Back to Journals » Clinical Ophthalmology » Volume 19
Is Thermal Pulsation Therapy Effective for Dry Eyes Before and After Cataract Surgery? A Systematic Review and Meta-Analysis
Authors Chen KY, Chan HC, Chan CM
Received 1 October 2024
Accepted for publication 17 December 2024
Published 6 January 2025 Volume 2025:19 Pages 19—33
DOI https://doi.org/10.2147/OPTH.S498869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kai-Yang Chen,1 Hoi-Chun Chan,2 Chi Ming Chan3,4
1Department of Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; 2Department of Pharmacy, School of Pharmacy, China Medical University, Taichung, Taiwan; 3Department of Ophthalmology, Cardinal Tien Hospital, New Taipei City, Taiwan; 4Department of Ophthalmology, School of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
Correspondence: Chi Ming Chan, Email [email protected]; [email protected]
Background: Meibomian gland dysfunction (MGD) is a primary cause of evaporative dry eye disease (DED), which is often exacerbated by cataract surgery due to surgical trauma and inflammation. Thermal pulsation therapy (TPT) aims to enhance meibomian gland function and relieve dry eye symptoms. We conducted a systematic review and meta-analysis to evaluate the effectiveness of TPT in managing dry eye symptoms associated with cataract surgery.
Methods: A systematic search was performed in December 2024 across PubMed, ScienceDirect, CINAHL, and the Cochrane Central Register of Controlled Trials to identify original research on the efficacy of TPT in addressing cataract surgery-related dry eye symptoms. The quality of the included studies was evaluated using the Risk of Bias in Non-Randomized Studies of Interventions tool, with results visualized through the Robvis 2.0 tool developed by the Cochrane Collaboration.
Results: The search yielded 365 records, of which seven studies met the inclusion criteria for this meta-analysis. Key outcomes analyzed included the meibomian gland yielding liquid secretion (MGYLS) score, tear break-up time (TBUT), ocular surface disease index (OSDI) score, and lipid layer thickness (LLT). The meta-analysis revealed a moderate effect of TPT, with a small but clinically significant improvement observed in MGYLS scores (Cohen’s d = 0.29, p = 0.033) and TBUT (Cohen’s d = 0.15, p = 0.029). However, the effects on OSDI scores and LLT were not statistically significant. Study heterogeneity varied, with some outcomes exhibiting considerable variability.
Conclusion: TPT provides moderate and clinically meaningful improvements in MGYLS scores and TBUT for patients experiencing dry eye symptoms after cataract surgery, although improvements in OSDI scores and LLT did not achieve statistical significance. The variability in study quality and heterogeneity highlights the need for well-designed, high-quality research to confirm these findings and evaluate the durability of TPT’s therapeutic effects both before and after cataract surgery.
Synopsis: This study investigated if thermal pulsation therapy (TPT) helps with dry eye symptoms after cataract surgery. The analysis of existing research suggests TPT improves tear quality and meibomian gland function, potentially improving patient comfort after cataract surgery.
Keywords: thermal pulsation therapy, dry eye symptoms, cataract surgery, meibomian gland dysfunction, systematic review, meta-analysis
Introduction
Meibomian glands produce an oily tear film layer to prevent evaporation, ensuring optimal comfort.1 Dysfunction of these glands often leads to dry eye syndrome and irritation.2 One of the emerging non-invasive, effective treatments is thermal pulsation therapy (TPT), which helps alleviate meibomian gland dysfunction (MGD) symptoms.3 TPT combines heat therapy and massage as a treatment for MGD.4 Furthermore, clinical trials have been conducted to assess the effectiveness and safety of TPT in treating MGD. Additionally, pulsations are applied to the eyelids to express the liquefied oil from the glands, enhancing oil flow and gland function.5
TPT is an effective treatment for MGD. Leading brands include Lipiflow, offering FDA-approved in-office therapy with heat and pulsation; iLux, combining localized heat with manual compression; and TearCare, featuring customizable heated patches with clinician-assisted expression. EyeXpress uses a goggle-like device for bilateral heating, while MiBo Thermoflo applies continuous thermoelectric heat via a handheld device. These technologies improve meibum secretion, alleviate dry eye symptoms, and vary in cost, duration, and patient suitability. These devices are intended to ease MGD symptoms and have been subjected to several clinical trials to determine their safety and effectiveness.6
MGD is a significant contributor to dry eye disease (DED). There are two major types of dry eye: aqueous-deficient dry eyes, caused by inadequate tear production, and evaporative dry eyes, for which MGD is the most common cause.7 Increased keratin production may also block gland openings.8 Similarly, chronic inflammation may damage the glands, altering normal function.9
Cataract surgery may also cause dry eye symptoms postoperatively due to surgical factors and recovery-related incidences.10 Surgical trauma due to perioperative corneal and ocular surface manipulation may disrupt the tear film and cause inflammation.11 Similarly, perioperative damage to the corneal nerves responsible for tear production stimulation may alter tear production.11,12 In addition, postoperative inflammation may also affect the ocular surface.13 Anti-inflammatory and antibiotic eye drops postoperatively may also irritate and worsen dry eye symptoms. Moreover, perioperative exposure to air may dry the ocular surface.
