Back to Journals » Infection and Drug Resistance » Volume 18
Metagenomic Next-Generation Sequencing Unravels Talaromyces Marneffei-Induced Bone Destruction in an HIV-Negative Woman: A Case Report with Fatal Outcome
Authors Chen J , Pang Z , Qin C, Huang Z, Chen Y
Received 14 February 2025
Accepted for publication 3 June 2025
Published 11 July 2025 Volume 2025:18 Pages 3481—3486
DOI https://doi.org/10.2147/IDR.S522851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sandip Patil
Jiayi Chen,* Zixiang Pang,* Chengyu Qin, Zuhai Huang, Yuanming Chen
Department of Spinal Orthopedics, The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanming Chen, Department of Spinal Orthopedics, The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China, Email [email protected]
Introduction: Talaromycosis is an opportunistic fungal disease caused by Talaromyces marneffei (TM), a significant pathogen predominantly affecting immunocompromised individuals, especially those with HIV infection. Although traditionally regarded as an HIV-associated infection, increasing cases have been reported among HIV-negative patients.
Case Presentation: This report details a case of a 36-year-old HIV-negative woman infected with Talaromyces marneffei. The patient presented with bone destruction and was diagnosed through metagenomic next-generation sequencing (mNGS). Despite receiving antifungal, anti-inflammatory, and symptomatic treatment, the infection remained uncontrolled, ultimately progressing to multi-organ failure and resulting in death.
Conclusion: Bone damage due to Talaromyces marneffei infection is very uncommon in HIV-negative patients. Thus, healthcare providers should be alert for possible skeletal lesions linked to this infection. Early diagnosis and appropriate antimicrobial treatment are vital for the patient’s prognosis.
Keywords: Talaromyces marneffei, HIV-negative, bone destruction, metagenomic next-generation sequencing, antifungal treatment
Background
Talaromyces marneffei is a dimorphic fungus predominantly found in Southeast Asia, including countries like Thailand, Vietnam, and China,1–3 and it can cause severe infections.4 While infections typically occur in HIV-positive individuals, there have been increasing reports of cases in non-HIV patients.5–7 Culturing T. marneffei is critical for diagnosis; however, the urgency of infections, along with the lengthy culture time and low yield, presents challenges for clinical diagnosis.8 Accurate identification of the pathogen is essential for timely initiation of appropriate antimicrobial treatment.9,10 Therefore, developing rapid and accurate identification methods is crucial.11 We describe a case of a 36-year-old woman from China, HIV-negative, who exhibited symptoms including cough and bone pain. During her hospital stay, she experienced septic shock, and T. marneffei infection, which had caused bone destruction, was eventually diagnosed through metagenomic next-generation sequencing (mNGS). We review the clinical presentation of this case to enhance awareness and understanding of this infection.
Case Presentation
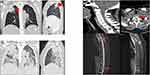
A 36-year-old Chinese woman was admitted to the hospital on June 22, 2023, due to a persistent cough lasting four months, bone pain for one month, and a recently discovered pulmonary mass four days prior. The patient reported that her cough began in February 2023, without sputum production, hemoptysis, chills, fever, chest tightness, or dyspnea, and the results of the nucleic acid test for the novel coronavirus are negative. After self-medicating, her cough somewhat improved. In May 2023, she developed pain in the right side of her back, which did not significantly improve with acupuncture and physiotherapy at a local clinic, and the pain spread to both sides of her back. On June 18, 2023, a chest CT scan at a local hospital revealed a mass lesion in the upper lobe of the right lung, with a suspicion of malignancy and possible invasion into adjacent ribs. There was also evidence of bone destruction in the T1 vertebra with a pathological compression fracture, and slight inflammation in both lungs was noted. The patient subsequently came to our hospital for further diagnosis and treatment. She reported no history of unprotected sexual intercourse or blood transfusions and had no personal or hereditary disease history. She also denied traveling outside her hometown in the past 10 years. Two years ago, she experienced cervical lymphadenopathy and was diagnosed with tuberculous lymphadenitis via lymph node fine-needle aspiration biopsy at a local hospital, for which she completed a six-month course of anti-tuberculosis therapy. Upon initial examination, her vital signs were: body temperature 36.5°C, blood pressure 92/65 mmHg, heart rate 84 beats per minute, and respiratory rate 20 breaths per minute. The physical examination was largely normal, except for small palpable lymph nodes on both sides of the neck, approximately 0.5 cm in diameter, clearly defined, slightly firm, moderately mobile, non-tender, and with no abnormal skin changes. After admission, routine tests were conducted, including blood biochemistry, urine