Back to Journals » Clinical Ophthalmology » Volume 19
Is the Meibopatch Heated Pad Useful in Treating Patients with Meibomian Gland-Related Dry Eye Disease? – Assessing Tolerability, Acceptance, and Effectiveness
Authors Bakhiet TE, Ansari AS , Williams GS, Awad MH , Thomas A
Received 11 December 2024
Accepted for publication 13 March 2025
Published 30 May 2025 Volume 2025:19 Pages 1743—1749
DOI https://doi.org/10.2147/OPTH.S508547
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tasneem Elghazali Bakhiet, Abdus Samad Ansari, Gwyn Samuel Williams, Mahmoud Husseiny Awad, Alistair Thomas
Swansea Eye Unit, Singleton Hospital, University of Swansea, Swansea, Wales, UK
Correspondence: Tasneem Elghazali Bakhiet, Email [email protected]
Background: Dry Eye Syndrome (DES) significantly impacts vision-related quality of life and poses a substantial financial burden in ophthalmology practice globally. Projected expenditure on DES treatments is estimated to exceed 6.6 billion USD by 2027. The Meibopatch (VISUfarma International, Valbonne, France) represents one of the latest devices designed to enhance treatment adherence and aid in managing Dry Eye Syndrome (DES). However, its long-term acceptance and efficacy among patients with posterior blepharitis, a condition closely linked with Meibomian gland dysfunction (MGD), has yet to be demonstrated.
Methods: Patients with symptomatic non-responsive blepharitis were enrolled during clinic visits and asked to self-administer Meibopatch treatment for four weeks. The ocular surface disease index (OSDI) questionnaire was utilized to evaluate symptoms both at baseline and following the four-week treatment period. Supplementary assessments on acceptability and tolerability were conducted through post-treatment questionnaires.
Results: A total of 43 patients were recruited. The baseline pre-treatment OSDI score ± SD (Standard Deviation) was 47.9± 16.4, while the post-treatment OSDI value + SD was 37.7± 14.9. Analysis revealed a significant mean difference between pre- and post-treatment OSDI scores (p=0.0005), indicating notable symptomatic improvement over the assessment duration. However, the findings diverged from evaluations of acceptability and overall patient satisfaction with the Meibopatch.
Conclusion: The results suggest that Meibopatch represents a safe therapeutic option for managing DES. However, the controversy arises from the incongruence between the substantial reduction in symptoms noted from the OSDI scores and the reported patient outcomes regarding satisfaction and effectiveness. Future efforts should focus on validating the OSDI in blepharitis patients and establishing patient-centered outcomes in the realm of DES.
Keywords: meibomian gland, dry eye syndrome, blepharitis, meibomian gland warming, patient-reported outcomes
Introduction
Dry eye syndrome (DES) is a multifaceted disease affecting the ocular surface, which is characterized by a loss of homeostasis of the tear film. DES is accompanied by a myriad of ocular symptoms that manifest as a result of deficiency in tear production. This leads to persistent dryness of both the cornea and conjunctiva. The prevalence of DES can be difficult to estimate due to the tendency for patients to self-treat when experiencing symptoms. Estimates of prevalence will also be directly affected by the source of data, thus rates of disease will differ between population-based estimates and those derived from optometry practices or eye hospitals. For instance, the prevalence of patient-diagnosed dry eye syndrome ranges from 14% to 33%, which is in stark contrast to the estimated prevalence of clinician-diagnosed dry eye (ranging from 1% to 22%).1,2 Despite the difficulties in estimating the magnitude of disease, there is no question as to its current cost to both patients and health resource utilization. A recent study established the total cost of DES to be 1.1 million US dollars per 1000 persons per year.3 These costs are largely driven by the multiple therapies available for patients with DES, of which gold lid weights appear to be the most prohibitive intervention.3
The degenerative process associated with DES stems from tear film instability and hyperosmolarity, as well as ocular surface inflammation and neurosensory abnormalities.4 The impact of these ocular insults will vary based on the aetiology of DES which can be broadly classified as aqueous deficient or evaporative.5 Aqueous deficient dry eye (ADDE) can be further sub-classified as Sjögren syndrome dry eye and non-Sjögren syndrome dry eye.5 Evaporative dry eye (EDE) is also further sub-divided into lid related or ocular surface related dry eye.5 Meibomian gland dysfunction (MGD) is the most common source for lid-related EDE.6 MGD is defined as a chronic diffuse abnormality of the Meibomian glands. Clinically, it is recognized by signs of terminal duct obstruction as well as changes in the character and quantity of glandular secretion. MGD often leads to alteration of the tear film, which prompts symptoms of ocular irritation, clinically apparent inflammation, visual disturbance, and ocular surface disease’.7 MGD is thought to be a major source of dry eye, accounting for up to 70% of DES cases reported among Asians, and 20% among Caucasians.8–10 As demonstrated, MGD varies greatly across ethnicities.