Dry eyes after cataract surgery can be managed through various approaches, including artificial tears to supplement natural tears.14 Warm compresses may also enhance meibomian gland function and improve tear quality.15 In addition, eyelid hygiene is essential to maintain ocular surface health. Some patients may experience prolonged dry eye symptoms after cataract surgery, necessitating effective management strategies.16,17
Thermal Pulsation Therapy (TPT) plays a crucial role in managing dry eye symptoms at various stages of cataract surgery. Using TPT for dry eye that occurred before cataract surgery helps address pre-existing dry eye conditions by improving tear film stability and meibomian gland function, enhancing patient comfort and reducing the risk of postoperative dry eye symptoms. This proactive approach optimizes ocular surface health, potentially improving surgical outcomes and minimizing complications. Using TPT after cataract surgery for pre-existing dry eye prepares the ocular surface for better healing by enhancing meibum secretion and stabilizing the tear film. This reduces the risk of exacerbated dry eye symptoms during recovery and helps manage postoperative inflammation, ensuring quicker recovery and better long-term comfort. Finally, using TPT for dry eye that occurred after cataract surgery effectively alleviates persistent dry eye symptoms by improving meibomian gland function and tear film quality. This intervention enhances ocular surface health, leading to better visual outcomes and increased patient satisfaction, while reducing the reliance on artificial tears and other short-term dry eye management solutions. Despite the high success rate of cataract surgery, patients often experience post-surgical dry eyes, which can affect visual outcomes. Current management strategies, including artificial tears, provide inadequate relief and may not address the underlying pathophysiology.18 Understanding the efficacy of TPT in managing dry eyes after cataract surgery is crucial for optimizing patient outcomes and facilitating personalized treatment. This study aims to systematically review the available literature on the effectiveness of TPT in treating dry eyes both before and after cataract surgery, highlighting its potential benefits in improving patient outcomes. The focus will be on evaluating how TPT influences the severity of dry eye symptoms in post-surgical patients, as well as its impact on meibomian gland function and ocular surface health throughout the perioperative period.
Methods
The study adhered to the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).19 A comprehensive search was performed using all available data on December 6, 2024. The primary protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42024557212).
Identification and Selection of Studies
We systematically searched databases including PubMed, ScienceDirect, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane Library for research articles evaluating the efficacy of TPT in managing dry eyes preceding and following cataract surgery.
Search Strategy
The search was designed to capture the most relevant studies using a combination of medical subject headings, keywords, and Boolean operators (AND, OR, NOT). Keywords included variations of terms related to TPT, cataract surgery, and dry eye management, such as “Thermal pulsation”, “LipiFlow treatment”, “meibomian gland dysfunction”, “cataract surgery”, and “dry eye. This strategy was applied across the selected databases to optimize the search results.
Eligibility Criteria
Articles were selected based on the modified PICO criteria. The population of interest included patients experiencing dry eye symptoms post-cataract surgery and treated with TPT. The primary outcome was the efficacy of TPT in alleviating these symptoms. Notably, this review focused exclusively on the effectiveness of TPT and did not include a comparison group. We aimed to include only original research articles that investigated TPT’s role in managing dry eyes, whether pre- or post-surgery. Articles such as reviews, meta-analyses, opinion pieces, or conference abstracts were excluded, as well as studies involving animal subjects or alternative treatments outside the scope of TPT.
Data Selection and Extraction
Data from eligible studies were independently abstracted by two reviewers (K.Y.C. and H.C.C.), and any discrepancies or misunderstandings were resolved through consensus with a third author (C.M.C.). Information extracted included study design, sample characteristics, baseline data, outcomes, and key findings. This data were compiled into an Excel workbook for further analysis.