and stool analysis, liver and kidney function tests, electrolytes, tumor markers, ECG, bone ECT, and percutaneous lung biopsy. The dynamic laboratory test results at key clinical time points are summarized in Table 1, with detailed immunological data provided in Supplementary Table 1. The blood HIV test was negative, and levels of squamous cell carcinoma antigen, non-small cell lung cancer antigen, neuron-specific enolase, as well as serum and urine immunofixation electrophoresis, were all normal. Abdominal and pelvic CT scans showed destruction of the L1 vertebral body and left pedicle, along with both sacral alae, and the formation of a soft tissue mass, suggesting a possible metastatic tumor. The chest CT revealed bone destruction of the right 3rd rib, C7-Th2 vertebrae, and the left 4th posterior rib, with associated pathological fractures, as well as multiple enlarged lymph nodes in the mediastinum and right hilum, possibly indicating malignancy (Figure 1A–C). To clarify the diagnosis, an ultrasound-guided lung lesion biopsy was performed on June 26, 2023. Pathology indicated inflammatory fibrous tissue hyperplasia, considered an inflammatory pseudotumor, and immunohistochemistry did not support IgG4-related inflammation, recommending further examination. Due to progressive weakness in both lower limbs caused by multiple bone destructions and pathological fractures, the likelihood of a malignant tumor was considered high, with a significant risk of paraplegia. Therefore, posterior thoracic lesion resection was performed on July 3, 2023. Postoperatively, the patient developed a high fever, with a maximum temperature of 40°C. Considering intraoperative findings, the possibility of tuberculosis infection could not be excluded, so empirical anti-tuberculosis treatment was initiated. Postoperative investigations showed persistent infection or inflammatory response, accompanied by significant anemia, liver dysfunction, hypoxemia, and electrolyte imbalances. Pathological examination and mNGS of the bone tissue obtained from the posterior thoracic lesion resection revealed T. marneffei (See Supplementary Material 1 for detailed MNGS methodology, with relevant pathological images shown in Supplementary Figure 1A–C), which was further confirmed by special culture, leading to the cessation of tuberculosis medication. After a confirmed diagnosis of T. marneffei infection, treatment with amphotericin B was initiated on July 5, 2023. The initial dose was 5 mg/day and was gradually escalated to a maintenance dose of 35 mg/day, administered via intravenous infusion over more than 6 hours each time to minimize drug-related toxicities. During the early phase of treatment, the patient experienced persistent fever with a peak temperature of 39.5°C, and oxygen saturation remained between 86% and 92% under nasal cannula oxygen, accompanied by dyspnea. Due to suboptimal infection control, intravenous meropenem at 1 g every 8 hours was added starting July 6. On July 11, follow-up chest CT indicated worsening inflammation in both lungs (Figure 1D), and the patient’s condition deteriorated, developing severe pneumonia and acute respiratory distress syndrome (ARDS), necessitating endotracheal intubation and mechanical ventilation. Linezolid at 0.6 g every 12 hours was concurrently initiated. Despite aggressive antimicrobial therapy, inflammatory markers continued to rise, circulatory status remained unstable, and the patient ultimately succumbed to multiple organ failure on July 12.
 |
Table 1 Laboratory Test Results |
Discussion
In the 1990s, increasing numbers of HIV-negative patients were diagnosed with T. marneffei infection, often exhibiting weakened cell-mediated immunity.12 TM is a dimorphic fungus that appears as a mycelium at 25°C and as a yeast form at 37°C.13–15 In southern China, T. marneffei infections are more common in individuals with immunodeficiency or those who are HIV positive. T. marneffei primarily affects the lungs and the mononuclear phagocyte system, and its clinical manifestations can be classified as either localized or disseminated.16,17 Localized infections typically enter through the respiratory tract, with symptoms mainly concentrated in the lungs. These symptoms resemble those of pulmonary tuberculosis, leading to frequent misdiagnosis, which can result in prolonged illness and adversely affect the patient’s quality of life.18,19 T. marneffei can proliferate extensively within human tissues, but the exact route of infection remains unclear. Some studies suggest that human infection may not result from direct contact with bamboo rats but rather from inhaling conidia spores present in rain-soaked soil.20 In this case, the HIV-negative patient without obvious immunodeficiency risk factors developed disseminated T. marneffei infection with unusual bone involvement, consistent with prior reports. A 10-year retrospective analysis showed that HIV-negative patients with T. marneffei infection had higher neutrophil counts, more severe organ damage, and increased mortality compared to those who were HIV-positive.21 Therefore, early diagnosis and treatment are crucial for reducing mortality rates.
