Back to Journals » Clinical Ophthalmology » Volume 19
Intraocular Pressure Control in Vitreoretinal Surgical Systems
Authors Charles M, Zhu Y, Garufis C, McDonell B, Wuyyuru VD
Received 28 February 2025
Accepted for publication 22 May 2025
Published 29 June 2025 Volume 2025:19 Pages 2047—2056
DOI https://doi.org/10.2147/OPTH.S525285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Martin Charles,1 Ying Zhu,2 Carrie Garufis,2 Brian McDonell,2 Varalakshmi D Wuyyuru2
1Charles Centro Oftalmológico, Buenos Aires, Argentina; 2Alcon Research LLC, Lake Forest, CA, USA
Correspondence: Martin Charles, Charles Centro Oftalmológico, Riobamba 841, C1116, CABA, Buenos Aires, Argentina, Email [email protected]
Purpose: A comparison was made between the Constellation® Vision System with intraocular pressure (IOP) control and the Enhancing Visual Acuity (EVA) system with automatic infusion compensation (AIC) during a simulated vitrectomy in a closed eye model.
Patients and Methods: An acrylic eye model was connected to a vitrectomy probe, an infusion cannula, and a pressure transducer. The Constellation and EVA systems were tested using 25-gauge probes in vacuum mode with vacuum settings at 250, 450, and 650 mmHg. The target IOP was 30 mmHg. Average IOP was assessed before aspiration (initial IOP), during vitreous removal (vitreous IOP), and after vitreous removal and replacement with Alcon’s BSS® sterile irrigation solution (BSS IOP).
Results: Using Constellation’s IOP control, target IOP (30± 2.5 mmHg) was maintained 93%, 74%, and 63% of the time at 250, 450, and 650 mmHg of vacuum, respectively. At 650 mmHg, initial IOP, vitreous IOP, and BSS IOP were 30.18± 0.18, 30.99± 0.66, and 31.55± 1.38 mmHg. Using EVA with AIC, target IOP was maintained 16%, 17%, and 23% of the time at 250, 450, and 650 mmHg of vacuum. At 650 mmHg, initial IOP, vitreous IOP, and BSS IOPs were 30.54± 0.08, 44.62± 1.24, and 29.92± 0.72 mmHg (P< 0.0001).
Conclusion: When confronted with dynamic fluid conditions during vitreous removal, the Constellation system with IOP control automatically maintained stable IOP in the target range 63% to 93% of the time at different vacuum settings versus ≤ 23% of the time with the EVA system with AIC.
Keywords: constellation, DORC EVA, automatic infusion compensation(AIC), IOP control, vitrectomy
Introduction
Unstable intraocular pressure (IOP) during vitrectomy can cause surgical complications and possible retinal damage, especially in patients with reduced retinal and optic nerve blood flow.1–6 A study that assessed intraoperative IOP during pars plana vitrectomy showed that most patients (78%) experienced substantial IOP fluctuations during the procedure.7 A separate clinical study that evaluated IOP in 61 eyes also reported that IOP fluctuations were common during routine vitrectomy.8
To improve safety and surgical outcomes during vitrectomy, a stable IOP should be maintained, particularly when using high-speed dual-blade vitrectomy probes with higher flow. Because the vitreous is a heterogeneous substance, flow can vary during vitrectomy depending on its viscosity2 and on the movement of the probe between regions of vitreous and irrigation solution, potentially affecting IOP.9 Ophthalmic surgical systems with built-in IOP control features provide intraoperative control over IOP and can bring enhanced stability and efficiency to vitreoretinal procedures.