Methodological Quality Assessment
Two reviewers (K.Y.C. and H.C.C.) independently assessed the methodological quality of each study. For non-randomized studies, the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used. For randomized trials, the Risk-of-Bias Visualization (Robvis 2.0) tool was employed, both developed by the Cochrane Collaboration.17–19
Data Analysis
The software used for the meta-analysis in this study was Comprehensive Meta-Analysis version 3.3. A random-effects model was applied, and all data were converted to means with standard deviations for the analysis. Pooled estimates were expressed as mean differences (MDs) with 95% confidence intervals (CIs). Heterogeneity across studies was assessed using the χ² and I² statistics, with a P value of <0.1 for χ² or >50% for I² indicating substantial heterogeneity. Publication bias was evaluated using Egger’s test, and if significant bias was detected, the trim and fill method was applied to validate the results. A sensitivity analysis using the leave-one-out method was conducted to assess the robustness of the pooled analysis. Statistical significance was defined as a two-tailed P value of <0.05. Finally, the data were summarized thematically to draw conclusions about the overall effectiveness of TPT in managing pre- and post-cataract dry eye.
Results
Study Selection
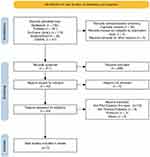
The literature search yielded 365 records, of which 54 duplicates were removed. Additionally, 268 records were excluded following title and abstract screening. The remaining 43 articles were sought for retrieval. These were further excluded from the study because they did not focus on pre- and post-cataract dry eye, thermal pulsation, or were protocols or unrelated studies. Finally, seven trials were included in this study, and their characteristics are summarized in Table 1. These consisted of one retrospective pilot study, four randomized controlled trials, one prospective cohort study, one case series, and one prospective study that met the eligibility criteria. The results of the study selection and screening process are presented in Figure 1.
 |
Table 1 Characteristics of Included Studies |
Methodological Quality Assessment
The methodological quality of non-randomized studies was assessed using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool. This tool evaluates the following domains: bias due to confounding factors, bias due to participant selection, bias due to the classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in outcome measurement, and bias in the selection of reported results. Conversely, the Risk-of-Bias Visualization (Robvis 2.0) tool, developed by the Cochrane Collaboration, was used to assess the risk of bias in randomized studies. The following parameters were evaluated: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in outcome measurement, and bias in the selection of reported results. Figures 2–5 illustrate the results of the risk of bias assessment for the included studies.
 |
Figure 2 Traffic Lights Plot of the Robvis Assessment of Randomized Studies. |
 |
Figure 3 Summary plot of ROB2 assessment of randomised studies. |
 |
Figure 4 Traffic lights plot of ROBINS-I assessment of non-randomized studies. |
 |
Figure 5 Summary plot of ROBINS-I assessment of non-randomized studies. |
Analysis of Outcomes
The analysis of outcomes is organized under several subheadings, including Meibomian Glands Yielding Liquid Secretion (MGYLS) score, Tear Break-up Time (TBUT), Ocular Surface Disease Index Score (OSDI), and Lipid Layer Thickness (LLT). The dominant themes for each outcome are reviewed in detail to provide a comprehensive understanding of the results.
MGYLS Score
A forest plot for three individual studies was constructed to analyze continuous data. A random-effects model was applied to calculate the deviation and differences in mean and standard deviation (SD). This forest plot summarized the quantitative data for each study and provided an estimated overall quantitative value for the combined effects. The overall effect size, calculated in terms of Cohen’s d, was found to be d = 0.29, CI = 95% (0.023, 0.435). Heterogeneity was estimated as follows: Tau² = 0.15, Chi² = 13.71, df = 3 (p = 0.004), and I² = 0%. The analysis for the overall effect yielded Z = 2.180 (p = 0.029) (Figure 6).
 |
Figure 6 Forest Plot of Comparison of MGYLS score. |
According to Matossian et al, there was a significant difference in MGYLS score between the test group receiving TPT and the control group. The test group demonstrated a mean MGYLS score of 1.6 ± 3.1 at one month post-surgery, whereas the control group reported a mean MGYLS score of 1.1 ± 3.3. However, the p-value for this difference was not statistically significant (p = 0.17). This finding suggests that although gland expressibility improved in the test group, the observed difference did not reach statistical significance at this time point.
Research by Park et al, similarly revealed no significant difference in MGYLS score between the control and TPT groups (p = 0.160). Notably, the MGYLS score worsened from the baseline in the control group after one month (p = 0.001) and further deteriorated at three months (p < 0.001).