Early and accurate diagnosis of T. marneffei infection is essential, yet difficult to achieve. Traditionally, identifying T. marneffei relied on isolating the fungus from clinical samples, which is not conducive to rapid diagnosis due to its long incubation period.22,23 However, with the advent of next-generation sequencing (NGS) technology, T. marneffei can be detected within 48 hours, even when pathologists cannot identify the pathogen through microscopic morphology alone.24–26 This is particularly important for early diagnosis in cases with atypical lesions and clinical presentations. In this patient, initial misdiagnosis as inflammatory pseudotumor and tuberculosis led to delayed initiation of targeted antifungal therapy, which likely contributed to disease progression and poor outcome. This case underscores the importance of early use of molecular diagnostic tools like NGS, especially in atypical lesions. The combination of NGS genetic testing with clinical presentation, imaging studies, and laboratory results ultimately confirmed the T. marneffei infection. Clinically, it is advisable to first conduct tissue pathology, staining, and culture. If no specific microorganism is identified, mNGS should be considered as a further diagnostic tool.
Several factors may have contributed to the fatal outcome. First, the delay in initiating antifungal therapy due to initial misdiagnosis possibly allowed extensive fungal proliferation and dissemination. Second, the persistent high fever and progressive respiratory failure despite broad-spectrum antibiotics suggest possible co-infections or overwhelming inflammatory response, which are known to worsen prognosis in T. marneffei infection. Third, although amphotericin B was administered and dose-escalated appropriately,27 the patient’s advanced disease stage, extensive bone destruction, and compromised respiratory status may have limited the efficacy of treatment.
In HIV-negative patients, bone involvement due to T. marneffei infection is relatively rare. Therefore, for patients presenting with recurrent fever, pulmonary masses, and bone lesions, it is important to consider not only tuberculosis and tumors but also the possibility of T. marneffei and other rare pathogenic infections. This case highlights the need for further research into T. marneffei, particularly regarding its manifestations and prognosis in atypical hosts. Additionally, developing more sophisticated methods for assessing immune function is crucial to identify individuals who may be more susceptible to such infections. A deeper understanding of this infection in HIV-negative patients could enhance timely diagnosis and treatment, ultimately improving health outcomes. This calls for heightened clinical awareness and consideration of a broader range of potential diagnoses in similar clinical scenarios.
Conclusions
This case report reminds clinicians that T. marneffei infection is not limited to HIV-positive patients and should also be vigilant for its possibility in non-HIV patients. Due to the difficulty in diagnosis, overlooking T. marneffei infection can lead to serious consequences if it spreads. Utilizing next-generation sequencing (NGS) technology allows for rapid and accurate diagnosis of T. marneffei infection, thereby improving patient outcomes.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
We confirm that written informed consent for publication from the patient’s legal guardian was obtained. The research was approved by the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University (2024-KY-0394) in accordance with the Helsinki Declaration of the World Medical Association and the ethical principles formulated by the Chinese GPC. This report was approved for publication by the Second Affiliated Hospital of Guangxi Medical University.
Consent for Publication
Informed consent was obtained from all individual participants included in this study.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Jiayi Chen and Zixiang Pang contributed equally to this work and should be considered co-first authors.
Funding
This work was supported by the General Program of the Natural Science Foundation of Guangxi (No.2024GXNSFAA010168) and the Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (No. S2022105).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Wang F, Han R, Chen S. An overlooked and underrated endemic Mycosis—Talaromycosis and the pathogenic fungus Talaromyces Marneffei. Clin Microbiol Rev. 2023;36(1). doi:10.1128/cmr.00051-22
2. Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in Mainland China. Mycopathologia. 2012;175(1–2):57–67. doi:10.1007/s11046-012-9577-0
3. Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341. doi:10.1016/j.simyco.2014.08.001
4. Hyde KD, Al-Hatmi AMS, Andersen B, et al. The world’s ten most feared fungi. Fungal Diversity. 2018;93(1):161–194. doi:10.1007/s13225-018-0413-9
