Back to Journals » Journal of Inflammation Research » Volume 17
MMP-12 and Periodontitis: Unraveling the Molecular Pathways of Periodontal Tissue Destruction
Authors Lin B, Fan Y, Yang X, Pathak JL , Zhong M
Received 9 July 2024
Accepted for publication 18 October 2024
Published 28 October 2024 Volume 2024:17 Pages 7793—7806
DOI https://doi.org/10.2147/JIR.S480466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam Bachstetter
Bingpeng Lin,1,2,* Yufei Fan,2,* Xuechao Yang,2 Janak L Pathak,2 Mei Zhong3
1Department of Orthodontics, School and Hospital of Stomatology, Guangzhou Medical University, Guangzhou, 510180, People’s Republic of China; 2Guangdong Engineering Research Center of Oral Restoration and Reconstruction, Guangzhou Key Laboratory of Basic and Applied Research of Oral Regenerative Medicine, Affiliated Stomatology Hospital of Guangzhou Medical University, Guangzhou, 510182, People’s Republic of China; 3Department of Prosthodontics, School and Hospital of Stomatology, Guangzhou Medical University, Guangzhou, 510180, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Janak L Pathak; Mei Zhong, Email [email protected]; [email protected]
Abstract: Periodontal disease is a common disorder affecting a wide range of people and has a high prevalence globally. Periodontitis comprises a series of inflammatory conditions affecting periodontal support tissue, which could ultimately lead to tooth loss and reduce life quality and add to the financial burden of society. Matrix metalloproteinase-12 (MMP-12) is an elastase that is produced mostly by macrophages and could degrade a wide spectrum of extracellular matrix (ECM) and also contribute to several systematic pathological conditions. Recently, researchers have reported higher expression of MMP-12 in chronic periodontitis patients. However, there are few reports on the role of MMP-12 in periodontitis pathogenicity, and the interaction between MMP-12, periodontal pathogens, and periodontal tissues remains unclear. In this review, we introduce the potentially unique role of MMP-12 in the context of periodontal inflammation earlier, summarize the possible effects of MMP-12 on the pathological process of periodontitis and the interaction of host response under the challenge of various inflammatory factors, and provide possible diagnostic and therapeutic strategies targeting MMP-12 for the management of periodontitis. Future research and policies should focus on and implement effective chairside testing methods to reduce the prevalence of periodontal diseases.
Keywords: matrix metalloproteinase-12, periodontitis, dysbiosis, epithelial barrier, immunity, osteoimmunology
Introduction
Periodontitis, one of the most common infectious diseases in humans, is characterized by inflammation of the periodontal tissue and subsequent destruction of the teeth-supporting apparatus, including the periodontal ligament and alveolar bone, ultimately leading to tooth loss.1,2 Periodontitis is a multifactorial oral disease that involves complex interactions among at least three microbial pathogens, a red complex including Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola3, destructive host immune responses, and environmental factors such as smoking.2 Specific gram-negative anaerobic pathogens initiate inflammation in the periodontal tissue. Simultaneously, a range of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and prostaglandin E2 (PGE2) are produced by macrophages and other inflammatory cells after stimulation with lipopolysaccharide (LPS) and other virulence factors from these bacteria.2,3 Subsequently, the secretion of matrix metalloproteinases (MMPs) leads to collagen fiber degradation and alveolar bone destruction, which ultimately results in clinical attachment loss, sites with deep probing depths, mobility, bleeding upon probing, and tooth mobility.2
The MMPs are categorized based on their subcellular distribution and specificity for components of the ECM into membrane-type matrix metalloproteases (MT-MMPs), collagenases, gelatinases, stromelysins, matrilysins, and other types of MMPs.4 Belonging to other type of MMPs, MMP-12 is a member of the family of zinc-dependent enzymes and is capable of degrading a wide spectrum of extracellular matrix (ECM) components, such as collagen IV, fibronectin, and laminin.5,6 MMP-12 is a 54-kDa pre-proenzyme with a final active form of 22-kDa.7,8 Similar to other different classes of MMPs, MMP-12 contains three common domains: pro-peptides, catalytic, and a hemopexin-like C-terminal domain that links to the catalytic domain through a flexible hinge region.9 Secreted by macrophages and trophoblasts, MMP-12 contributes to several inflammatory diseases and has a potential function in tissue remodeling10–12 and wound healing.13,14 Thus, MMP-12 is involved in several pathological conditions of various diseases, such as viral infection,9 respiratory diseases, including chronic obstructive pulmonary disease (COPD) and emphysema,15 cardiovascular diseases,14,16,17 inflammatory bowel disease18 and cancers.19 Increased pulmonary MMP-12 levels and MMP-12 gene expression have been related to disease severity in asthma and COPD. Targeting MMP-12 showed potential in animal models of pulmonary diseases.8 In addition, MMP-12 is involved in tissue remodeling in inflammatory respiratory diseases such as chronic obstructive pulmonary diseases, including emphysema. Recent studies using MMP-12 inhibitors have demonstrated a reduction in both the inflammatory process and airspace enlargement in lung tissue.7 MMPs are enzymes involved in periodontium destruction.20 According to most studies, MMP-8 concentrations was 3.6 times higher in patients with periodontitis than healthy subjects and the levels of this enzyme positively correlated with the clinical parameters of periodontitis severity such as plaque index and CAL (clinical attachment level).21 Interestingly, periodontal treatments in combination with anti-inflammatory drugs could decrease the MMP-8 levels in gingival pockets of patients.22 MMP-8 has high potential not only for diagnosing periodontitis, but also for determining its severity and assessing the effectiveness of treatment.4
Similar to previous studies of MMP-8, elevated levels of MMP-12-mediated degradation of ECM proteins are found in the saliva and gingival tissue of patients with chronic periodontitis (CP).23–25 Moreover, high levels of MMPs in gingival crevicular fluid, including MMP-12, were previously reported in African-American children with localized aggressive periodontitis, but scaling and root planing (SRP) and systemic antibiotic treatment reduced the levels of MMPs.26 MMP-12 enzyme has the ability to activate other MMPs such as pro-MMP-2 and pro-MMP-3, which in turn could activate pro-MMP-1 and pro-MMP-9 and indirectly regulating the development of periodontitis.27 The above-mentioned facts from the literature indicate that MMP-12, in addition to other MMPs, may play a role in chronic periodontitis pathogenicity.
