Back to Journals » Journal of Inflammation Research » Volume 18
Macrophages as Multifaceted Orchestrators of Tissue Repair: Bridging Inflammation, Regeneration, and Therapeutic Innovation
Authors Wang L , Yang K, Xie X, Wang S, Gan H, Wang X, Wei H
Received 12 March 2025
Accepted for publication 20 June 2025
Published 8 July 2025 Volume 2025:18 Pages 8945—8959
DOI https://doi.org/10.2147/JIR.S527764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qing Lin
Li Wang,1,2 Kai Yang,2 Xinxin Xie,2 Shaohui Wang,2 Huiping Gan,3 Xiaoli Wang,4 Hailiang Wei2
1College of Basic Medicine, Shaanxi University of Chinese Medicine, Xianyang, 712046, People’s Republic of China; 2Department of General Surgery, Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, 712000, People’s Republic of China; 3Department of Anorectal Surgery, Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, 712000, People’s Republic of China; 4Department of Dermatology, Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, 712000, People’s Republic of China
Correspondence: Hailiang Wei, Email [email protected] Xiaoli Wang, Email [email protected]
Abstract: Macrophages play pivotal roles in tissue repair through remarkable functional plasticity, orchestrated by their developmental origins and local microenvironmental cues. Embryonically derived resident macrophages primarily maintain tissue homeostasis, while monocyte-derived macrophages respond predominantly to inflammation and extracellular matrix remodeling. Effective tissue repair requires precise temporal regulation of macrophage polarization, balancing inflammation resolution, angiogenesis, and scar formation. Metabolic reprogramming further enhances macrophage plasticity, enabling adaptation to fluctuating energy demands at injury sites. Emerging evidence also highlights that macrophages integrate biomechanical forces—such as matrix stiffness and shear stress—with biochemical signals to fine-tune their inflammatory and reparative programs. Recognizing this mechanoregulation broadens therapeutic avenues for precisely modulating macrophage behavior in regenerative medicine. Targeting macrophage subsets, polarization states, or metabolic pathways has emerged as a promising therapeutic strategy to optimize healing outcomes. However, the inherent complexity of macrophage heterogeneity presents considerable challenges to therapeutic precision. This review systematically summarizes the multifaceted roles of macrophages in tissue repair, emphasizing how developmental origins dictate functional specificity, dynamic phenotypic transitions, and metabolic adaptability, aiming to advance macrophage-based precision therapeutics for regenerative medicine.
Keywords: macrophage, tissue repair, macrophage polarization, therapeutic strategy
Introduction
Following tissue injury, necrotic cell debris and invading pathogens release damage-associated molecular patterns and pathogen-associated molecular patterns, triggering an inflammatory response.1 Immune cells—including macrophages, neutrophils, and natural killer cells—are recruited to the injury site to clear cellular debris and pathogens, while simultaneously collaborating with epithelial cells, endothelial cells, fibroblasts, and stem cells to facilitate tissue repair.2 During this process, the innate immune response is activated to recruit inflammatory cells that initiate the repair cascade.3 As the inflammatory phase subsides, macrophages shift from a pro-inflammatory phenotype to a reparative phenotype, eventually departing the injury site or being cleared to restore tissue homeostasis.4 Disruption of macrophage function during this process can lead to various pathological conditions, including the overproduction of inflammatory mediators and fibroblasts, which may result in chronic wounds and eventually progress to pathological fibrosis.5
Macrophages play a dual regulatory role in tissue repair. In the early inflammatory phase, macrophages secrete chemotactic factors that enhance the inflammatory response following injury.6 Once the inflammatory phase concludes, the macrophage population undergoes a phenotypic transformation into a pro-reparative state.7 At this stage, these cells secrete large amounts of pro-proliferative factors such as platelet-derived growth factor, which not only accelerates the regeneration of damaged cells but also promotes the formation of new vascular networks.8 By releasing specific signaling molecules, macrophages can also induce fibroblasts to transform into contractile myofibroblasts—a process that is essential for wound closure and extracellular matrix (ECM) remodeling.9 During the later stages of repair, macrophages gradually adopt an anti-inflammatory phenotype.10 These cells, by sensing inhibitory signals such as IL-10, secrete their own anti-inflammatory mediators and express immune regulatory molecules like PD-L1.11 This immunomodulatory function is critical for terminating the inflammatory cascade; however, if dysregulated, it may cause secondary tissue damage and significantly delay the healing process.
By elucidating the interplay between macrophage ontogeny, phenotypic plasticity, and metabolic reprogramming, this review highlights the critical regulatory roles macrophages play in tissue regeneration. Specifically, we discuss their contributions to inflammation resolution, fibrosis attenuation, angiogenesis promotion, and interactions with mesenchymal stem cells and fibroblasts. Furthermore, we examine the mechanisms underlying macrophage phenotype transitions and metabolic reprogramming during tissue repair. These insights may transform current therapeutic paradigms in regenerative medicine by enabling more precise modulation of macrophage functions, thus enhancing tissue integrity and facilitating targeted tissue restoration.
Origins and Phenotypic Heterogeneity of Macrophages
Macrophage Polarization: Beyond the Classical M1/M2 Paradigm
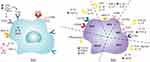
Macrophages exhibit remarkable plasticity, with their phenotypes being profoundly influenced by the local tissue microenvironment, thereby adopting multiple distinct states. The traditional M1/M2 dichotomy is primarily derived from in vitro studies (as shown in Figure 1). Under experimental conditions, macrophages stimulated by lipopolysaccharide (LPS), TNF-α, or IFN-γ typically polarize towards a pro-inflammatory M1 phenotype. These cells highly express TLR-2, TLR-4, CD80, CD86, iNOS, and MHC-II, accompanied by the activation of NF-κB and STAT1 signaling pathways. During wound repair, M1 macrophages promote the inflammatory response by releasing cytokines such as IL-6, IL-12, TNF-α, reactive oxygen species (ROS), and antimicrobial peptides.12,13 Additionally, M1 macrophages possess a robust phagocytic capacity, facilitating the clearance of debris and bacteria from the wound environment.14
As the acute inflammatory phase wanes, macrophages tend to transition into a reparative M2 phenotype, characterized by high expression levels of CD206, CD163, CD209, and FIZZ1. M2 macrophages enhance tissue repair through the secretion of anti-inflammatory cytokines like IL-10 and growth factors such as PDGF and TGF-β.15,16 Notably, the M2 phenotype can be further subdivided into distinct subtypes (M2a, M2b, M2c, M2d, and M2eff) based on differences in activating factors and surface markers.17 Specifically, the M2a subtype is predominantly activated by IL-4 or IL-13, exhibiting high expression of CD206 and CD163 alongside the secretion of IL-10 and TGF-β, and is associated with type II inflammation, ECM deposition, fibrosis, and angiogenesis.18 The M2b subtype is typically induced by immune complexes, LPS, or IL-1β, characterized by the expression of MHC-II and CD86, and the secretion of TNF-α, IL-1, IL-6, and IL-10, thereby participating in immune regulation and TH2 activation.19 The M2c subtype is mainly induced by IL-10 or TGF-β1, with markers including CD163, CCR2, TLR1, and TLR8, which enhance the expression of IL-10 and TGF-β, contributing to MMP secretion, ECM remodeling, fibrosis, angiogenesis, and apoptotic cell clearance.20 The M2d subtype emerges following stimulation by TLR ligands or IL-10, accompanied by the secretion of IL-10 and VEGF, thus promoting angiogenesis and eliciting immunosuppression.21 The M2eff subtype is activated via efferocytosis and participates in the resolution of inflammation and tissue repair by secreting cytokines such as TGF-β, PGE2, and PAF.22