Techniques for diagnosis of MGD include patient questionnaires, tear break-up time, ocular surface staining, eyelid examination, meibomian gland expression and the Schirmer test.11 The most commonly utilized patient questionnaire includes the Ocular Surface Disease Index (OSDI).12 The OSDI is a validated tool comprising twelve questions on three areas assessing dry eye symptoms regarding environmental triggers, ocular symptoms and vision symptoms.12 Clinicians rely on this tool when assessing the baseline severity of symptoms. The OSDI also provides patients an opportunity to record their symptoms overtime, providing clinicians with evidence individual response to treatment.
Current therapies for MGD include eyelid hygiene, artificial lubricants, antibiotics, anti-inflammatory drugs, omega-3 dietary supplementation, topical steroids and surgery.11 Traditionally, therapies involving eyelid-warming achieved the best results by improving tear film stability as well as reducing ocular surface damage and tear evaporation.13 These therapies –eyelid warming and hygiene—include warming and massage of meibomian glands to express meibomian lipids.14 This was often achieved with use of a warm damp towel compressed on the patient’s closed eyes, however this proved cumbersome, requiring the repeated warming of the towel mid-treatment. Compliance with this technique has been reported to be as low as 10%.13
Proprietary devices have since been developed that improve effectiveness and compliance of such treatment.15–17 Amongst the newest devices, the Meibopatch (VISUfarma International, Valbonne, France) encompasses an eye-mask with grape seed filling. Designed for daily use, it helps alleviate symptoms of dry eye syndrome (DES). The mask is heated in the microwave to an optimal temperature, verified using a proprietary thermometer strip, and then applied to closed eyelids for 7 to 10 minutes. Patients are advised to perform eyelid massage immediately after use, with best results achieved through twice-daily application. Compared to single-use warm compresses, the MeiboPatch is reusable, making it a more cost-effective and environmentally friendly option. Its microwaveable design allows for quick and consistent heating, ensuring therapeutic warmth. The integrated temperature test strip enhances both safety and efficacy by preventing overheating.
The long-term acceptance and effectiveness of this device has yet to be demonstrated amongst a subgroup of patients with posterior blepharitis, a condition highly associated with MGD, marked by changes in medium quality and expressibility.7
Aims
We aim to describe the effectiveness, tolerability, and patient reported acceptance of the Meibopatch among a cohort of patients with posterior blepharitis.
Methods
Study Design and Participants
This study included patients recruited during ophthalmology clinic appointments at Singleton Hospital, Swansea, UK between December 2017 and August 2018. Patients were required to be 18 years of age or older to be included. All included participants required a diagnosis of blepharitis by an ophthalmologist within the last year. Diagnosis of blepharitis was made following accepted criteria. Patients were required to demonstrate one or more of the following symptoms: pruritic eyelids, conjunctival injection, ocular irritation with or without associated lacrimation, flaking and crusting at the base of the eyelashes, sensations of burning, stinging, or grittiness, or photophobia. Patients were required to be symptomatic, despite one or more previous therapies. Patients with any co-existing or previous ocular surface diseases were excluded. Sample size was determined based on the availability of Meibopatch devices within the department. Verbal consent was taken prior to the enrollment of patients.
To assess the tolerability, safety, and effectiveness of the Meibopatch, patients were asked to complete an OSDI12 questionnaire during their initial baseline interview, and four weeks post-therapy. Additional baseline measures include the collection of demographic characteristics such as age, sex, educational background, employment, marital status, past medical history, previous therapies used, number of previous treatment attempts, and current medications. A full ophthalmologic examination was performed under slit lamp as part of the baseline assessment of a full medical history. Participants were followed up by onsite nursing staff at four weeks post treatment. Follow-up assessments include clinical examination and questionnaires.
The post-treatment OSDI questionnaire was given to patients during the recruitment, patients were asked to return this to our recruitment offices in a pre-paid envelope via standard postal service. Patients were also required to complete a further questionnaire regarding their opinion of the device. We aimed to use this additional questionnaire to evaluate intervention acceptability and patient satisfaction. This was also to be completed and returned via post in the post-treatment period. Any patients uncomfortable with returning their questionnaires via post were also given the option to have a researcher telephone them to collect this information during the post-treatment follow-up. A copy of this additional questionnaire is included in Appendix A.