5. He L, Mei X, Lu S, et al. Talaromyces marneffei infection in non–HIV‐infected patients in mainland China. Mycoses. 2021;64(10):1170–1176. doi:10.1111/myc.13295
6. Chan JFW, Lau SKP, Yuen K-Y, Woo PCY. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerging Microbes Infect 2019;5(1):1–9. doi:10.1038/emi.2016.18
7. Qiu Y, Liu A-L, Huang J, et al. Comparison of the clinical features of HIV-positive and HIV-negative hosts infected with Talaromyces marneffei: a multicenter, retrospective study. Inter J Infect Dis. 2023;132:93–98. doi:10.1016/j.ijid.2023.04.398
8. Ruan Z, Ning C, Lai J, et al. Accuracy of rapid diagnosis of Talaromyces marneffei: a systematic review and meta-analysis. PLoS One. 2018;13(4). doi:10.1371/journal.pone.0195569
9. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/s0140-6736(18)30696-2
10. Liu L, Sun B, Ying W, et al. Rapid diagnosis of Talaromyces marneffei infection by metagenomic next-generation sequencing technology in a Chinese cohort of inborn errors of immunity. Front Cell Infect Microbiol. 2022;12. doi:10.3389/fcimb.2022.987692
11. Liu G-N, Huang J-S, Zhong X-N, et al. Penicillium marneffei infection within an osteolytic lesion in an HIV-negative patient. Inter J Infect Dis. 2014;23:1–3. doi:10.1016/j.ijid.2013.12.019
12. Pruksaphon K, Amsri A, Jeenkeawpieam J, Thammasit P, Nosanchuk JD, Youngchim S. The microbial damage and host response framework: lesson learned from pathogenic survival trajectories and immunoinflammatory responses of Talaromyces marneffei infection. Front Immunol. 2024;15. doi:10.3389/fimmu.2024.1448729
13. Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffeiInfection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi:10.1128/cmr.19.1.95-110.2006
14. Andrianopoulos A. Laboratory maintenance and growth of Talaromyces marneffei. Curr Protocol Microbiol. 2020;56(1). doi:10.1002/cpmc.97
15. Pruksaphon K, Nosanchuk JD, Ratanabanangkoon K, Youngchim S. Talaromyces marneffei infection: virulence, intracellular lifestyle and host defense mechanisms. J Fungi. 2022;8(2):200. doi:10.3390/jof8020200
16. Ranjana KH, Priyokumar K, Singh TJ, et al. Disseminated Penicillium marneffei infection among HIV-infected patients in Manipur State, India. J Infect. 2002;45(4):268–271. doi:10.1053/jinf.2002.1062
17. Zhou F, Bi X, Zou X, Xu Z, Zhang T. Retrospective analysis of 15 cases of Penicilliosis marneffei in a Southern China Hospital. Mycopathologia. 2014;177(5–6):271–279. doi:10.1007/s11046-014-9737-5
18. De Monte A, Risso K, Normand AC, et al. Chronic pulmonary Penicilliosis due toPenicillium marneffei: late presentation in a French traveler. J Travel Med. 2014;21(4):292–294. doi:10.1111/jtm.12125
19. Lau SKP, Xing F, Tsang CC, et al. Clinical characteristics, rapid identification, molecular epidemiology and antifungal susceptibilities of Talaromyces marneffei infections in Shenzhen, China. Mycoses. 2019;62(5):450–457. doi:10.1111/myc.12887
20. Kappagoda S, Deresinski S. Anticytokine autoantibodies and fungal infections. J Fungi. 2023;9(8):782. doi:10.3390/jof9080782
21. Yu Q, Wei M, Xiao R, et al. Clinical characteristics, course, and long-term outcomes in patients with Talaromyces marneffei infection: a 10-year retrospective cohort study. Infect Dis Ther. 2023;12(5):1283–1297. doi:10.1007/s40121-023-00801-5
22. Wong SSY, Wong KH, Hui WT, et al. Differences in clinical and laboratory diagnostic characteristics of Penicilliosis Marneffei in Human Immunodeficiency Virus (HIV)- and Non-HIV-infected patients. J Clin Microbiol. 2001;39(12):4535–4540. doi:10.1128/jcm.39.12.4535-4540.2001
23. Supparatpinyo K, Khamwan C, Baosoung V, Sirisanthana T, Nelson KE. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344(8915):110–113. doi:10.1016/S0140-6736(94)91287-4
24. Tsang -C-C, Teng JLL, Lau SKP, Woo PCY. Rapid genomic diagnosis of fungal infections in the age of next-generation sequencing. J Fungi. 2021;7(8):636. doi:10.3390/jof7080636
25. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Ann Rev Pathol. 2019;14(1):319–338. doi:10.1146/annurev-pathmechdis-012418-012751
26. Chen J, Liu Y, Huang S, et al. Spinal infections? mNGS combined with microculture and pathology for answers. Infect Drug Resist. 2024;Volume 17:3025–3034. doi:10.2147/idr.S466738
27. Le T, Kinh NV, Cuc NTK, et al. A trial of Itraconazole or Amphotericin B for HIV-associated Talaromycosis. N Engl J Med. 2017;376(24):2329–2340. doi:10.1056/NEJMoa1613306
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Identification of Talaromyces marneffei Infection in an HIV-Negative Patient by ITS Sequencing
Sun A, Gou X, Zhu Y, Lv H, Ge Y
Infection and Drug Resistance 2023, 16:5275-5282
Published Date: 14 August 2023
18F-FDG PET/CT Imaging of Talaromyces marneffei Infection with Bone Destruction in an HIV-Negative Patient: Case Report and Review
He W, Wang S, Xiong X, Dai W
Infection and Drug Resistance 2025, 18:1745-1752
Published Date: 8 April 2025