The Constellation® Vision System (Alcon Vision LLC, Fort Worth, TX, USA) was the first vitrectomy system developed with the ability to correct pressure levels in real time and maintain target IOP.10 For the Constellation system with IOP control, users set a desired IOP level (eg, 30 mmHg). During the procedure, the flow sensor in the system is used to predict the pressure drops in the eye as they occur and compensates by adjusting the infusion pressure automatically to maintain the desired IOP level. No other setting adjustments are necessary for Constellation to maintain an IOP level. In two separate studies, Constellation system’s IOP control was tested using enucleated porcine eyes and was found to substantially reduce fluctuations in IOP.11,12
The automatic infusion compensation (AIC) of the Enhancing Visual Acuity (EVA) system (DORC [Dutch Ophthalmic Research Center] International, Zuidland, Netherlands) requires users to manually select both infusion pressure start and end points. In the vacuum mode, AIC provides IOP compensation by elevating the infusion pressure settings as the aspiration vacuum of the vitrectomy increases. To compensate for pressure fluctuations during the surgical procedure, the device adjusts irrigation pressure between the 2 preset values (“start” and “end” pressure) in proportion to the level of aspiration controlled by the footswitch position.13
The use of vitrectomy systems with IOP compensation, such as Constellation with IOP control and EVA with AIC, can help maintain an optimal and stable IOP during surgical procedures. The purpose of this study was to evaluate and compare the IOP-stabilizing features of the Constellation Vision System IOP control and EVA with AIC during benchtop core vitrectomy experiments. IOP was quantified using a closed eye model with porcine vitreous during a simulated vitrectomy under different vacuum settings.
Materials and Methods
Study Design and Setup
A benchtop testing setup was designed at Alcon Laboratories (Lake Forest, CA, USA). A hollow acrylic sphere simulating the basic geometry of an eye (Figure 1A and B) was used to hold a vitrectomy probe and an infusion cannula. The pressure transducer (PX409-005GUSBH; Omega Engineering, Norwalk, CT, USA) was connected to the bottom of the eye model to detect the pressure change under different system settings and conditions. At the beginning of the experiment, the model eye was completely filled with porcine vitreous, with the infusion line delivering Alcon’s proprietary BSS® sterile irrigation solution (Alcon Vision LLC). Vitreous probes were operated at their maximum speed to remove the vitreous. During this process, BSS replaced vitreous until the solution inside the model eye was completely replaced with BSS.
 |
Figure 1 Experimental setup. The pressure transducer and vitrectomy probes were connected to the acrylic eye model (A), and the fixture was connected to the vitrectomy instrument (B). |
Constellation Vision System was used with the IOP control setting turned on or off. Testing was performed with 25-gauge (G) Hypervit® 20,000 cuts per minute (cpm) dual-blade vitrectomy probes (Alcon Vision LLC) at 3 vacuum levels (250, 450, and 650 mmHg). The IOP level was set at 30 mmHg (Table 1).
 |
Table 1 Instrument Configurations |
The EVA vitrectomy system was used with the AIC setting turned on or off. Testing was performed with 25 G Two-Dimensional Cutter (TDC) 8000 cpm DORC Continuum range probes with 16,000 cpm effective cut rate (Dutch Ophthalmic Research Center International) vitrectomy probes at 3 vacuum levels (250, 450, and 650 mmHg). The ideal AIC setting changed based on fluid properties and the aspiration pressure. Because there was no universal setting for all conditions, different AIC settings were selected in the study for the 3 vacuum levels to reach a stable IOP level in BSS. The start infusion IOP was set at 30 mmHg, and the end infusion pressure was set to different levels so that IOP was constantly maintained at 30 mmHg when aspirating BSS at the specific maximum vacuum levels being tested (Table 1).
There were no human participants in this study, and informed consent was not required.
Intraocular Pressure Control
The Constellation system with IOP control automatically compensated for IOP changes to maintain the preset IOP level selected by the users. During Constellation system priming, IOP change was characterized as a function of flow using an infusion flow sensor in the Constellation console. With IOP control enabled, the console automatically compensated for the pressure drop under different infusion flow conditions encountered during surgery.
The EVA system with the AIC turned on required users to manually select 2 separate pressure parameters for the irrigation pressure start point and end point (Table 1). With AIC enabled, the infusion pressure would be automatically adjusted within the selected AIC range in proportion to the current aspiration. The AIC could be configured to provide IOP compensation during surgery by elevating the pressure settings as the aspiration vacuum of the vitrector increased.
Average IOP was assessed before aspiration (initial IOP), during vitreous removal (vitreous IOP), and after vitreous removal and replacement with BSS (BSS IOP; Figure 2) using Constellation with 25 G Hypervit probes or EVA with 25 G TDC probes.