On the other hand, Zhao et al, reported a statistically significant improvement in MGYLS score among patients who underwent preoperative treatment with TPT compared to those who did not receive this treatment. Specifically, the TPT group showed a significant increase at three months post-treatment, with values of 2.06 ± 1.77 compared to 1.81 ± 1.83 in the non-TPT group (p = 0.003). This finding indicates that preoperative management of MGD with TPT significantly enhanced the functional secretion of the meibomian glands, thereby contributing to improved ocular surface health both before and after cataract surgery.
TBUT
The forest plot for three individual studies was generated for continuous data, employing a random-effects model to calculate deviations and differences in mean and standard deviation (SD). The overall effect size was determined using Cohen’s d, which was found to be d = 0.15, CI = 95% (0.491, 0.917). Heterogeneity was assessed as follows: Tau² = 0.06; Chi² = 8.71, df = 2 (p = 0.08); I² = 54%. The analysis of the overall effect yielded Z = 2.18 (p = 0.029) (Figure 7).
 |
Figure 7 Forest Plot of Comparison of TBUT (seconds). |
According to Zhao et al, there was a statistically significant difference in TBUT between the TPT group and the non-TPT group (p = 0.019). The TPT group exhibited TBUT values of 4.47 ± 2.77 seconds and 4.72 ± 2.78 seconds after one week and one month, respectively, compared to 3.44 ± 2.23 seconds and 3.44 ± 2.24 seconds in the non-TPT group. However, no statistically significant difference in TBUT was observed between the control group and the TPT group (p = 0.505), with TBUT values of 3.65 ± 1.49 seconds and 3.46 ± 1.48 seconds, respectively.
Over time, TBUT in the control group worsened to 3.32 ± 1.57 seconds after one month but returned to baseline values after three months. In contrast, the TPT group demonstrated progressive improvement in TBUT, with values increasing to 3.93 ± 1.94 seconds after one month and 4.37 ± 1.83 seconds after three months. This improvement in TBUT indicates enhanced tear film stability and a healthier ocular surface environment, as observed in the test group receiving TPT compared to the control group after three months.
OSDI Score
The forest plot for two individual studies was generated for continuous data, with a random-effects model selected to calculate the deviation and differences in mean and standard deviation. The overall effect size was calculated in terms of Cohen’s d, which was found to be d = −0.19, 95% CI (0.061, 0.67). The heterogeneity was estimated as Tau² = 0.01; Chi² = 2.30, df = 2 (p-value = 0.42); I² = 43%. The analysis for the overall effect yielded Z = 2.35 (p = 0.019), as shown in Figure 8.
 |
Figure 8 Forest plot of Comparison of LLT. |
In the study by Park et al, a statistically significant difference was observed in the OSDI score between the test group receiving TPT and the control group. The test group exhibited a mean OSDI score of 18.5 ± 10.2 at 3 months post-surgery, compared to 24.3 ± 12.1 in the control group, with a p-value of 0.02. This suggests that TPT significantly improved the symptoms of ocular surface disease in patients undergoing cataract surgery. In the study by Vasudevan et al, the treatment group demonstrated a 65% reduction in OSDI, while the control group showed a 60% reduction. This improvement in OSDI was significantly greater in the treatment group than the control group (p = 0.05) at visit 1, but did not reach statistical significance at visit 2 (p = 0.11), as depicted in Figure 9.
 |
Figure 9 Forest plot of Comparison of OSDI Score. |
LLT
A forest plot for two individual studies was plotted for continuous data. A random-effects model was selected to calculate the deviations and differences in means and standard deviations. The overall effect size was calculated in terms of Cohen’s d, which was found to be d = −0.22 (95% CI: −0.144, 0.523). The heterogeneity was estimated as follows: Tau² = 0.14; Chi² = 1.89, df = 3 (p-value = 0.42); I² = 43%. The analysis for the overall effect yielded a Z-value of 1.114 (p = 0.265) (Figure 8).
In Park et al, no significant difference in LLT was observed between the control group and the TPT group, with thickness values of 90.40 ± 12.90 μm and 86.76 ± 16.83 μm, respectively. However, the LLT in the control group decreased to 81.94 ± 11.57 μm after one month and returned to baseline values after three months. According to Zhao et al, no statistically significant difference in average LLT was found between the TPT-surgery eyes and the non-TPT-surgery eyes at any of the measured time points, including one week, one month, and three months post-treatment (p > 0.05). These values remained relatively stable, suggesting that while TPT improved other parameters of meibomian gland function, it did not result in a significant change in LLT in the context of cataract surgery (Figure 8).