It is important to note that this simplified classification has certain limitations in vivo, as macrophages rarely exist in purely M1 or M2 states. Instead, they often adopt a spectrum of intermediate phenotypes in response to dynamic changes in the local microenvironment to meet diverse physiological and pathological demands.23
Developmental Origin Dictates Macrophage Function in Tissue Homeostasis and Injury
In addition to the functional variations resulting from different activation states, the developmental origin of macrophages significantly influences their behavior. There are two ontogenic sources for macrophages.24 The first source arises during embryonic development, where macrophages are initially generated in the yolk sac or fetal liver, subsequently migrating to specific tissues where they differentiate into tissue-resident macrophages (TRMs) with region-specific characteristics. These cells play a critical role in maintaining organ homeostasis.25 The second stems from the bone marrow hematopoietic system, in which macrophages differentiate from monocytes (moMs), representing the primary source of macrophages postnatally. These cells are capable of homing to sites of injury and, in response to local biological signals, differentiating into specialized functional phenotypes that execute a range of biological roles.26
Lineage-tracing studies have demonstrated that most adult cardiac macrophages are derived from the embryonic yolk sac, whereas CCR2-positive monocytes that infiltrate following injury dominate the early inflammatory response.27 Notably, TRMs of embryonic origin accelerate the repair process by promoting cardiomyocyte proliferation and vascular regeneration. When the migration of monocytes into the injured heart is inhibited, the retention of resident macrophages significantly enhances tissue healing through their anti-inflammatory and reparative functions.28 Subsequently, monocytes and moMs become the predominant mononuclear phagocyte population at the site of injury, performing functions such as antigen presentation, regulation of inflammation, and replacement of depleted TRMs.29 Research has revealed that the transition from monocytes to moMs involves a series of continuous phenotypic states—a process referred to as the “monocyte waterfall”—during which monocytes gradually lose their original characteristics and acquire macrophage functions.30 Recent studies further indicate that once Ly6Chi monocytes enter tissues, they can differentiate into two distinct stromal macrophage subpopulations: one associated with nerve bundles and the other with blood vessels, a binary differentiation observed across multiple tissues.31 In addition to the phenotypic differences emerging from the gradual maturation of monocytes into moMs, monocytes themselves exist in multiple subsets. For example, in mice, classical Ly6Chi monocytes and non-classical Ly6Clo monocytes (corresponding to human CD14+CD16⁻ and CD14loCD16+, respectively) perform distinct functions under both homeostatic and inflammatory conditions.32,33
This suggests that bone marrow-derived monocytes may exacerbate injury by releasing destructive mediators, whereas resident macrophages coordinate the resolution of inflammation and structural remodeling. In a spinal cord injury model, functionally distinct pro-inflammatory and reparative macrophages were observed to migrate to the injury site via specific molecular signals. Pro-inflammatory Ly6ChiCX3CR1lo monocytes depend on the interaction between the CCR2 receptor and the chemokine CCL2 for migration, while reparative Ly6CloCX3CR1hi cells are directed via a molecular pathway involving vascular cell adhesion molecule VCAM-1, integrin receptor VLA-4, and ecto-enzyme CD73.34 Lörchner et al demonstrated that following cardiac injury, TNF released by inflammatory monocytes stimulates damaged cardiomyocytes to secrete substantial amounts of regenerating islet-derived protein 3β. This protein recruits specific macrophage subpopulations to clear neutrophils, effectively preventing excessive degradation of the cardiac matrix and structural damage.35
Mechanical Cues, Cytoskeletal Dynamics, and Macrophage Phenotype in Tissue Repair
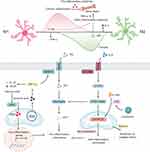
A growing body of evidence indicates that macrophages are mechanosensitive cells capable of perceiving diverse mechanical stresses—such as substrate stiffness and shear stress—present in host tissues.36 These physical signals are transduced into intracellular biochemical cues through plasma-membrane mechanoreceptors, cytoskeletal force transmission, and nuclear mechanotransduction pathways.37 Consequently, they modulate infiltrating macrophage behavior, influencing the switch between pro- and anti-inflammatory phenotypes and altering phagocytic activity.38
Extracellular Mechanosensing and Phenotypic Polarization
Focal adhesions (FAs) link the extracellular matrix (ECM) to the cytoskeleton and serve as mechanoreceptors. Upon integrin clustering, ECM components engage integrins, which interface with multiple intracellular proteins—including focal-adhesion kinase (FAK). The mechanical activity of integrins is coupled to actin dynamics through FAs.38 Integrins are heterodimeric receptors composed of non-covalently associated α- and β-subunits;39 the β-propeller domain of the α-subunit and the βA domain of the β-subunit form the principal ligand-binding site.40 Mechanical deformation of the ECM exerts tensile forces on bound integrins, inducing conformational extension and activation.41 In macrophages, expression levels of β2 and β3 integrins correlate positively with adhesion and motility; inhibition of these integrins markedly suppresses both processes.42 Integrin signalling can also restrain inflammatory activation: macrophages from αM‐integrin-deficient mice secrete significantly more TNF-α than wild-type cells after LPS stimulation.43 Activated integrins recruit and activate downstream mediators such as FAK.44 FAK is essential for macrophage trafficking; FAK-deficient macrophages exhibit impaired recruitment to inflammatory sites and attenuated chemotaxis toward CSF-1.45 FAK activation via phosphorylation propagates to downstream molecules such as Rac.46 Deletion of Rac1 or Rac2 in mice alters macrophage morphology: Rac1−/− bone-marrow-derived macrophages (BMDMs) become elongated with reduced spreading, whereas Rac2−/− cells are likewise elongated but spread to a similar extent. Rac2 deficiency lowers F-actin content and abolishes podosome formation, whereas Rac1 deficiency merely disrupts podosome assembly. Notably, loss of Rac1 does not impair migration, whereas Rac2 deficiency reduces the number of migrating cells without affecting velocity; invasion through matrix substrates is diminished in both Rac1- and Rac2-null macrophages.47 Integrin-triggered activation of FAK and Src also stimulates ROCK and, via a Rac1/ROCK-dependent mechanism, remodels cell morphology and promotes anti-inflammatory polarization.48 Under LPS/ATP stimulation, low-stiffness substrates augment mitochondrial ROS production in macrophages; the ROCK inhibitor Y-27632 limits this response and suppresses inflammasome activation and cell migration.49 Within the 11–88 kPa stiffness range, macrophages up-regulate the anti-inflammatory gene IL-10 in a RhoA/ROCK-dependent manner.50