Statistical Analysis
Data from 6 samples were recorded and analyzed using descriptive statistics at each system setting. Statistical significance when comparing different IOP control settings and fluid properties was assessed using one-way analysis of variance and the Welch t test.
Results
IOP Control Off
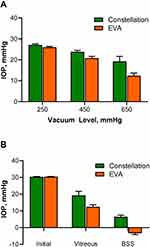
Without IOP compensation, the Constellation, using 25 G Hypervit probes, and the EVA, using 25 G TDC probes, were affected by system settings and fluid properties. When using Constellation with the IOP control off, the average vitreous IOP was 27.09±0.46, 23.81±0.70, and 19.25±2.39 mmHg at 250, 450, and 650 mmHg of vacuum, respectively (Figure 3A). When using EVA with the AIC off, the average vitreous IOP was 26.01±0.37, 20.80±0.86, and 12.42±1.24 mmHg.
At 650 mmHg of vacuum, average initial IOP, vitreous IOP, and BSS IOP were 30.34±0.16, 19.25±2.39, and 6.53±0.92 mmHg, respectively, with the Constellation system and 30.45±0.15, 12.42±1.24, and −3.32±0.84 mmHg with the EVA system (Figure 3B).
IOP Control On
With the IOP control on, the Constellation system maintained pressure at the desired level, achieving average vitreous IOP and BSS IOP levels within the target range (27.5–32.5 mmHg) and with <1 mmHg variation between the average vitreous IOP and BSS IOP. However, substantially higher average vitreous IOP than the initial 30 mmHg was observed using EVA with AIC at 250, 450, and 650 mmHg of vacuum (Figure 4A and B).
There was a substantial increase in the percentage of time spent at the target IOP (~30 mmHg) during the vitreous removal procedure using the Constellation with IOP control versus using EVA with AIC. With the Constellation system, IOP remained in the target range 93%, 74%, and 63% of the time at 250, 450, and 650 mmHg of vacuum, respectively (Figure 5A). For the EVA system with AIC, IOP remained in the target range 16%, 17%, and 23% of the time (Figure 5B).
For the Constellation system with IOP control, there were no significant differences in IOP in the 250-, 450-, and 650 -mmHg vacuum settings during either the vitreous or BSS stages (P>0.3; Figure 6A). However, for the EVA system with AIC, there was significant variation in IOP when removing vitreous at different vacuum settings (P<0.05; Figure 6B), but BSS IOP remained at the desired level for all 3 vacuum settings.
When using Constellation with IOP control, vitreous IOP was not statistically different compared with BSS IOP and initial IOP at any of the tested vacuum levels (P>0.05 for all; Figure 7A). At 250 mmHg of vacuum, initial, vitreous, and BSS IOPs were 30.19±0.14, 30.39±0.47, and 30.93±1.28 mmHg, respectively. At 450 mmHg, the IOPs were 30.16±0.17, 30.88±0.89, and 31.68±1.74 mmHg and at 650 mmHg were 30.18±0.18, 30.99±0.66, and 31.55±1.38 mmHg.
When using EVA with AIC, there were significant differences among the initial IOP, vitreous IOP, and BSS IOP at all vacuum levels (P<0.0001 for all; Figure 7B). At 250 mmHg of vacuum, initial IOP, vitreous IOP, and BSS IOP were 30.51±0.21, 39.59±0.87, and 30.75 ±0.47 mmHg, respectively (P<0.0001). At 450 mmHg, they were 30.43±0.15, 43.34±1.15, and 30.48±0.37 mmHg (P<0.0001) and at 650 mmHg they were 30.54±0.08, 44.62±1.24, and 29.92±0.72 mmHg (P<0.0001).