Publication Bias
To evaluate the presence of publication bias in our meta-analysis, we assessed the symmetry of funnel plots and conducted statistical tests, including Egger’s test and Begg’s test. The funnel plots appeared visually symmetric, indicating a low likelihood of significant publication bias for both MGYLS score and TBUT outcomes (Figures 10 and 11).
 |
Figure 10 Funnel Plot of MGYLS score. |
 |
Figure 11 Funnel Plot of TBUT. |
Discussion
This study explored the efficacy of TPT in managing dry eye symptoms after cataract surgery. Compared with other analyses, our study focused specifically on the efficacy of TPT in managing dry eyes before and after cataract surgery, addressing key questions related to the severity of symptoms and meibomian gland function. Pang et al27 compared vectored thermal pulsation treatment (VTPT) and warm compress treatment (WCT) for MGD, while Hu et al26 evaluated the effectiveness of Lipiflow for MGD and dry eye disease. Our study found moderate benefits of TPT in improving MGYLS score and TBUT, with no significant publication bias. As shown in Table 2, all studies suggest positive impacts on symptom relief and gland function but also highlight the need for more robust trials.
 |
Table 2 A Comprehensive Table Highlighting Specific Methodological Differences, Patient Demographics, and Other Nuances Compared to Prior Analyses |
Patients often report dry eye symptoms following cataract surgery. The incidence is typically high during the early postoperative period. However, some cases remain symptomatic with dry eye problems for up to six months after this initial acute phase.30 Cataract surgery is associated with dry eyes due to traumatic procedures during surgery, causing injury to the associated structures.31 Additionally, inflammation and disruption of the susceptible ocular surface promote dry eye symptoms.32 Moreover, preexisting dry eye and other ocular surface diseases may predispose a patient to become more prone to developing marked symptoms following surgical procedures.33 Similarly, age is a significant factor in the development of dry eye symptoms.34 The elderly, the primary age group that undergoes cataract surgery, are more likely to experience dry eye due to age-related changes in tear production and the health of the ocular surface. In other cases, medications administered after the surgery, such as topical antibiotics and steroids, can further disrupt tear production and increase dry eye symptoms.25
Dry eyes are associated with MGD, a chronic ocular disease of the meibomian glands frequently accompanied by terminal duct obstruction and alterations in glandular production.28 MGD is a fairly common condition that affects many patients.29
MGD is a prevalent condition that affects the structure and function of the meibomian glands, which are responsible for producing the lipid component of the tear film.35 This dysfunction significantly contributes to evaporative DED, resulting in a range of ocular surface symptoms. Across global demographics, MGD is a leading cause of DED, playing a substantial role in many cases.36 However, prevalence rates vary among different populations and age groups. Despite these variations, MGD is widely recognized as a major condition affecting a significant portion of the adult population. Its prevalence is further exacerbated by prolonged computer use, an aging population, and environmental changes.
The most common cause of MGD is gland obstruction.37 Meibum will continue to be secreted by the gland even if it is blocked, increasing pressure within the gland and resulting in ductal dilation, duct dropout, acinar degeneration, and eventually, meibocyte death.38,39 Most patients with MGD are asymptomatic, however, some are symptomatic and present with dry eye symptoms such as itching, a burning sensation, eye discomfort, eyelid swelling, foreign body sensation, watering, redness, irritation, or blurred vision.40,41
This study’s results show a positive impact of TPT on meibomian gland function. Most included studies reported significant improvements in meibomian gland scores, secretion quality, and gland-yielding liquid secretion scores after TPT compared to control groups.21,23,24 In addition, there was a statistically significant difference in meibomian gland liquid secretion between TPT and other interventions, including manual expression, lipid-based eye drops, or other devices designed to treat MGD. The findings emphasize the efficacy of thermal pulsation mechanisms involving controlled heat application and pulsations to liquefy obstructed meibum.
This study also emphasizes the role of TPT in enhancing tear film quality and ocular surface health. A significant property of the TBUT is that it assesses the stability of the tear film, the comfort of the eye, and vision in general. Tear film stability would result in less evaporation of tears and consequently decrease the dryness of the cornea’s surface. However, corneal and conjunctival staining was assessed as a critical index in ocular surface health assessment. Less staining means the purpose of the TPT for corneal and conjunctival cells has been achieved.