Mechanosensitive ion channels also transduce mechanical cues by altering intracellular ion concentrations and membrane potential.51 Key channels include the Piezo family and transient receptor potential (TRP) channels. Piezo1 and Piezo2, trimeric propeller-shaped channels, sense mechanical deformation and induce Ca²+ influx.52 Macrophages exhibit Piezo1-dependent mechanosensitivity; periodic hydrostatic pressure drives Ca²+ entry, activates AP-1, and induces EDN1 transcription, thereby stabilizing HIF-1α, up-regulating inflammatory genes, and facilitating neutrophil recruitment and pathogen clearance.53 Piezo1-mediated Ca²+ signals also initiate NF-κB-dependent transcriptional programmes that influence macrophage polarization; channel inhibition dampens inflammation and accelerates wound healing.54 Mechanical stretch suppresses Piezo1 expression and attenuates inflammatory activation, whereas the Piezo1 agonist Yoda1 heightens inflammation, underscoring the channel’s mechanosensory role.55 TRP channels are likewise expressed in macrophages. TRPV4, a mechanosensitive, non-selective Ca²+-permeable cation channel, triggers Ca²+-dependent cytoskeletal remodelling and gene transcription, thereby influencing macrophage phenotype and function.56 Increased matrix stiffness drives TRPV4-dependent polarization toward the pro-inflammatory M1 state.57 TRPV4 also mediates NLRP3 inflammasome activation and IL-1β production, provoking TRPV4-dependent inflammatory responses in vivo.58
Cytoskeletal–Nuclear Mechanotransduction of Inflammatory Programs
The nucleus, harbouring the genetic material, acts as a mechanosensitive organelle that directly modulates gene expression. Mechanical signals are relayed from the cytoskeleton to the nuclear envelope and interior via the LINC (linker of nucleoskeleton and cytoskeleton) complex.59 External forces induce conformational changes in nuclear-membrane proteins, tension the envelope, and open nuclear-pore complexes (NPCs). The same forces stretch, relax, or compact chromatin, initiate DNA and histone modifications, and consequently modulate transcription-factor accessibility.60 Alterations in actin dynamics orchestrate the nucleocytoplasmic shuttling of transcriptional regulators such as YAP/TAZ and myocardin-related transcription factor A (MRTF-A).61 On stiff substrates, elevated cytoskeletal tension promotes actin polymerization, dissociating G-actin–MRTF complexes; MRTF subsequently translocates to the nucleus to activate MRTF–SRF target genes. Simultaneously, NF-κB subunits p50 and p65, together with histone deacetylase 3 (HDAC3), are exported to the cytoplasm, down-regulating NF-κB target genes. Conversely, under low-tension conditions, actin depolymerization retains NF-κB and HDAC3 within the nucleus.62 Reduced actin polymerization also diminishes MRTF-A–SRF activity, thereby attenuating inflammatory responses.36
Functional Diversity of Macrophages During Tissue Repair
Temporal Dynamics of Macrophage Polarization: Balancing Inflammation and Regeneration
The timing and duration of M1 and M2 macrophage responses are critical for successful tissue repair, with factors such as injury severity, duration of inflammation, macrophage activation state, tissue type, and the host’s overall health all influencing the repair process.63 In bone repair, depletion of macrophages inhibits the osteoinductive effects of β-tricalcium phosphate.64 This observation has been confirmed in a mouse tibial injury model, where Alexander et al demonstrated that bone macrophages are essential for type I collagen matrix deposition and bone mineralization. Colony-stimulating factor 1 (CSF-1) significantly increases macrophage numbers at the injury site, concomitantly enhancing type I collagen matrix deposition and promoting mineralization.65 An initial inflammatory response dominated by M1 macrophages is necessary for bone healing;66 however, the clearance of macrophages before or during the acute inflammatory phase67 or the premature suppression of the immune response68 negatively affects bone repair. Although M1-polarized macrophage-mediated inflammation is generally detrimental to tissue repair, in vitro studies have shown that M1 macrophages can promote the osteogenic differentiation and mineralization of mesenchymal stem cells, suggesting an additional role in bone repair.69 Nevertheless, sustained chronic inflammation mediated by M1 macrophages is highly deleterious to bone repair; the persistent production of pro-inflammatory cytokines enhances osteoclast activity, leading to bone resorption and inhibition of osteoblast-mediated bone formation.70 While the reparative functions of M2 macrophages are considered indispensable in tissue healing, their presence during the acute inflammatory phase can be counterproductive.71 In vitro stimulation of bone marrow-derived macrophages with IL-4 or IL-10 to generate M2a or M2c subsets, followed by injection of these polarized macrophages into full-thickness skin wounds in diabetic db/db mice, revealed that despite exhibiting an anti-inflammatory phenotype in vitro, M2 macrophages did not improve wound healing in wild-type mice and, in fact, delayed wound closure in diabetic mice.72 Similarly, Dreymueller et al reported that embryonic stem cell-derived macrophages with an M2-like phenotype prolonged the healing time of deep skin wounds created by tail root flaps in mice.73 Since M0 macrophages in the control group were not driven toward an M1 phenotype by the early pro-inflammatory wound microenvironment, the delayed healing observed with M2 macrophages is more likely attributable to their interference with the early pro-inflammatory phase.73 These findings suggest that a certain degree of initial pro-inflammatory response is not only benign but necessary to activate subsequent phases of healing, while uncontrolled, prolonged, or excessive inflammatory activation is truly harmful. This conclusion is further supported by the work of Shinozaki et al, which demonstrated that the absence of tumor necrosis factor-α (TNFα) promotes granulation tissue formation yet delays re-epithelialization in mouse skin wounds.74
Macrophages also play a vital role in fibrosis regression through the secretion of matrix metalloproteinases (MMPs) that degrade the ECM.75 In liver injury, MMPs secreted by macrophages are inhibited by TIMPs produced by myofibroblasts and activated hepatic stellate cells, resulting in continuous ECM deposition and scar formation.76 Once the injurious stimulus subsides, macrophages undergo a phenotypic switch via efferocytosis, transitioning towards an anti-fibrotic, resolution-promoting state.77 These resolution-phase macrophages not only clear cellular debris but also secrete fibrotic degradation enzymes, including MMP9, MMP12, and MMP13, thereby accelerating the breakdown of fibrotic matrix.78,79 This indicates that the phenotypic transition of macrophages is crucial in the resolution of fibrosis. Furthermore, studies have shown that the timing of the M2 response directly determines whether soft tissue injury ultimately returns to normal homeostasis or evolves into chronic fibrosis.80 For example, depleting macrophages during the liver injury phase can reduce the deposition of myofibroblasts and ECM; however, removing them during the fibrosis resolution phase weakens the capacity for ECM degradation,81 underscoring the critical role of macrophages in orchestrating the temporal regulation of the injury response.
Moreover, the dynamic balance of macrophage phenotypes has been implicated in key aspects of central nervous system repair. During early demyelination, microglia and infiltrating peripheral macrophages exhibit an M(IFN-γ) phenotype, secreting pro-inflammatory cytokines such as IFN-γ. As repair mechanisms are initiated, these cells transition to an M2 phenotype, secreting anti-inflammatory cytokines such as IL-4 and releasing reparative mediators like activin A.82 Similarly, in liver injury models, IL-4 and IL-10 have been reported to facilitate the conversion of inflammatory monocytes to a reparative phenotype.83 In a parasitic lung inflammation model, IL-4 binds to IL-4 receptors on macrophages and drives the production of IGF-1 and IL-10, thereby mitigating IL-17-mediated tissue damage.84 These findings underscore the importance of IL-4R-mediated signaling in promoting the transition of macrophages from a pro-inflammatory to an anti-inflammatory phenotype. In a mouse model with macrophage-specific IL-4R knockout, it was found that the loss of IL-4R in resident macrophages exacerbates liver inflammation without altering fibrosis, whereas the loss of IL-4R in recruited monocytes significantly worsens liver fibrosis without intensifying inflammation.85 This suggests that the M2 phenotype of resident versus recruited macrophages plays distinct roles in inflammation resolution and fibrosis.