Discussion
Fluctuations in IOP during vitrectomy have been associated with suprachoroidal hemorrhage, surgical complications, and retinal damage, particularly in patients with proliferative diabetic retinopathy and retinal vein occlusion and in eyes with impaired retinal and optic blood flow.1,4 High IOP can cause a decrease in choroidal blood flow and could exacerbate retinal ischemia or cause corneal edema in predisposed eyes.2,3 Low IOP can cause choroidal hemorrhage and may result in retinal damage.2 Maintaining stable IOP can help improve surgical outcomes and reduce the risks associated with IOP fluctuations, such as decreased vascular perfusion pressure and compromised blood flow to the optic nerve and retina.1
In this bench study comparing the IOP-stabilizing features of the Constellation system with IOP control with those of the EVA system with AIC, we found that the Constellation system was able to detect an IOP drop during the vitreous removal process and automatically compensate for the drop, keeping IOP in a stable range throughout the procedure whereas, with the EVA system, IOP during vitreous removal was substantially higher than the target 30 mmHg and had larger fluctuation when dealing with a BSS and vitreous mixture. Additionally, we found that the EVA system was not as effective as the Constellation system in maintaining stable IOP levels when the vacuum settings or fluid properties changed.
Also of note, the EVA system with the AIC feature in vacuum mode required users to manually enter a pressure parameter in addition to the desired IOP, unlike the Constellation system. While the addition of a second “end” pressure setting reduced IOP errors during the procedure, there was no specific setting that would achieve the desired IOP throughout the variety of flow conditions. To reach the target IOP level, changes in AIC settings would be required to balance changes in the aspiration settings and fluidic properties. For example, the AIC setting could be optimized based on the BSS aspiration flow rate, but the IOP level in BSS might significantly differ from that in the porcine vitreous, and vice versa. Thus, noticeable IOP fluctuations were observed during the dynamic vitreous removal process using EVA with AIC.
The results of this study are consistent with previous findings. Two earlier studies showed that the IOP control features of vitreoretinal surgical systems can effectively compensate for IOP fluctuations, and the Constellation Vision System with IOP control was demonstrated to effectively compensate for a decrease in pressure with an increased infusion flow in enucleated porcine eyes.11,12 Also similar to the current results, the drop in IOP has previously been shown to be significantly smaller when using Constellation with versus without IOP control for 23 G and 25 G probes (P<0.0001).12 In a clinical study that monitored IOP in 61 eyes, IOP fluctuations were larger and severe adverse effects (hypotony and partial ocular collapse) were more frequent when using a vitrectomy system without IOP control.8
Under the current study conditions, IOP compensation with the EVA system with AIC was affected by vacuum settings and fluidic changes. In a previous bench study, EVA with AIC stabilized IOP in the vacuum mode when using a vitreous chamber model with BSS and a customized artificial vitreous. Under these experimental conditions, EVA with AIC compensated for the IOP drop when the minimum and maximum infusion pressure was set at 30 and 55 mmHg. The study reported that the ideal AIC settings would depend “on the rheological properties of the vitreous humor and the aspiration pressure”.13 However, without knowing the property of the vitreous during the procedure, the selection of the ideal AIC setting to achieve a constant stable and controllable IOP level will be challenging.
This study provided a careful assessment of IOP control when using the current Alcon Constellation and the DORC EVA systems. In 2022, DORC launched a newer vitrectomy platform, the EVA NEXUSTM. Unlike the current EVA system, the EVA NEXUS includes a “SmartIOPTM” feature that measures and stabilizes anterior and posterior chamber pressures during surgery, designed to enable a more responsive AIC control and constant IOP during vitrectomy.14,15 Therefore, the results of this study may not be applicable to the newer system, and future testing should be done with the latest version of the EVA NEXUS. In addition, it should be noted that the use of the model eye with porcine vitreous has intrinsic limitations, and the results of this bench study may not be applicable to clinical settings. Future studies are needed to evaluate IOP control in human eyes during vitrectomy in real-world clinical settings.
Conclusion
In conclusion, the Constellation system with IOP control automatically compensated for the IOP drop during the vitreous removal process and consistently maintained the IOP in the target range (~30 mmHg). Stable IOP was maintained independent of the changes in system settings and fluid properties (ie, during BSS and vitreous aspiration). The EVA system with AIC required manual selection of 2 infusion pressure set points to reduce IOP error during the procedure and was less effective than the Constellation system in keeping IOP within a stable range during vitrectomy.