Management of MGD is aimed at treating signs and symptoms of the disease. Changes in daily routines can help patients with MGD prevent disease exacerbation. Monitoring computer use and maintaining proper eyelid hygiene are essential components of effective disease management.42 Reducing time spent on computers can help decrease the rate of tear film evaporation, thereby improving dry eye symptoms. In addition, regular eyelid hygiene using warm water and local shampoo is advised to reduce the gland’s obstruction.43 Patients with severe symptoms may use topical anti-inflammatory drugs and eye ointment for lubrication at bedtime to reduce the severity of their symptoms.44 Additionally, if the patients still have persistent bothering symptoms, they can be offered to be treated surgically through surgical procedures like intraductal probing.44
Although the present meta-analysis had taken measures to analyze risk biases and heterogeneity, some limitations should be noted. The mean age of the trial participants ranged from 48.9 to 73.4 years, with one study not reporting age information, making our findings less applicable to younger individuals. Most patients with DED and needing cataract are older.
Research Implications
This study sheds light on the potential benefits of TPT in controlling dry eye problems after cataract surgery. This study, which focuses on particular outcomes such as meibomian gland function, TBUT, and ocular surface health, has the potential to improve post-surgical dry eye therapy. The data indicates that TPT could be a useful therapy option for enhancing meibomian gland secretion of meibum, tear film stability, and overall ocular surface health in post-cataract patients. This may help ophthalmologists adjust pre- and post-cataract dry eye therapy, potentially leading to improved patient outcomes and satisfaction.
Strengths and Limitations
One strength of this systematic review is its comprehensive methodology, following PRISMA guidelines and utilizing robust risk-of-bias assessment tools such as ROBINS-I and Robvis 2.0. Including various study designs, including randomized controlled trials, prospective cohort studies, and retrospective studies, enhances the generalizability of the results. Additionally, the use of random-effects models for data analysis ensures that the heterogeneity between studies is accounted for. However, there are notable limitations. The study included a relatively small number of trials (seven), limiting the findings’ power and generalizability. The heterogeneity in some outcomes, such as MGYLS score and LLT, also suggests variability in study methodologies and patient populations.
Conclusion
This systematic review and meta-analysis highlight the potential benefits of TPT in improving meibomian gland function, tear film stability, and symptoms of dry eye following cataract surgery. TPT shows moderate efficacy in improving meibomian gland function and alleviating dry eye symptoms. The analysis of MGYLS score revealed a small but statistically significant effect with Cohen’s d = 0.29 and no significant heterogeneity (I² = 0%). TPT also improved TBUT, with Cohen’s d = 0.15, indicating enhanced tear film stability. However, the effects on OSDI score and LLT were less pronounced, with the OSDI score showing significant improvement in some studies (p = 0.02) and LLT showing no significant change (Cohen’s d = −0.22, p = 0.265). These results suggest that while TPT is effective in improving MGYLS and TBUT, it can be an effective non-invasive treatment for post-surgical dry eye, particularly in enhancing gland secretion and TBUT. Further well-designed randomized controlled trials with larger sample sizes are needed to confirm these findings and establish clearer guidelines for clinical practice.
Ethics Approval and Consent to Participate
Not applicable. This study does not involve human participants, human data or human tissue.
Consent for Publication
Not applicable. This study does not involve individual data.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No specific funding was received from any funding bodies in the public, commercial, or not-for-profit sectors to conduct the work described in this manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Lam PY, Shih KC, Fong PY, et al. A review on evidence-based treatments for meibomian gland dysfunction. Eye Contact Lens. 2020;46:3–16. doi:10.1097/ICL.0000000000000680
2. Teo CHY, Ong HS, Liu Y-C, Tong L. Meibomian gland dysfunction is the primary determinant of dry eye symptoms: analysis of 2346 patients. Ocular Surf. 2020;18:604–612. doi:10.1016/j.jtos.2020.06.008
3. Meng Z, Chu X, Zhang C, et al. Efficacy and Safety evaluation of a single thermal pulsation system treatment (Lipiflow®) on meibomian gland dysfunction: a randomized controlled clinical trial. Intl Ophthalmol. 2022;43:1175–1184. doi:10.1007/s10792-022-02516-x