Metabolic Reprogramming in Macrophage Activation and Repair Efficiency
Recent study indicates that the metabolic characteristics of immune cells influence their plasticity and play a role in the tissue repair processes regulated by the immune system.86 Although macrophages are known to rapidly adapt to the local microenvironment at injury sites—combating infections while promoting repair—they must activate multiple metabolic pathways, including glycolysis, the tricarboxylic acid (TCA) cycle, and fatty acid synthesis, to meet the biosynthetic demands of tissue repair (as shown in Figure 2).87 The precise impact of these pathways on the repair process remains an active area of investigation.
Activation of hypoxia-inducible factor 1-alpha (HIF-1α) is crucial for the infiltration and activation of myeloid cells in vivo. When HIF-1α is absent, cellular ATP levels dramatically decrease, which impairs myeloid cell aggregation, motility, invasiveness, and bacterial killing. Conversely, loss of HIF-1α’s negative regulator, VHL, leads to a markedly increased acute inflammatory response.88 Under inflammatory conditions, the concentration of the TCA cycle intermediate succinate in macrophages is significantly elevated. This increase in succinate sustains HIF-1α levels and promotes a pro-inflammatory phenotype in macrophages.89 For instance, upon LPS stimulation, mitochondrial succinate dehydrogenase facilitates succinate oxidation, driving ROS production and inducing the expression of pro-inflammatory genes.90 In contrast, itaconate can inhibit succinate dehydrogenase, thereby suppressing succinate oxidation and exerting anti-inflammatory effects.91 Moreover, the phenomenon of trained immunity in myeloid cells is characterized by high glucose consumption and an increased glycolytic rate. This metabolic shift depends on the activation of the HIF-1α pathway; inhibition of HIF-1α markedly reduces the expression of the glucose transporter GLUT1, thereby lowering glycolysis. Consequently, under LPS stimulation, macrophages generate less ATP, which diminishes their bactericidal capacity.92 In addition, the synthesis of key mediators involved in wound healing, such as IL-1β and vascular endothelial growth factor (VEGF), is significantly reduced in HIF-1α-deficient macrophages.88 These findings underscore HIF-1α’s role in regulating macrophage glycolysis and the TCA cycle, which in turn affects the antibacterial and pro-inflammatory functions of M1 macrophages.
Under the influence of Th2 cytokines—including interleukin-4 (IL-4), as well as transcription factors such as STAT6 and PPARγ coactivator-1β (PGC-1β)—macrophages activate pathways of fatty acid oxidation and mitochondrial oxidative phosphorylation.93 Expression of PGC-1β promotes the M2 polarization of macrophages while strongly inhibiting pro-inflammatory cytokine expression.93 IL-4-stimulated macrophages exhibit increased rates of fatty acid oxidation, improved mitochondrial quality, and enhanced respiratory activity.93 This metabolic reprogramming from glycolysis toward oxidative phosphorylation is closely associated with the shift from a pro-inflammatory to a reparative (M2) phenotype, suggesting that modulation of macrophage metabolism could be a promising strategy to regulate inflammation. Additionally, lysosomal acid lipase plays a pivotal role in macrophage lipid degradation by promoting fatty acid oxidation. Studies have demonstrated that the lipolytic function of lysosomal acid lipase enhances mitochondrial oxidative phosphorylation and facilitates M2 polarization.94 Inhibition of lysosomal lipolysis within macrophages has been shown to block IL-4 receptor α-mediated M2 polarization.94 M2 macrophages activated via IL-4 receptor α further promote tissue repair by facilitating the assembly of collagen fibers. This process involves the transmission of IL-4 receptor α signals from macrophages to fibroblasts, leading to the induction of lysyl hydroxylase 2 expression.95 Moreover, research indicates that Relm-α participates in this signaling cascade, with its release from macrophages being dependent on proper mitochondrial function.96
Anti-Inflammatory and Anti-Fibrotic Roles of Macrophages in Tissue Regeneration
Macrophage anti-inflammatory and anti-fibrotic activities are considered key to the resolution phase of most wound healing responses. As illustrated in Figure 3, De Nardo et al demonstrated that high-density lipoproteins can induce the expression of the transcriptional regulator ATF3 in macrophages, which in turn downregulates the expression of Toll-like receptor (TLR)-induced pro-inflammatory cytokines.97 Yuk et al found that mice deficient in ERRα are more susceptible to endotoxin-induced septic shock, exhibiting a more severe pro-inflammatory response compared to controls. This is because ERRα negatively regulates TLR-induced inflammation by directly binding to the promoter region of the deubiquitinase Tnfaip3 involved in TLR signaling, as well as by controlling metabolic reprogramming.98 In addition, IL-4 and IL-13 promote the phosphorylation of STAT6 and the expression of pro-fibrotic genes by upregulating miR-142-5p and downregulating miR-130a-3p in macrophages, which leads to the inhibition of SOCS1 and the de-repression of PPARγ.99 Furthermore, n-3 polyunsaturated fatty acids, which possess anti-inflammatory properties, exert cardioprotective effects by inhibiting macrophage-mediated cardiac fibroblast activation.100 These findings suggest that multiple mechanisms contribute to the formation of a pro-healing, anti-inflammatory macrophage phenotype. Westphalen et al discovered that a subset of alveolar macrophages (AMs) adherent to the alveolar wall can form gap junction channels with alveolar epithelial cells via connexin 43. These channels permit the direct intercellular transfer of signaling molecules such as Ca²+, which in turn activates the Akt pathway and attenuates the inflammatory response.101 In addition, AMs have been reported to secrete exosomes and vesicles containing suppressors of cytokine signaling (SOCS) proteins, including SOCS1 and SOCS3, which are then taken up by alveolar epithelial cells. This uptake suppresses the activation of STAT signaling within epithelial cells, thereby limiting the expression of pro-inflammatory cytokines.102 Macrophages also influence inflammation by modulating intercellular communication among epithelial cells. Knuever et al found that insulin receptor (IR) and IGF-1R signaling in myeloid cells are crucial for regulating the interactive inflammatory response between the epidermis and dermis. When these receptors are absent in myeloid cells, the propagation of inflammatory signals is curtailed, providing a degree of protection against dermatitis.103 Furthermore, anti-inflammatory macrophages help maintain the resolution of inflammation across multiple tissues by modulating regulatory T cells (Tregs) that secrete IL-10 and TGF-β1.104
Recent studies indicate that macrophages activated by type 2 cytokines also exhibit anti-fibrotic activity, particularly during the chronic repair phase. Models of chronic fibrosis and tumors have shown that M(IL-4) polarized macrophages delay fibrosis by inhibiting local CD4+ T cell responses and reducing the ECM production by myofibroblasts.105,106 In both tumors and granulomas, M(IL-4) macrophages compete with T cells and myofibroblasts in hypoxic regions for arginine and ornithine, thereby creating an immunosuppressive microenvironment.107 This nutritional competition has been identified as an important immunosuppressive mechanism of regulatory macrophages.108 Additionally, IL-6, IL-10, and IL-21 have all been shown to enhance the expression of IL-4R on macrophages, thereby promoting their anti-inflammatory and anti-fibrotic functions following IL-4/IL-13 stimulation.109–111