Acknowledgments
Medical writing assistance was provided by Natalia Zhukovskaya, PhD, of ICON plc (Blue Bell, PA), and was funded by Alcon.
Funding
This study was funded by Alcon Research LLC.
Disclosure
Martin Charles received funding from Alcon. Varalakshmi Wuyyuru, Ying Zhu, Brian McDonell, and Carrie Garufis are employees of Alcon. In addition, Dr Brian McDonell has patents US9693898B2, US10639197B2, US11583441B2 and US12059374B2 issued to Alcon. The authors report no other conflicts of interest in this work.
References
1. Moorhead LC, Gardner TW, Lambert HM, et al. Dynamic intraocular pressure measurements during vitrectomy. Arch Ophthalmol. 2005;123(11):1514–1523. doi:10.1001/archopht.123.11.1514
2. Steel DH, Charles S. Vitrectomy fluidics. Ophthalmologica. 2011;226(suppl 1):27–35. doi:10.1159/000328207
3. Findl O, Strenn K, Wolzt M, et al. Effects of changes in intraocular pressure on human ocular haemodynamics. Curr Eye Res. 1997;16(10):1024–1029. doi:10.1076/ceyr.16.10.1024.9024
4. Tabandeh H, Sullivan PM, Smahliuk P, Flynn HW Jr, Schiffman J. Suprachoroidal hemorrhage during pars plana vitrectomy. Risk factors and outcomes. Ophthalmology. 1999;106(2):236–242. doi:10.1016/S0161-6420(99)90062-3
5. Gass JD, Parrish R. Outer retinal ischemic infarction--a newly recognized complication of cataract extraction and closed vitrectomy. Part 1. A case report. Ophthalmology. 1982;89(12):1467–1471. doi:10.1016/s0161-6420(82)34615-1
6. Brubaker RF. Intraocular surgery and choroidal hemorrhage. Arch Ophthalmol. 1984;102(12):1753–1754. doi:10.1001/archopht.1984.01040031417013
7. Rossi T, Querzoli G, Angelini G, et al. Ocular perfusion pressure during pars plana vitrectomy: a pilot study. Invest Ophthalmol Vis Sci. 2014;55(12):8497–8505. doi:10.1167/iovs.14-14493
8. Yang HS, Yun YI, Park JH, Choi S, Woo JM. In vivo intraocular pressure monitoring during microincision vitrectomy with and without active control of infusion pressure. Eur J Ophthalmol. 2017;27(5):601–606. doi:10.5301/ejo.5000956
9. Takashina H, Watanabe A, Tsuneoka H. Perioperative changes of the intraocular pressure during the treatment of epiretinal membrane by using 25- or 27-gauge sutureless vitrectomy without gas tamponade. Clin Ophthalmol. 2017;11:739–743. doi:10.2147/opth.S133775
10. Kim YJ, Park SH, Choi KS. Fluctuation of infusion pressure during microincision vitrectomy using the constellation vision system. Retina. 2015;35(12):2529–2536. doi:10.1097/iae.0000000000000625
11. Sugiura Y, Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Intraocular pressure fluctuation during microincision vitrectomy with Constellation Vision System. Am J Ophthalmol. 2013;156(5):941–947e941. doi:10.1016/j.ajo.2013.06.016
12. Falabella P, Stefanini FR, Lue JC, et al. Intraocular pressure changes during vitrectomy using constellation vision system’s intraocular pressure control feature. Retina. 2016;36(7):1275–1280. doi:10.1097/IAE.0000000000000911
13. Nepita I, Stocchino A, Dodero A, et al. Dynamic pressure measurements during vitrectomy in a model of the eye. Transl Vis Sci Technol. 2022;11(5):21. doi:10.1167/tvst.11.5.21
14. Feldhaus L, Ohlmann A, Kassumeh S, Priglinger S, Mayer W. EVA NEXUS-Phaco performance study. Int J Ophthalmol. 2024;17(8):1447–1452. doi:10.18240/ijo.2024.08.09
15. Peter S. Clinical trial to test the safety of the EVA nexus surgical platform. Int J Retina Vitreous. 2024;10(1):45. doi:10.1186/s40942-024-00563-3
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.