4. D’Souza S, Basu S, Narang P, Donthineni P. Evaporative dry eye disease due to meibomian gland dysfunction: preferred practice pattern guidelines for diagnosis and treatment. Indian J Ophthalmol. 2023;71:1348. doi:10.4103/ijo.ijo_2841_22
5. Sabeti S, Kheirkhah A, Yin J, Dana R. Management of meibomian gland dysfunction: a review. Surv Ophthalmol. 2020;65:205–217. doi:10.1016/j.survophthal.2019.08.007
6. Tauber J, Owen J, Bloomenstein M, Hovanesian J, Bullimore MA. Comparison of the iLUX and the lipiflow for the treatment of meibomian gland dysfunction and symptoms: a randomized clinical trial. Clin Ophthalmol. 2020;14:405–418. doi:10.2147/opth.S234008
7. Findlay Q, Reid K. Dry eye disease: when to treat and when to refer. Aust Prescr. 2018;41:160–163. doi:10.18773/austprescr.2018.048
8. Gupta PK, Periman LM, Lain E, et al. Meibomian gland dysfunction: a dermatological perspective on pathogenesis and treatment outlook. Clin Ophthalmol. 2021;15:4399–4404. doi:10.2147/opth.S327407
9. Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124:S20–s26. doi:10.1016/j.ophtha.2017.05.031
10. Cho YK, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009;23:65–73. doi:10.3341/kjo.2009.23.2.65
11. Mikalauskiene L, Grzybowski A, Zemaitiene R. Ocular surface changes associated with ophthalmic surgery. J Clin Med. 2021;10. doi:10.3390/jcm10081642
12. Sharma B, Soni D, Saxena H, et al. Impact of corneal refractive surgery on the precorneal tear film. Indian J Ophthalmol. 2020;68:2804. doi:10.4103/ijo.ijo_2296_19
13. Medeiros CS, Santhiago MR. Corneal nerves anatomy, function, injury and regeneration. Exp Eye Res. 2020;200:108243. doi:10.1016/j.exer.2020.108243
14. Labetoulle M, Benitez-del-Castillo JM, Barabino S, et al. Artificial tears: biological role of their ingredients in the management of dry eye disease. Int J Mol Sci. 2022;23:2434. doi:10.3390/ijms23052434
15. Bilkhu P, Vidal-Rohr M, Trave-Huarte S, Wolffsohn JS. Effect of meibomian gland morphology on functionality with applied treatment. Contact Lens Anterior Eye. 2022;45:101402. doi:10.1016/j.clae.2020.12.065
16. Schechter B, Mah F. Optimization of the ocular surface through treatment of ocular surface disease before ophthalmic surgery: a narrative review. Ophthalmol Ther. 2022;11:1001–1015. doi:10.1007/s40123-022-00505-y
17. Chen K-Y, Chan H-C, Chan C-M. Is retinal vein occlusion highly associated with an increased risk of myocardial infarction? A systematic review and meta-analysis. Int J Retina Vitreous. 2024;10:86. doi:10.1186/s40942-024-00606-9
18. Rolando M, Merayo-Lloves J. Management strategies for evaporative dry eye disease and future perspective. Curr Eye Res. 2022;47:813–823. doi:10.1080/02713683.2022.2039205
19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi:10.1186/s13643-021-01626-4
20. Matossian C. Impact of thermal pulsation treatment on astigmatism management and outcomes in meibomian gland dysfunction patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2283–2289. doi:10.2147/opth.s263046
21. Park J, Yoo Y-S, Shin K, et al. Effects of lipiflow treatment prior to cataract surgery: a prospective, randomized, controlled study. Am J Ophthalmol. 2021;230:264–275. doi:10.1016/j.ajo.2021.04.031
22. Zhao Y, Li J, Xue K, et al. Preoperative management of MGD with vectored thermal pulsation before cataract surgery: a prospective, controlled clinical trial. Semin Ophthalmol. 2021;36:2–8. doi:10.1080/08820538.2021.1881567
23. Mencucci R, Mercuri S, Cennamo M, Morelli A, Favuzza E. Efficacy of vector thermal pulsation treatment in reducing post-cataract surgery dry eye disease in patients affected by meibomian gland dysfunction. J Cataract Refract Surg. 2022. doi:10.1097/j.jcrs.0000000000001124
24. Matossian C, Chang DH, Whitman J, et al. Preoperative treatment of meibomian gland dysfunction with a vectored thermal pulsation system prior to extended depth of focus IOL implantation. Ophthalmol Ther. 2023;12:2427–2439. doi:10.1007/s40123-023-00740-x
25. Afsharkhamseh N, Movahedan A, Motahari H, Djalilian AR. Cataract surgery in patients with ocular surface disease: an update in clinical diagnosis and treatment. Saudi J Ophthalmol. 2014;28:164–167. doi:10.1016/j.sjopt.2014.06.013
26. Hu J, Zhu S, Liu X. Efficacy and safety of a vectored thermal pulsation system (Lipiflow®) in the treatment of meibomian gland dysfunction: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260:25–39. doi:10.1007/s00417-021-05363-1
27. Pang SP, Chen YT, Tam KW, Lin IC, Loh EW. Efficacy of vectored thermal pulsation and warm compress treatments in meibomian gland dysfunction: a meta-analysis of randomized controlled trials. Cornea. 2019;38:690–697. doi:10.1097/ico.0000000000001907