The Abertawe Bro Morgannwg Joint Study Review Committee examined this study and determined that it constituted an audit of current management as it is local practice to hand patients Meibopatch heated pads for recalcitrant MGD. This study adheres to the STROBE guidelines.18
Intervention
All patients meeting the above-described eligibility criteria were offered Meibopatch treatment. Patients were asked to self-administer Meibopatch treatment twice a day for 7 to 10 minutes, as directed by the manufacturer for 4 weeks. A demonstration of the Meibopatch treatment was given for all patients during the recruitment period. Assessment of appropriate technique was required prior to study enrollment.
Statistical Analysis
Baseline demographic characteristics including responses to both the OSDI and tolerability questionnaires are reported for baseline and post-treatment assessment periods.
Continuous measures are summarized using means and standard deviations (SD), while dichotomous measures are reported by percentage. Box-plots were constructed to identify outlier observations. Sensitivity analyses were performed after removing outlier observations. All continuous variables were assessed for normal distribution, whereby proper transformations were applied when necessary.
The two tailed paired t-test was used for comparison of pre- and post-treatment OSDI scores. A p value of ≤0.05 was considered statistically significant. Post-hoc power calculation revealed 90% power to detect difference between groups. We did not perform any sensitivity analyses to evaluate the effect of different imputations for missing data or loss to follow-up. All patients included in the analyses were required to have completed the study without missing information. Statistical analysis was carried out using STATA Version 13.1.19
Results
A total of 43 patients meeting the previously described eligibility criteria were recruited at clinic appointments from Singleton Hospital, Swansea, UK between 2017 and 2018. All recruited participants were deemed eligible for inclusion into the final analysis. No adverse reactions or safety concerns were raised during this study. The baseline mean pre-treatment OSDI score ± standard deviation (SD) of all patients was 47.9±16.4. The mean post-treatment OSDI value + SD of all patients was 37.7±14.9. A t-test evaluating the mean difference between scores in the pre- and post-treatment setting was demonstrated to be statistically significant, favoring a reduction in OSDI scores in the four weeks following treatment with the Meibopatch (p=0.0005).
We received 43 completed responses to our secondary questionnaires assessing patient satisfaction, tolerability, and acceptability of the Meibopatch. Patient responses to this questionnaire are summarized in Figure 1. Amongst the 43 patients trialing the Meibopatch for DES, less than 50% (n=19, 44.2%) reported the treatment to be effective at reducing symptoms. While the majority of patients (88.4%) found the Meibopatch easy to administer, only 37.2% report willingness to continue use of the treatment if prescribed by a physician. In fact, the majority of patients (74.4%) reported they would not continue to use this treatment if they were required to purchase from a pharmacy out of pocket. Despite more than half of patients reporting they found the Meibopatch ineffective for alleviating their DES, only 11.6% stated they found the Meibopatch to be less effective than previous self-administered therapies (eg hot compress treatment). In addition, 41.9% of patients stated they found the Meibopatch equally effective to previous hot compress treatments. It was difficult to fully compare Meibopatch to previous interventions since 18.6% of patients had not self-administered hot compress previously.
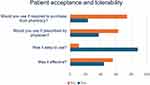 |
Figure 1 Additional Patient Questionnaire Responses. |
Discussion
Findings from this study suggest that Meibopatch is a safe therapy for the treatment of DES. When assessing the effectiveness of this therapy using a comparison of pre- and post-treatment OSDI scores, the Meibopatch significantly reduces symptoms of DES. These findings are however in direct conflict with patient reported outcomes, whereby the majority of patients found the treatment ineffective at reducing symptoms of dry eye and report they would not continue to use this therapy if required to purchase out of pocket. This is an important finding, as it demonstrates the ill reflective nature of the OSDI questionnaire when compared to patient values and treatment preferences and also highlights that what may be statistically significant may not be clinically significant in the eyes of the patient.