Macrophage-Driven Angiogenesis: Mechanisms and Temporal Regulation
Establishing a microvascular network through neovascularization at the wound site is a critical component of tissue repair. However, if this process is not precisely regulated, it may result in either aberrant vascular hyperplasia or insufficient vascular formation/regression—both conditions closely associated with the progression of various diseases.112 As key modulators of the microenvironment, macrophages are involved throughout vascular regeneration and have been shown to regulate crucial events such as vascular sprouting, elongation, and branching.113
Traditionally, M2 macrophages have been regarded as the main promoters of angiogenesis, while M1 macrophages are thought to inhibit vessel formation.114,115 Yet, recent research has begun to reveal distinct roles for M1 and M2 macrophages at different stages of neovascularization. For example, Willenborg et al demonstrated that during the early phase of repair, a subset of inflammatory CCR2(+)Ly6C(+) macrophages expresses VEGF-A. Macrophage-derived VEGF-A is subsequently converted into epithelial-derived VEGF-A, thereby promoting physiological tissue vascularization and healing.116 Similarly, other studies indicate that M1 macrophages can release high levels of angiogenic stimulators, including VEGF.117 Moreover, while a short-term presence (1 day) of M1 macrophages has been shown to promote angiogenesis, their prolonged presence (3 days) leads to vascular regression.118 This observation aligns with other reports suggesting that M1 macrophages can both enhance117 and inhibit16,119 angiogenesis. Notably, vascular regression is an integral part of healthy angiogenesis,120 implying that M1 macrophages may support vascular formation by modulating their behavior over time. In addition, in vitro studies and in vivo imaging in zebrafish models have shown that TNFα+ M1 macrophages are closely associated with vascular tips, suggesting that these cells not only promote vascular repair through VEGF secretion but also guide endothelial cell migration.121 The same study further indicated that if pro-inflammatory signals persist or are reactivated in the later stages of repair, proper vascular regression is impaired, a defect linked to the continued expression of VEGF by pro-inflammatory macrophages.121 Collectively, these findings suggest that the timely regulation of macrophage function in vascular repair is crucial. An early pro-inflammatory phase to initiate sprouting, followed by a timely transition to an anti-inflammatory phase to permit vascular regression, is essential for successful vascular repair. In addition to M1 macrophages, M2 macrophages also play unique roles during vascular maturation. For instance, M2a macrophages secrete the pericyte chemoattractant PDGF-BB, which stabilizes pericytes and promotes the anastomosis of budding endothelial cells.117 Meanwhile, M2c macrophages secrete MMPs to degrade the basement membrane, thereby creating space for lumen formation.122 These data suggest that different M2 phenotypes contribute to various stages of vascular stabilization, remodeling, and maturation, emphasizing the need for further studies to elucidate their functions and dynamic changes in vivo.
Overall, M1 and M2 macrophages cooperate via spatiotemporal regulatory mechanisms to complete angiogenesis: M1 macrophages predominantly drive early vascular sprouting, while M2 macrophages are responsible for later lumen maturation. However, the dynamic regulatory networks governing different macrophage polarization states in complex in vivo environments still require further multidimensional investigation.
Intercellular Crosstalk: Macrophage Interactions with Stromal and Immune Cells During Repair
As core drivers of tissue repair, macrophages interact with mesenchymal stem cells (MSCs) to direct the development of specific cell types.123 MSCs, which originate from the mesoderm, are multipotent adult stem cells with self-renewal capability that can differentiate into various cell types including chondrocytes, osteoblasts, and adipocytes.124 The crosstalk between macrophages and MSCs is mediated through paracrine signaling, direct cell–cell contact, and even mitochondrial transfer.125 MSCs exhibit chemotactic properties, and the initial inflammation and activation of pro-inflammatory macrophages following tissue injury facilitate the recruitment of MSCs to the injury site. Once activated, MSCs secrete a range of chemokines such as CCL2 and CCL4, which further mediate macrophage infiltration.126
Direct interactions between macrophages and MSCs via the CD200–CD200R pathway have been shown to enhance the expression of the anti-inflammatory factor TNF-stimulated gene 6 in MSCs.127 This upregulation, in turn, suppresses the activation of pro-inflammatory macrophages, playing a critical role in protecting tissues from damage induced by excessive inflammation.128 Moreover, MSCs are capable of forming transient tunneling nanotubes with macrophages, facilitating the transfer of mitochondria.129 It is reported that the transfer of mitochondria from MSCs to AMs enhances their phagocytic function and promotes their polarization toward a reparative phenotype.130
Fibroblasts, which differentiate from MSCs during embryonic development, are responsible for the production of the ECM.131 Interactions between macrophages and fibroblasts via juxtacrine and paracrine mechanisms promote tissue remodeling.132 For instance, macrophage-derived TGF-β1 can stimulate fibroblast activation, driving their differentiation into myofibroblasts. These myofibroblasts exhibit high expression of smooth muscle actin and secrete large amounts of collagen, thereby enhancing ECM deposition.133 Furthermore, as antigen-presenting cells, macrophages interact with T cells to modulate disease outcomes. Within the immune microenvironment, macrophages and T cells play pivotal roles; typically, M1 macrophages are associated with TH1 responses, whereas M2 macrophages are linked with TH2 cells and Tregs. In turn, cytokines secreted by T helper cells also influence macrophage polarization.134
Macrophage-Targeted Therapies: Emerging Strategies and Translational Opportunities
Macrophages play complex and essential roles in both disease pathogenesis and tissue repair, and modulation of macrophage functions has become a major focus of preclinical and clinical research.135 The proliferation, differentiation, and function of monocytes and macrophages are highly dependent on the CSF1/CSF1 receptor (CSF1R) signaling pathway, making this axis an attractive therapeutic target.136 CSF1 regulates not only macrophage migration, proliferation, and survival, but also their functional heterogeneity and tissue regenerative capacity.137 Several strategies, including CSF1-Fc fusion proteins, neutralizing antibodies against CSF1 or CSF1R, and CSF1R kinase inhibitors, are currently under extensive evaluation and have begun clinical testing to modulate macrophage populations within pathological tissues.138 Anti-CSF1R therapies primarily deplete tissue-resident monocytes and macrophages, impairing their maturation and renewal without substantially affecting pro-inflammatory monocytes, potentially limiting their efficacy in inflammation driven by bone marrow-derived populations.139 Moreover, activation of the CSF1 pathway notably enhances tissue repair and regeneration. For instance, CSF1-Fc therapy significantly accelerates recovery from acute liver injury and liver regeneration post-hepatectomy by expanding resident macrophage and recruited monocyte populations.140 Additionally, CSF1-Fc stimulates macrophages to secrete mediators such as urokinase, TNF-α, and IL-6, directly promoting hepatocyte proliferation, although overactivation poses risks of hepatosplenomegaly.141 Following acute kidney injury, CSF1 similarly accelerates tissue repair by promoting macrophage-derived epithelial regenerative mediators.142
