = 30.86%, INTmed = 38.89%, and PIE = 3.60%), suggests that the association between high inflammatory score and poor functional outcome is notably amplified among patients with DCI. In other words, DCI escalated the effect of inflammation on the risk of poor functional outcome. The proportion of mediated interaction (INTmed, excess relative risk = 2.82, 95% CI: −0.62–6.27) attributed to mediation and interaction was calculated to be 38.89%, indicating that DCI partially mediated the effect. This suggests that patients with high inflammatory score are more likely to develop DCI and higher risks of poor functional outcome. In addition, the mediating effects between high inflammatory score and DCI on poor functional outcome (the INTmed plus the PIE) accounted for 42.49% of the total effect, while the interaction effect between high inflammatory score and DCI on poor functional outcome (the INTref plus the INTmed) was 65.53%. Overall, the proportion eliminated (the INTref plus the INTmed plus the PIE) was 69.13% (Figure 4).
Incremental Predictive Value of Inflammatory Score for Unfavorable Outcome and Nomogram Establishment
The inflammatory score evaluated separately showed moderate discriminative powers to distinguish between favorable and unfavorable outcome at 6 months (Figure 5). DeLong test indicated that the hybrid model demonstrated a significant incremental prediction (p < 0.001). On the other hand, reclassification analyses showed that the hybrid model had a higher reclassification capacity (Table S9). In addition, calibration curves and decision curve analyses indicated that the hybrid model had better performance (Figures S7 and S8). Lastly, a prediction nomogram was established to inform the individual prediction of poor functional outcome for aSAH patients (Figure S9). To make this nomogram easier to use, a free web calculation was created here (Figure S10).
 |
Figure 5 ROC curve analysis of inflammatory score for predicting poor outcome in patients with aSAH. Abbreviations: ROC, receiver operating characteristic; aSAH, aneurysmal subarachnoid hemorrhage. |
Discussion
In the current study, we performed ML algorithms for variable selection from easily obtainable inflammatory biomarkers and developed a risk score. As expected, inflammatory score is independently associated with DCI and poor outcome in patients with aSAH. Additionally, there might be some positive interaction effects between high inflammatory score and DCI on poor outcome, and DCI serves as a mediator. Importantly, the hybrid model with inflammatory score had significantly better discrimination ability for poor outcome and could serve in clinical decision-making.
Indeed, these associations between the involvement of systemic inflammation and aneurysmal pathophysiology are now evident.23,24 These peripheral blood parameters and derived indices are well suited for the task of host immune and inflammatory responses as they are scalability, cost-effectiveness, and availability. However, single indicator cannot fully reflect comprehensive characteristics with unsatisfactory sensitivity and specificity. In addition, some studies incorporated these indicators of strong collinearity and correlation into a multivariate regression analysis to investigate independent prognostic factors, causing interference between variables and certain statistical problems. Hence, the current study first has done an initial dimensionality reduction and used the resulting orthogonal components as input for the least absolute shrinkage and selection operator analysis, and it is expected to deliver more precise parameter estimates. As shown in the result, key variables that differentiated the clinical outcomes in aSAH patients included lymphocyte, PIV, RDW, and LDH. Consistent with previous reports, emerging studies have shown these biomarkers to be informative in the pathophysiology after aSAH and the subsequent risk of poor functional outcome.25–28 Thereafter, the selected features were entered into various supervised ML classifiers to construct the best inflammatory score, which is akin to the canonical approach used in polygenetic risk studies.29,30 It is important to note that we found that the SVM model of these 5 biomarkers had great performance in identifying the risk of poor outcome in patients with aSAH.
Furthermore, we combined the inflammatory score and established clinical parameters to find out whether this changed the risk estimate. It was proved that an improvement in risk prediction by inclusion of the inflammatory score can most probably be accomplished for individuals who suffered a poor outcome after aSAH. Next, we developed a nomogram with good calibration based on the hybrid model, which can be used to identify patients at risk of the poor outcome and select more appropriate treatment options. Finally, we have created an easy-to-use web calculator to make it easily accessible to the general public. Briefly, it is satisfying that the inflammatory score, which is derived from the various available serological parameters, can be used in routine clinical practice. On the other hand, the dynamic nomogram could facilitate translation of this work to clinical trials to determine whether patients are on the risk of poor functional outcome. Indeed, the use of the nomogram and its benefit in patients with aSAH remains to be validated in an independent cohort.