28. Kaur K, Stokkermans T. Meibomian gland disease. StatPearls. 2024.
29. Akowuah PK, Kobia-Acquah E, Donkor R, et al. Prevalence of meibomian gland dysfunction in Africa: a systematic review and meta-analysis of observational studies. Ophthalmic Epidemiol. 2021;29:1–10. doi:10.1080/09286586.2021.1958351
30. Starr CE, Dana R, Pflugfelder SC, et al. Dry eye disease flares: a rapid evidence assessment. Ocular Surf. 2021;22:51–59. doi:10.1016/j.jtos.2021.07.001
31. Cagini C, Di Lascio G, Torroni G, et al. Dry-eye and inflammation of the ocular surface after cataract surgery. J Cataract Refract Surg. 2021;47:1430–1435. doi:10.1097/j.jcrs.0000000000000652
32. Asiedu K. Role of ocular surface neurobiology in neuronal-mediated inflammation in dry eye disease. Neuropeptides. 2022;95:102266. doi:10.1016/j.npep.2022.102266
33. Noor NA, Rahayu T, Gondhowiardjo TD. Prevalence of dry eye and its subtypes in an elderly population with cataracts in Indonesia. Clin Ophthalmol. 2020;14:2143–2150. doi:10.2147/OPTH.S240057
34. Wang M, Muntz A, Lim J, et al. Ageing and the natural history of dry eye disease: a prospective registry-based cross-sectional study. Ocul Surf. 2020;18:736–741. doi:10.1016/j.jtos.2020.07.003
35. Moreno I, Verma S, Gesteira TF, Coulson- Thomas VJ. Recent advances in age-related meibomian gland dysfunction (ARMGD). Ocular Surf. 2023;30:298–306. doi:10.1016/j.jtos.2023.11.003
36. Abu EK, Ofori AO, Boadi-Kusi SB, et al. Dry eye disease and meibomian gland dysfunction among a clinical sample of type 2 diabetes patients in Ghana. Afr Health Sci. 2022;22:293–302. doi:10.4314/ahs.v22i1.36
37. Bukhari AA. Prevalence of obstruction meibomian gland disease among ophthalmology patients. Med Sci. 2009;16.
38. Hwang HS, Parfitt GJ, Brown DJ, Jester JV. Meibocyte differentiation and renewal: insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp Eye Res. 2017;163:37–45. doi:10.1016/j.exer.2017.02.008
39. Rojtinić IŠ. Meibomian gland dysfunction. zir.nsk.hr. 2020.
40. Bilkhu P. Management of allergic conjunctivitis and dry eye. publications.aston.ac.uk. 2014.
41. Chen KY, Chan HC, Wei LY, Chan CM. Efficacy of gabapentin and pregabalin for treatment of post refractive surgery pain: a systematic review and meta-analysis. Int Ophthalmol. 2024;44:409. doi:10.1007/s10792-024-03300-9
42. Lee BS, Kabat AG, Bacharach J, Karpecki P, Luchs J. Managing dry eye disease and facilitating realistic patient expectations: a review and appraisal of current therapies. Clin Ophthalmol. 2020;14:119–126. doi:10.2147/opth.s228838
43. Zhang L, Wang J, Gao Y. Eyelid cleaning: methods, tools, and clinical applications. Indian J Ophthalmol. 2023;71:3607–3614. doi:10.4103/ijo.ijo_1457_23
44. Guillon M, Shah S. Rationale for 24-hour management of dry eye disease: a review. Contact Lens Anterior Eye. 2019;42:147–154. doi:10.1016/j.clae.2018.11.008
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Prevalence of Dry Eye Disease Among Individuals Scheduled for Cataract Surgery in a Norwegian Cataract Clinic
Graae Jensen P, Gundersen M, Nilsen C, Gundersen KG, Potvin R, Gazerani P, Chen X, Utheim TP, Utheim ØA
Clinical Ophthalmology 2023, 17:1233-1243
Published Date: 27 April 2023