Conclusions
Although macrophages are traditionally classified into discrete M1 and M2 states, accumulating evidence indicates that in vivo macrophages predominantly exist in transitional phenotypes, dynamically responding to microenvironmental stimuli. During tissue repair, macrophages undergo sequential transitions from pro-inflammatory to anti-inflammatory and pro-regenerative states, orchestrating processes such as inflammation resolution, fibrosis inhibition, and angiogenesis through precise cytokine-mediated signals. Their intrinsic metabolic reprogramming further underpins this functional plasticity, enabling macrophages to meet the bioenergetic demands of the repair milieu. Interactions between macrophages and stromal cells represent additional critical modulators of macrophage activity, influencing their polarization and regenerative efficacy. Targeting macrophage functions, particularly via the CSF1/CSF1R signaling axis, has emerged as a promising therapeutic approach to enhance tissue healing. Nevertheless, defining macrophage subsets, their dynamic transitional states, and context-specific molecular markers remain critical. Future studies integrating single-cell profiling with functional validation in tissue-specific injury models will be instrumental in refining macrophage-targeted therapeutic strategies, ultimately achieving precise modulation of tissue integrity and regeneration under pathological conditions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hassanshahi A, Hassanshahi M, Khabbazi S, et al. Adipose-derived stem cells for wound healing. J Cell Physiol. 2019;234(6):7903–7914. doi:10.1002/jcp.27922
2. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/j.immuni.2010.05.007
3. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi:10.1002/path.2277
4. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
5. Manole E, Niculite C, Lambrescu IM, et al. Macrophages and stem cells-two to tango for tissue repair? Biomolecules. 2021;11(5):697. doi:10.3390/biom11050697
6. Yu Y, Yue Z, Xu M, et al. Macrophages play a key role in tissue repair and regeneration. PeerJ. 2022;10:e14053. doi:10.7717/peerj.14053
7. Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18(3):579–587. doi:10.1038/s41423-020-00541-3
8. Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 2019;97(3):258–267. doi:10.1111/imcb.12236
9. Murray LA, Chen Q, Kramer MS, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43(1):154–162. doi:10.1016/j.biocel.2010.10.013
10. Chazaud B. Inflammation and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. 2020;41(6):481–492. doi:10.1016/j.it.2020.04.006
11. Ramachandran P, Iredale JP, Fallowfield JA. Resolution of liver fibrosis: basic mechanisms and clinical relevance. Semin Liver Dis. 2015;35(2):119–131. doi:10.1055/s-0035-1550057
12. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi:10.1038/nri2448
13. Ogle ME, Segar CE, Sridhar S, et al. Monocytes and macrophages in tissue repair: implications for immunoregenerative biomaterial design. Exp Biol Med. 2016;241(10):1084–1097. doi:10.1177/1535370216650293
14. Hesketh M, Sahin KB, West ZE, et al. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18(7):1545. doi:10.3390/ijms18071545
15. Kiss M, Van Gassen S, Movahedi K, et al. Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol. 2018;330:188–201. doi:10.1016/j.cellimm.2018.02.008
16. Jetten N, Verbruggen S, Gijbels MJ, et al. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. doi:10.1007/s10456-013-9381-6
17. Yunna C, Mengru H, Lei W, et al. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi:10.1016/j.ejphar.2020.173090
18. Ross EA, Devitt A, Johnson JR. Macrophages: the good, the bad, and the gluttony. Front Immunol. 2021;12:708186. doi:10.3389/fimmu.2021.708186
19. Wang LX, Zhang S-X, Wu H-J, et al. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106(2):345–358. doi:10.1002/JLB.3RU1018-378RR
20. Nakai K. Multiple roles of macrophage in skin. J Dermatol Sci. 2021;104(1):2–10. doi:10.1016/j.jdermsci.2021.08.008
21. Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care. 2012;1(1):10–16. doi:10.1089/wound.2011.0307
22. Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi:10.1172/JCI1112
23. Xue J, Schmidt S, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–288. doi:10.1016/j.immuni.2014.01.006
24. Gordon S, Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15(1):53. doi:10.1186/s12915-017-0392-4
25. Kierdorf K, Prinz M, Geissmann F, et al. Development and function of tissue resident macrophages in mice. Semin Immunol. 2015;27(6):369–378. doi:10.1016/j.smim.2016.03.017
26. Makdissi N. Macrophage development and function. Methods Mol Biol. 2024;2713:1–9.
27. Epelman S, Lavine K, Beaudin A, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi:10.1016/j.immuni.2013.11.019
28. Lavine KJ, Epelman S, Uchida K, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111(45):16029–16034. doi:10.1073/pnas.1406508111
29. Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49(4):595–613. doi:10.1016/j.immuni.2018.10.005
30. Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Front Immunol. 2018;9:2733. doi:10.3389/fimmu.2018.02733
31. Chakarov S, Lim HY, Tan L, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 2019;363(6432). doi:10.1126/science.aau0964
32. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi:10.1016/S1074-7613(03)00174-2
33. Jakubzick C, Gautier E, Gibbings S, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39(3):599–610. doi:10.1016/j.immuni.2013.08.007
34. Shechter R, Miller O, Yovel G, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38(3):555–569. doi:10.1016/j.immuni.2013.02.012
35. Lörchner H, Pöling J, Gajawada P, et al. Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat Med. 2015;21(4):353–362. doi:10.1038/nm.3816
36. McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72(7):1303–1316. doi:10.1007/s00018-014-1796-8
37. Hou Y, Xie W, Yu L, et al. Surface roughness gradients reveal topography-specific mechanosensitive responses in human mesenchymal stem cells. Small. 2020;16(10):e1905422. doi:10.1002/smll.201905422
38. Zhou H, Xue Y, Dong L, et al. Biomaterial-based physical regulation of macrophage behaviour. J Mater Chem B. 2021;9(17):3608–3621. doi:10.1039/D1TB00107H
39. Pang X, He X, Qiu Z, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8(1):1. doi:10.1038/s41392-022-01259-6
40. Kanchanawong P, Calderwood DA. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat Rev Mol Cell Biol. 2023;24(2):142–161. doi:10.1038/s41580-022-00531-5
41. Chastney MR, Kaivola J, Leppänen V-M, et al. The role and regulation of integrins in cell migration and invasion. Nat Rev Mol Cell Biol. 2025;26(2):147–167. doi:10.1038/s41580-024-00777-1