Another advantage of our study is that the present investigation is one of the pioneer studies for exploring the interplay between inflammation and DCI on the risk of poor outcome, and DCI as a mediator within the chain of events. According to the literatures, the cascaded inflammatory responses are a predominant characteristic of aSAH, participating in the complex process of pathophysiology.23,24,31 Sudden ruptures of intracranial aneurysm dissolved subarachnoid clots entering cerebrospinal fluid circulation, and catecholamine surge may trigger the inflammation activation, which in turn regulates and is regulated by the inflammatory process. However, fewer studies investigated the potential mechanism of inflammation within the event chain. Our findings provide new evidence that there might be a strong and synergistic interaction between inflammation and DCI on the risk of poor functional outcome, and DCI plays an important mediating mechanism. This implies that the consequent derailment of inflammatory response is a devastating event in aSAH development and the potential therapeutic implications by eliminating inflammatory reactions. Note that due to the mediation effect of DCI, the possible reduction in risk would occur if DCI was treated.32
Therefore, based on a potential causal chain of high inflammatory score-DCI-poor outcome, more intensive measures for DCI prevention should be considered for aSAH patients with high inflammatory score at admission.32,33 Regretfully, the INTref, INTmed and PIE were not statistically significant in the four-way decomposition methods. We speculate that this may be due to the sample size, which could not provide sufficient statistical strength to perform sophisticated models. With a larger sample size, it is possible that statistical significance may be achieved. Nonetheless, we believe that incorporating this analysis underscores the potential significance of these interactions. Using mediation analysis within a counterfactual framework aimed at dissecting the relationships between inflammation, DCI, and outcomes in a nuanced way, this methodology has been shown to provide deeper insights into causal pathways in similar studies.6,21,34 Overall, our study shows that interaction and mediation analysis in clinical research may provide insights into the pathophysiology of clinical outcomes and offers valuable insights into public health interventions, including the great potential of the prioritizing DCI prevention and personalized risk assessment and management strategies for aSAH patients. Certainly, we agree that the retrospective design and sample size limit the robustness of the findings. In the future, further studies are also warranted to clarify the biological features of brain inflammation after aSAH and its impact on pathology mechanisms.
Strengths and Limitations
The present study has several strengths. First, our study comprehensively takes into account the various blood inflammatory biomarkers and derived parameters at admission. Moreover, we used for proper adjustment in the ML methods and developed an inflammatory score. On the other hand, this study represents a pioneering effort in simultaneously examining the relationship between high inflammatory score, DCI, and poor outcome using a four-way decomposition method. Additionally, we observed a clear dose–response relationship between the combined effect of high inflammatory score and DCI on the risk of poor outcome and the additive predictive value of inflammatory score. Nevertheless, several limitations should also be noted. First, this study is retrospective, which potentially limits our interpretation. Hence, we employ PSM and IPTW techniques to minimize these biases, which, while helpful, cannot completely eliminate the limitations of retrospective design. Second, no independent cohorts from other centers are available for our analyses, preventing validation of the associations found in this study. Third, even though the 22 markers measured in this study cover a broad spectrum of important and promising inflammatory markers, many other immune and inflammatory markers were not included, such as those in the interleukin family. Considering the cost-effectiveness and easy availability, we focused mainly on these markers referred to as peripheral blood-based parameters. Indeed, research focusing on the significance of interleukin family members should be conducted in the future. Additionally, we only measured inflammatory markers available at the time of admission, whereas a multi-time-point evaluation is probably more appropriate to validate the predictive value of biomarkers. Lastly, the number of patients enrolled in this study was relatively small. Therefore, caution should be exercised in generalizing its results, and future studies are needed. Currently, we plan to carry out a multi-centre prospective observational study to evaluate the longitudinal association between the inflammatory biomarkers and the prognosis as well as DCI of aSAH patients. Hope we have future opportunities to share the research results with you.
Conclusion
In this study, we developed an inflammatory score consisting of appropriate integration of lymphocyte, PIV, RDW, and LDH that is independently associated with the DCI and poor outcome in patients with aSAH. In addition, the effect of high inflammatory score on the risk of poor outcome may partly be explained by the DCI, where there is both pure mediation and mediated interaction. Importantly, the inflammatory score showed to be an incremental predictive value for poor outcome. However, the effects between them were rather complex. Future studies are warranted to verify our findings and elucidate their exact role in aSAH pathophysiology.