The Meibopatch in Comparison to Other Adjunct Eyelid Warming Devices
When focusing specifically on the results attained from OSDI scoring, the current study demonstrates significant improvement in dry eye symptoms after repeated administration of the Meibopatch for the duration of four weeks. These findings are consistent with previous research by Baumann et al,16 who also demonstrate significant improvement in ODSI scores after treatment with the Meibopatch. Interestingly, the effectiveness of Meibopatch in the study by Baumann et al16 was only achieved at 3 months, a significant reduction in symptoms was not maintained at one-month post therapy.16 Such differences are likely due to the significantly smaller sample (n=15) evaluated in the Baumann et al study.16
Further efforts aimed to establish the effectiveness of other eyelid warming adjuncts have been limited by ineffective treatment administration protocols and insufficient follow-up durations.15,17,20 For instance, Arita et al15 assessed the improvement of dry eye symptoms at 30 minutes post administration of a single treatment with one of following five devices: Azuki no Chikara (Kiribai Chemical, Osaka, Japan), Eye Hot R (Cept, Tokyo, Japan), traditional style widely available hot towel, Hot Eye Mask (Kao, Tokyo, Japan), and Memoto Este (Panasonic, Osaka, Japan). Furthermore, Wang et al²² reported a notable improvement in both OSDI scores and subjective symptoms after using a disposable eyelid warming mask, similar to the Meibopatch, compared to the control group. While all devices demonstrated significant benefit for symptoms of dry eye, the limited follow-up period calls to question their true effectiveness. Findings from the study by Arita et al15 stands to benefit from an extended follow-up period, whereby assessment of dry eye symptoms could occur over a period of days after a multiple treatment administrations.
In the study by Sim et. al, adjunct eyelid warming devices (the EyeGiene; Eyedetec Medical Inc. California, USA, and Blephasteam; Spectrum Thea Pharmaceuticals LTD, Macclesfield, UK) were evaluated in comparison to traditional style hot towels. Sim et al20 were only able to establish the Blephasteam as an effective device for reducing symptoms of dry eye at 3 months post-treatment. This study is affected by both the limited sample size and variability in hot compress temperatures used within the control arm.20
While the results of our study appear to be consistent with the current literature, such that adjunct devices aimed at improving the utility eyelid warming therapy are shown to improve patient compliance and reduce symptoms of dry eye. Major differences between studies are likely secondary to variability in duration of follow-up. It would appear patients may gain greater benefit after longer durations of treatment administration, such that the majority of studies were unable to establish significant reduction in symptoms after 4 weeks of use.
When considering the acceptability and tolerability of the Meibopatch, it is essential to note 88.4% of patients in the current study stated they found Meibopatch treatment to be easy to administer. This is an important finding in light of the poor compliance noted amongst patients utilizing traditional hot towel therapies.11 While we are unable to determine the factors leading to poor compliance with traditional hot towels, it is a useful objective for further investigation to determine the source of such poor acceptability for traditional eyelid warming techniques.
The Utility of Current Measures of Effectiveness
When utilizing scores from the OSDI questionnaire –a validated tool used to assess the symptoms of DES—findings from our study suggest the Meibopatch provides significant benefit for patients with dry eye, reducing symptoms by one-month post therapy. These findings are however in direct conflict with our secondary questionnaire, aimed to gauge patient satisfaction, as well as treatment tolerability and acceptability. Within our secondary questionnaire, the majority of patients reported the treatment to be ineffective at reducing symptoms of dry eye. Such recognizable inconsistencies imply the OSDI questionnaire is ineffective at measuring a key aspect of patient satisfaction, or moreover fails to capture important symptoms which bear flagrant effects on patient symptomatology. When only 44.2% of patients in the current study responded they found Meibopatch treatment to be effective, a major doubt is cast concerning the validity of previous research utilizing the OSDI to establish efficacy.
Limitations
Results from this study may be confounded by the lack of a controlled comparator group. Without use of a reasonably matched control group, findings concerning the effectiveness of the Meibopatch may be confounded by both selection and volunteer bias. We are unfortunately unable to draw conclusions as to its relative effectiveness in comparison to other therapies. In addition, we were unable to adequately control for the impact of confounding due to our inability to access important demographic information including age, sex, number of previous treatments, and previous medical history. Unfortunately this information was not collected as part of the study, limiting any chance for adjustment using stratification or multi-variable modelling.
Additionally, no objective parameters were recorded as part of this study. Findings from clinical inspection, visual acuity, ocular pressure, as well as slit-lamp examination may have aided in our ability to accurately assess the physiologic impact of the Meibopatch.
Due to the prospective nature of the study design, a number of patients were lost-to-follow-up during the post-treatment time period. We must consider the potential outcomes for this subgroup of patients lost to follow-up, as their inclusion may ultimately effect the primary analysis to increase or diminish the magnitude of observed effect.