42. Wang Y, Shi R, Zhai R, et al. Matrix stiffness regulates macrophage polarization in atherosclerosis. Pharmacol Res. 2022;179:106236. doi:10.1016/j.phrs.2022.106236
43. Han C, Jin J, Xu S, et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11(8):734–742. doi:10.1038/ni.1908
44. Zaveri TD, Lewis JS, Dolgova NV, et al. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35(11):3504–3515. doi:10.1016/j.biomaterials.2014.01.007
45. Owen KA, Pixley FJ, Thomas KS, et al. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179(6):1275–1287. doi:10.1083/jcb.200708093
46. Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi:10.1126/science.279.5350.509
47. Wheeler AP, Wells CM, Smith SD, et al. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J Cell Sci. 2006;119(Pt 13):2749–2757. doi:10.1242/jcs.03024
48. Zheng X, Xin L, Luo Y, et al. Near-infrared-triggered dynamic surface topography for sequential modulation of macrophage phenotypes. ACS Appl Mater Interfaces. 2019;11(46):43689–43697. doi:10.1021/acsami.9b14808
49. Chuang YC, Chang H-M, Li C-Y, et al. Reactive oxygen species and inflammatory responses of macrophages to substrates with physiological stiffness. ACS Appl Mater Interfaces. 2020;12(43):48432–48441. doi:10.1021/acsami.0c16638
50. Sridharan R, Cavanagh B, Cameron AR, et al. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47–59. doi:10.1016/j.actbio.2019.02.048
51. Jin P, Jan LY, Jan YN. Mechanosensitive ion channels: structural features relevant to mechanotransduction mechanisms. Annu Rev Neurosci. 2020;43:207–229. doi:10.1146/annurev-neuro-070918-050509
52. Parpaite T, Coste B. Piezo channels. Curr Biol. 2017;27(7):R250–r252. doi:10.1016/j.cub.2017.01.048
53. Solis AG, Bielecki P, Steach HR, et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573(7772):69–74. doi:10.1038/s41586-019-1485-8
54. Atcha H, Jairaman A, Holt JR, et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat Commun. 2021;12(1):3256. doi:10.1038/s41467-021-23482-5
55. Atcha H, Meli VS, Davis CT, et al. Crosstalk between CD11b and Piezo1 mediates macrophage responses to mechanical cues. Front Immunol. 2021;12:689397. doi:10.3389/fimmu.2021.689397
56. Michalick L, Kuebler WM. TRPV4-A missing link between mechanosensation and immunity. Front Immunol. 2020;11:413. doi:10.3389/fimmu.2020.00413
57. Dutta B, Goswami R, Rahaman SO. TRPV4 plays a role in matrix stiffness-induced macrophage polarization. Front Immunol. 2020;11:570195. doi:10.3389/fimmu.2020.570195
58. Lan Z, Chen L, Feng J, et al. Mechanosensitive TRPV4 is required for crystal-induced inflammation. Ann Rheum Dis. 2021;80(12):1604–1614. doi:10.1136/annrheumdis-2021-220295
59. Crisp M, Liu Q, Roux K, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172(1):41–53. doi:10.1083/jcb.200509124
60. Maurer M, Lammerding J. The driving force: nuclear mechanotransduction in cellular function, fate, and disease. Annu Rev Biomed Eng. 2019;21:443–468. doi:10.1146/annurev-bioeng-060418-052139
61. Song Y, Soto J, Chen B, et al. Cell engineering: biophysical regulation of the nucleus. Biomaterials. 2020;234:119743. doi:10.1016/j.biomaterials.2019.119743
62. Uhler C, Shivashankar GV. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol. 2017;18(12):717–727. doi:10.1038/nrm.2017.101
63. O’Brien EM, Risser GE, Spiller KL. Sequential drug delivery to modulate macrophage behavior and enhance implant integration. Adv Drug Deliv Rev. 2019;149-150:85–94. doi:10.1016/j.addr.2019.05.005
64. Davison NL, Gamblin A-L, Layrolle P, et al. Liposomal clodronate inhibition of osteoclastogenesis and osteoinduction by submicrostructured beta-tricalcium phosphate. Biomaterials. 2014;35(19):5088–5097. doi:10.1016/j.biomaterials.2014.03.013
65. Alexander KA, Chang MK, Maylin ER, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26(7):1517–1532. doi:10.1002/jbmr.354
66. Maruyama M, Rhee C, Utsunomiya T, et al. Modulation of the inflammatory response and bone healing. Front Endocrinol. 2020;11:386. doi:10.3389/fendo.2020.00386
67. Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133–143. doi:10.1038/nrrheum.2012.1
68. Pountos I, Georgouli T, Calori GM, et al. Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. Sci World J. 2012;2012:606404. doi:10.1100/2012/606404
69. Guihard P, Danger Y, Brounais B, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30(4):762–772. doi:10.1002/stem.1040
70. Schmidt-Bleek K, Schell H, Schulz N, et al. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. 2012;347(3):567–573. doi:10.1007/s00441-011-1205-7
71. Whitaker R, Sung S, Tylek T, et al. Effects of injury size on local and systemic immune cell dynamics in volumetric muscle loss. NPJ Regen Med. 2025;10(1):9. doi:10.1038/s41536-025-00397-z
72. Jetten N, Roumans N, Gijbels MJ, et al. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PLoS One. 2014;9(7):e102994. doi:10.1371/journal.pone.0102994
73. Falanga V, Schrayer D, Cha J, et al. Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad3 knock-out mice. Wound Repair Regen. 2004;12(3):320–326. doi:10.1111/j.1067-1927.2004.012316.x
74. Shinozaki M, Okada Y, Kitano A, et al. Impaired cutaneous wound healing with excess granulation tissue formation in TNFalpha-null mice. Arch Dermatol Res. 2009;301(7):531–537. doi:10.1007/s00403-009-0969-z
75. Geervliet E, Bansal R. Matrix metalloproteinases as potential biomarkers and therapeutic targets in liver diseases. Cells. 2020;9(5):1212. doi:10.3390/cells9051212
76. Roderfeld M. Matrix metalloproteinase functions in hepatic injury and fibrosis. Matrix Biol. 2018;68-69:452–462. doi:10.1016/j.matbio.2017.11.011
77. Popov Y, Sverdlov DY, Bhaskar KR, et al. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G323–34. doi:10.1152/ajpgi.00394.2009
78. Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178(8):5288–5295. doi:10.4049/jimmunol.178.8.5288
79. Hironaka K, Sakaida I, Matsumura Y, et al. Enhanced interstitial collagenase (matrix metalloproteinase-13) production of Kupffer cell by gadolinium chloride prevents pig serum-induced rat liver fibrosis. Biochem Biophys Res Commun. 2000;267(1):290–295. doi:10.1006/bbrc.1999.1910
80. Martin KE, García AJ. Macrophage phenotypes in tissue repair and the foreign body response: implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021;133:4–16. doi:10.1016/j.actbio.2021.03.038
81. Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi:10.1172/JCI200522675
82. Miron VE, Boyd A, Zhao J-W, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. doi:10.1038/nn.3469
83. Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(46):E3186–95. doi:10.1073/pnas.1119964109
84. Chen F, Liu Z, Wu W, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18(2):260–266. doi:10.1038/nm.2628
85. Sandler NG, Mentink-Kane MM, Cheever AW, et al. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171(7):3655–3667. doi:10.4049/jimmunol.171.7.3655
86. Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. doi:10.1146/annurev-immunol-032713-120236
87. O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–565. doi:10.1038/nri.2016.70
88. Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi:10.1016/S0092-8674(03)00154-5
89. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242. doi:10.1038/nature11986
90. Mills EL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457–470.e13. doi:10.1016/j.cell.2016.08.064
91. Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24(1):158–166. doi:10.1016/j.cmet.2016.06.004
92. Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684. doi:10.1126/science.1250684
93. Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. doi:10.1016/j.cmet.2006.05.011
94. Huang SC, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(9):846–855. doi:10.1038/ni.2956
95. Nozaki R, Kasamatsu A, Moss J, et al. Lysyl hydroxylase 2 deficiency promotes filopodia formation and fibroblast migration. Biochem Biophys Res Commun. 2022;587:146–152. doi:10.1016/j.bbrc.2021.11.100
96. Knipper JA, Willenborg S, Brinckmann J, et al. Interleukin-4 receptor α signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity. 2015;43(4):803–816. doi:10.1016/j.immuni.2015.09.005
97. De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol. 2014;15(2):152–160. doi:10.1038/ni.2784
98. Yuk JM, Kim T, Kim S, et al. Orphan nuclear receptor ERRα controls macrophage metabolic signaling and A20 expression to negatively regulate TLR-induced inflammation. Immunity. 2015;43(1):80–91. doi:10.1016/j.immuni.2015.07.003
99. Su S, Zhao Q, He C, et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015;6:8523. doi:10.1038/ncomms9523
100. Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44(3):450–462. doi:10.1016/j.immuni.2016.02.015
101. Westphalen K, Gusarova GA, Islam MN, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506(7489):503–506. doi:10.1038/nature12902
102. Bourdonnay E, Zasłona Z, Penke LRK, et al. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med. 2015;212(5):729–742. doi:10.1084/jem.20141675
103. Knuever J, Willenborg S, Ding X, et al. Myeloid cell-restricted Insulin/IGF-1 receptor deficiency protects against skin inflammation. J Immunol. 2015;195(11):5296–5308. doi:10.4049/jimmunol.1501237
104. Soroosh P, Doherty TA, Duan W, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210(4):775–788. doi:10.1084/jem.20121849
105. Ostuni R, Kratochvill F, Murray PJ, et al. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–239. doi:10.1016/j.it.2015.02.004
106. Pesce JT, Ramalingam TR, Mentink-Kane MM, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4):e1000371. doi:10.1371/journal.ppat.1000371
107. Hesse M, Cheever AW, Jankovic D, et al. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157(3):945–955. doi:10.1016/S0002-9440(10)64607-X
108. Murray PJ, Rathmell J, Pearce E. SnapShot: immunometabolism. Cell Metab. 2015;22(1):190–190.e1. doi:10.1016/j.cmet.2015.06.014
109. Lang R, Patel D, Morris JJ, et al. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169(5):2253–2263. doi:10.4049/jimmunol.169.5.2253
110. Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15(5):423–430. doi:10.1038/ni.2865
111. Pesce J, Kaviratne M, Ramalingam TR, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116(7):2044–2055. doi:10.1172/JCI27727
112. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi:10.1038/nm0603-653
113. Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi:10.1182/blood-2009-12-257832
114. Peng H, Xian D, Liu J, et al. Regulating the polarization of macrophages: a promising approach to vascular dermatosis. J Immunol Res. 2020;2020:8148272. doi:10.1155/2020/8148272
115. Martin P, Gurevich DB. Macrophage regulation of angiogenesis in health and disease. Semin Cell Dev Biol. 2021;119:101–110. doi:10.1016/j.semcdb.2021.06.010
116. Willenborg S, Lucas T, van Loo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–625. doi:10.1182/blood-2012-01-403386
117. Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–4488. doi:10.1016/j.biomaterials.2014.02.012
118. Graney PL, Ben-Shaul S, Landau S, et al. Macrophages of diverse phenotypes drive vascularization of engineered tissues. Sci Adv. 2020;6(18):eaay6391. doi:10.1126/sciadv.aay6391
119. Zajac E, Schweighofer B, Kupriyanova TA, et al. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood. 2013;122(25):4054–4067. doi:10.1182/blood-2013-05-501494
120. Korn C, Augustin HG. Mechanisms of Vessel Pruning and Regression. Dev Cell. 2015;34(1):5–17. doi:10.1016/j.devcel.2015.06.004
121. Gurevich DB, Severn CE, Twomey C, et al. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018;37(13). doi:10.15252/embj.201797786
122. Lurier EB, Dalton D, Dampier W, et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology. 2017;222(7):847–856. doi:10.1016/j.imbio.2017.02.006
123. Manneken JD, Currie PD. Macrophage-stem cell crosstalk: regulation of the stem cell niche. Development. 2023;150(8). doi:10.1242/dev.201510
124. Fu X, Liu G, Halim A, et al. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8(8):784. doi:10.3390/cells8080784
125. Velarde F, Ezquerra S, Delbruyere X, et al. Mesenchymal stem cell-mediated transfer of mitochondria: mechanisms and functional impact. Cell Mol Life Sci. 2022;79(3):177. doi:10.1007/s00018-022-04207-3
126. Seebach E, Freischmidt H, Holschbach J, et al. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014;10(11):4730–4741. doi:10.1016/j.actbio.2014.07.017
127. Li Y, Zhang D, Xu L, et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16(12):908–920. doi:10.1038/s41423-019-0204-6
128. Day AJ, Milner CM. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78-79:60–83. doi:10.1016/j.matbio.2018.01.011
129. Mohammadalipour A, Dumbali SP, Wenzel PL. Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front Cell Dev Biol. 2020;8:603292. doi:10.3389/fcell.2020.603292
130. Koyanagi M, Brandes RP, Haendeler J, et al. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96(10):1039–1041. doi:10.1161/01.RES.0000168650.23479.0c
131. Davidson S, Coles M, Thomas T, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21(11):704–717. doi:10.1038/s41577-021-00540-z
132. Song L, Li K, Chen H, et al. Cell cross-talk in alveolar microenvironment: from lung injury to fibrosis. Am J Respir Cell Mol Biol. 2024;71(1):30–42. doi:10.1165/rcmb.2023-0426TR
133. Witherel CE, Abebayehu D, Barker TH, et al. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv Healthc Mater. 2019;8(4):e1801451. doi:10.1002/adhm.201801451
134. Song M, Xu S, Zhong A, et al. Crosstalk between macrophage and T cell in atherosclerosis: potential therapeutic targets for cardiovascular diseases. Clin Immunol. 2019;202:11–17. doi:10.1016/j.clim.2019.03.001
135. Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119(8):1810–1820. doi:10.1182/blood-2011-09-379214
136. Hume DA, Freeman TC. Transcriptomic analysis of mononuclear phagocyte differentiation and activation. Immunol Rev. 2014;262(1):74–84. doi:10.1111/imr.12211
137. Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. doi:10.1146/annurev-physiol-022516-034356
138. Sehgal A, Irvine KM, Hume DA. Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. Semin Immunol. 2021;54:101509. doi:10.1016/j.smim.2021.101509
139. MacDonald KP, Palmer JS, Cronau S, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116(19):3955–3963. doi:10.1182/blood-2010-02-266296
140. Stutchfield BM, Antoine DJ, Mackinnon AC, et al. CSF1 restores innate immunity after liver injury in mice and serum levels indicate outcomes of patients with acute liver failure. Gastroenterology. 2015;149(7):1896–1909.e14. doi:10.1053/j.gastro.2015.08.053
141. Gow DJ, Sauter KA, Pridans C, et al. Characterisation of a novel Fc conjugate of macrophage colony-stimulating factor. Mol Ther. 2014;22(9):1580–1592. doi:10.1038/mt.2014.112
142. Zhang MZ, Yao B, Yang S, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122(12):4519–4532. doi:10.1172/JCI60363
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.