Data Sharing Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Ethics Approval
This retrospective study was approved by the institutional review board (KY2024-R162), and informed consent was waived because all data were anonymized and de-identified. All procedures performed were in accordance with the 1964 helsinki Declaration and its later amendments or comparable ethical standards.
Acknowledgments
We thank all the staff and participants for their contribution to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Wenzhou Municipal Sci-Tech Bureau Program (Grant No. 2024Y0475).
Disclosure
The authors declare that they have no competing interests.
References
1. Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet. 2022;400(10355):846–862. doi:10.1016/s0140-6736(22)00938-2
2. Savarraj JPJ, Hergenroeder GW, Zhu L, et al. Machine Learning to Predict Delayed Cerebral Ischemia and Outcomes in Subarachnoid Hemorrhage. Neurology. 2021;96(4):e553–e62. doi:10.1212/wnl.0000000000011211
3. Signorelli F, Sela S, Gesualdo L, et al. Hemodynamic Stress, Inflammation, and Intracranial Aneurysm Development and Rupture: a Systematic Review. World Neurosurg. 2018;115:234–244. doi:10.1016/j.wneu.2018.04.143
4. Labeyrie PE, Goulay R, Martinez de Lizarrondo S, et al. Vascular Tissue-Type Plasminogen Activator Promotes Intracranial Aneurysm Formation. Stroke. 2017;48(9):2574–2582. doi:10.1161/strokeaha.117.017305
5. Signorelli F, Pailler-Mattei C, Gory B, et al. Biomechanical Characterization of Intracranial Aneurysm Wall: a Multiscale Study. World Neurosurg. 2018;119:e882–e9. doi:10.1016/j.wneu.2018.07.290
6. Li T, Li R, Lin F, et al. A Mediation Analysis of the Association Between Systemic Inflammation Response Index, in-Hospital Complications, and Poor Long-Term Functional Outcomes in Patients with Aneurysmal Subarachnoid Hemorrhage: insights from a Large Prospective Cohort Study. J Inflamm Res. 2024;17:3697–3708. doi:10.2147/jir.S460364
7. Chai CZ, Ho UC, Kuo LT. Systemic Inflammation after Aneurysmal Subarachnoid Hemorrhage. Int J Mol Sci. 2023;24(13). doi:10.3390/ijms241310943
8. Zhang P, Li Y, Zhang H, et al. Prognostic value of the systemic inflammation response index in patients with aneurismal subarachnoid hemorrhage and a Nomogram model construction. Br J Neurosurg. 2023;37(6):1560–1566. doi:10.1080/02688697.2020.1831438
9. Hoh BL, Ko NU, Amin-Hanjani S, et al. Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: a Guideline From the American Heart Association/American Stroke Association. Stroke. 2023;54(7):e314–e70. doi:10.1161/str.0000000000000436
10. Fraser JF, Heit JJ, Mascitelli JR, et al. Decoding the data: a comment on the American Heart Association/American Stroke Association (AHA/ASA) 2023 Guideline for the Management of patients with Aneurysmal Subarachnoid Hemorrhage. J Neurointerv Surg. 2023;15(9):835–837. doi:10.1136/jnis-2023-020675
11. Fuga M, Ishibashi T, Aoki K, et al. Intermediate catheter use is associated with complete occlusion and dense packing in coil embolization of unruptured cerebral aneurysms: a propensity score matched study. J Neurointerv Surg. 2024:
12. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–2395. doi:10.1161/strokeaha.110.589275
13. Tong Y, Cai R, Li JX, et al. Liver resection versus microwave ablation for hepatocellular carcinoma in ideal candidates for ablation per Barcelona Clinic Liver Cancer staging: a propensity score matching and inverse probability of treatment weighting analysis. Aliment Pharmacol Ther. 2022;56(11–12):1602–1614. doi:10.1111/apt.17263
14. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72(6):558–569. doi:10.4097/kja.19087
15. Wang Q, Zhang J, Zhao K, et al. Hyperhomocysteinemia is an independent risk factor for intracranial aneurysms: a case-control study in a Chinese Han population. Neurosurg Rev. 2020;43(4):1127–1134. doi:10.1007/s10143-019-01138-9
16. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163–94. doi:10.7326/0003-4819-147-8-200710160-00010-w1
17. Rashid R, Sohrabi C, Kerwan A, et al. The STROCSS 2024 guideline: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2024. doi:10.1097/js9.0000000000001268
18. Kouda K, Iki M, Fujita Y, et al. Trends in Serum Lipid Levels of a 10- and 13-Year-Old Population in Fukuroi City, Japan (2007-2017). J Epidemiol. 2020;30(1):24–29. doi:10.2188/jea.JE20180164
19. Zhang P, Wang H, Bao H, et al. Non-invasive Liver Fibrosis Scores Are Associated With Recurrence of Postoperative Chronic Subdural Hematoma. Front Neurol. 2022;13:873124. doi:10.3389/fneur.2022.873124
20. Knol MJ, VanderWeele TJ, Groenwold RH, et al. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26(6):433–438. doi:10.1007/s10654-011-9554-9
21. Discacciati A, Bellavia A, Lee JJ, et al. Med4way: a Stata command to investigate mediating and interactive mechanisms using the four-way effect decomposition. Int J Epidemiol. 2018. doi:10.1093/ije/dyy236
22. Demler OV, Pencina MJ, Cook NR, et al. Asymptotic distribution of ∆AUC, NRIs, and IDI based on theory of U-statistics. Stat Med. 2017;36(21):3334–3360. doi:10.1002/sim.7333
23. Coulibaly AP, Provencio JJ. Aneurysmal Subarachnoid Hemorrhage: an Overview of Inflammation-Induced Cellular Changes. Neurotherapeutics. 2020;17(2):436–445. doi:10.1007/s13311-019-00829-x
24. Zeyu Z, Yuanjian F, Cameron L, et al. The role of immune inflammation in aneurysmal subarachnoid hemorrhage. Exp Neurol. 2021;336:113535. doi:10.1016/j.expneurol.2020.113535
25. Li S, Zhang J, Li N, et al. Predictive nomogram models for unfavorable prognosis after aneurysmal subarachnoid hemorrhage: analysis from a prospective, observational cohort in China. CNS Neurosci Ther. 2023;29(11):3567–3578. doi:10.1111/cns.14288
26. Han W, Yi HJ, Shin DS, et al. Pan-immune-inflammation value predict delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2024;121:47–52. doi:10.1016/j.jocn.2024.02.003
27. Siegler JE, Marcaccio C, Nawalinski K, et al. Elevated Red Cell Distribution Width is Associated with Cerebral Infarction in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care. 2017;26(1):26–33. doi:10.1007/s12028-016-0306-2
28. Zan X, Deng H, Zhang Y, et al. Lactate dehydrogenase predicting mortality in patients with aneurysmal subarachnoid hemorrhage. Ann Clin Transl Neurol. 2022;9(10):1565–1573. doi:10.1002/acn3.51650
29. Morgenroth CL, Kleymann P, Ripke S, et al. Polygenetic risk scores and phenotypic constellations of obsessive-compulsive disorder in clozapine-treated schizophrenia. Eur Archiv Psychiat Clin Neurosci. 2024;274(1):181–193. doi:10.1007/s00406-023-01593-y
30. Zhou D, Yu D, Scharf JM, et al. Contextualizing genetic risk score for disease screening and rare variant discovery. Nat Commun. 2021;12(1):4418. doi:10.1038/s41467-021-24387-z
31. Gris T, Laplante P, Thebault P, et al. Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J Neuroinflammation. 2019;16(1):253. doi:10.1186/s12974-019-1629-7
32. Wang X, Zhang Y, Chong W, et al. Association of Rebleeding and Delayed Cerebral Ischemia with Long-term Mortality Among 1-year Survivors After Aneurysmal Subarachnoid Hemorrhage. Curr Neurovasc Res. 2022;19(3):282–292. doi:10.2174/1567202619666220822105510
33. Ahn SH, Savarraj JPJ, Parsha K, et al. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J Neuroinflammation. 2019;16(1):213. doi:10.1186/s12974-019-1578-1
34. Tönnies T, Schlesinger S, Lang A, et al. Mediation Analysis in Medical Research. Dtsch Arztebl Int. 2023;120(41):681–687. doi:10.3238/arztebl.m2023.0175
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