Future Research
To appropriately establish the effectiveness of the Meibopatch in comparison to traditional eye warming therapies as well as other adjunct therapies, a randomized controlled trial is required. This method of design would not only allow for the establishment of intervention superiority, or even non-inferiority to traditional therapies, it would also allow for an equal distribution of prognostic variables, thus reducing any impact of confounding. However, prior to commencing a randomized trial, the literature would truly stand to benefit from a study aimed at 1) re-establishing the validity of the OSDI within this cohort of patients, and 2) determining outcomes which reflect patient values and preferences within DES population. As demonstrated in our current study, there is clear inconsistency in the measures used to evaluate the effectiveness of interventions for DES. Within this study, we call to question the accuracy of the OSDI in reflecting patient values and preferences regarding DES symptomatology. The OSDI neither reflects nor acknowledges the values and preferences of the populations it is meant to serve in the context of our current study. This is particularly concerning as individual and population-level decision making is being guided by a standard of effect considered useful to researchers yet in direct conflict with what patients deem important. Future research should be aimed at determining patient goals for therapy when treating DES.
Conclusion
Results of this study suggest that Meibopatch is a safe therapy for the treatment of DES that is tolerated well by patients. The effectiveness of this therapy is contentious as the significant reduction in symptoms noted from the ODSI scores is not reflective of the patient reported outcomes concerning satisfaction and effectiveness. Future efforts are needed to establish 1) the validity of OSDI in patients with blepharitis, and 2) patient important outcomes in the field of DES.
Abbreviations
DES, Dry eye syndrome; MGD, Meibomian gland dysfunction; OSDI, ocular surface disease index; EDE, evaporative dry eye; ADDE, Aqueous deficient dry eye.
Ethical Statement
This study adheres to the principles of the Declaration of Helsinki. The Swansea University Ethics Board reviewed the study and determined that no additional ethical approval was necessary, as the data was collected during normal service delivery and is an audit of current practice.
Funding
A grant was obtained from Abertawe Bro Morgannwg University Health Board research and development funding, and Health and Care Research Wales. Roche Products Ltd. supported with funding for the article submission charges by a hands-off grant (Grant Number reference EPRF032887). Roche Products Ltd did not have any involvement in the preparation, drafting or editing of this manuscript, or in the choice of authors.
Disclosure
All authors have no conflicts of interest to declare for this work.
References
1. Moss SE, Klein R, Klein BEK. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264. doi:10.1001/archopht.118.9.1264
2. Shimmura S, Shimazaki J, Tsubota K. Results of a population-based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea. 1999;18(4):408–411. doi:10.1097/00003226-199907000-00003
3. Clegg J, Guest J, Lehman A, Smith A. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13(4):263–274. doi:10.1080/09286580600801044
4. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
5. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011
6. Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–164. doi:10.1016/S1542-0124(12)70150-7
7. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi:10.1167/iovs.10-6997a
8. Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investig Ophthalmol Vis Sci. 2011;52(4):1994. doi:10.1167/iovs.10-6997e
9. Siak JJK, Tong L, Wong WL, et al. Prevalence and risk factors of meibomian gland dysfunction: the Singapore Malay eye study. Cornea. 2012;31(11):1223–1228. doi:10.1097/ICO.0b013e31823f0977
10. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi:10.1097/ICO.0b013e318225415a
11. Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocul Surf. 2017;15(2):179–192. doi:10.1016/j.jtos.2017.01.006
12. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
13. Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008;85(8):675–683. doi:10.1097/OPX.0b013e318181adef
14. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–2064. doi:10.1167/iovs.10-6997g
15. Arita R, Morishige N, Shirakawa R, Sato Y, Amano S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf. 2015;13(4):321–330. doi:10.1016/j.jtos.2015.04.005
16. Baumann A, Cochener B. Évaluation des moyens modernes de prise en charge du dysfonctionnement meibomien. J Fr Ophtalmol. 2014;37(4):303–312. doi:10.1016/j.jfo.2013.12.007
17. Mori A, Shimazaki J, Shimmura S, Fujishima H, Oguchi Y, Tsubota K. Disposable eyelid-warming device for the treatment of meibomian gland dysfunction. Jpn J Ophthalmol. 2003;47(6):578–586. doi:10.1016/S0021-5155(03)00142-4
18. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/s0140-6736(07)61602-x
19. StataCorp. Stata statistical software: release 12. 2009.
20. Sim HS, Petznick A, Barbier S, et al. A randomized, controlled treatment trial of eyelid-warming therapies in meibomian gland dysfunction. Ophthalmol Ther. 2014;3(1–2):37–48. doi:10.1007/s40123-014-0025-8
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