MMP-12 has been well-researched in certain systemic diseases. Recently, researchers have focused on the role of MMP-12 in oral diseases, and several bioinformatics studies have suggested that MMP-12 can be used as a therapeutic target and prognostic biomarker for certain oral diseases, such as periodontitis and squamous cell carcinoma.28 The up-regulated MMP-12 by orthodontic force was demonstrated in periodontal ligament and could induced angiogenesis via decomposing the Col-IV in the endothelial basement membrane of vascular.29 MMP-12, induced by CSF2 and modulated by CD200/ CD200R pathway, was produced by monocyte-derived cells and could destroy the gingival epithelial integrity with reduced basal cell proliferation.30 However, the exact role of MMP-12 in the development and progression of periodontitis remains unclear. Based on these facts, this review aimed to discuss the possible relationship between MMP-12 and its function in the oral epithelial barrier, interaction with immune cells, and regulation of osteoimmunology attempting to elucidate the role of MMP-12 in the development and progression of periodontitis.
Role of MMP-12 in Oral Microbiome-Induced Inflammation in Epithelial Barrier Function
Oral microbial dysbiosis activates inflammation and destroys gingival epithelium
Periodontal disease occurs when the balance between the microbial biofilm and the host immune response is disrupted.31 Periodontal pathogens can be found both in subjects with periodontitis and in healthy individuals; however, the structure of microbiota changes from healthy to diseased individuals with periodontitis have a higher diversity of microbial communities and a variety of taxa associated with health depletion in periodontitis.32,33 The imbalance between oral microorganisms and host immunity activates inflammation. The persistent chronic inflammatory response leads to the overwhelming of the oral epithelial barrier, which eventually results in alveolar bone resorption by osteoclasts, ligament fiber degradation by matrix metalloproteinases, and granulation tissue formation.31
Crucial Role of MMP-12 in Regulating Epithelium Integrity
Several studies demonstrated that MMP-12 plays a key role in intestinal inflammation by mediating the degradation of basement membrane laminin, macrophage transmigration, and loss of the intestinal tight junction barrier. Nighot et al showed that both colonic permeability and MMP-12 expression were increased in the colonic mucosa of experimental colitis mice, but the severity of experimental colitis was alleviated and epithelial permeability was attenuated in MMP-12−/− mice.34 Chen et al found that MMP-12 was activated by Early Growth Response Protein 1 (Egr1) and plays a role in the inflammation process of IBD.35 Another study showed that small intestinal epithelial tight junction impairment is found in high-fat diet mice, which is reversed by MMP-12 knockdown.6 Additionally, MMP-12 in keratinocytes was considered as a downstream effector of Agrin’s mechanoperception and could coordinate the wound healing tissue microenvironment.13,36 These studies suggest that MMP-12 plays an important role in the regulation of intestinal epithelial integrity and wound healing. Moreover, the downregulation of MMP-12 improves gut health by altering the gut microbiota composition and reduces the expression levels of pro-inflammatory cytokines, including IL-1β and TNF-α.6
Interaction of MMP-12 with Pathogenic Infection
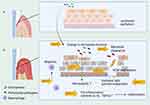
The onset of periodontitis is initiated by dental plaque, and the dysregulation of oral flora leads to the destruction of the gingival epithelial barrier. Previous studies have shown that higher levels of MMPs can be found in patients with chronic and aggressive periodontitis.26 Research on infectious diseases demonstrated that MMP-12 plays a role in complex processes during pathogenic infections. An in vitro study showed that the concentration of MMP-12 was higher in the supernatants of Mycoplasma bovis-infected marrow-derived macrophages than in uninfected cells.37 Vitamin D-deficient (VDD) mice challenged by airway infection with non-typeable Haemophilus influenzae exhibit enhanced bacterial clearance.38,39 Lower levels of pro-inflammatory cytokines (IL-6 and TNF-α) were detected in the bronchoalveolar lavage fluid (BALF). Upregulated MMP-12 as well as a higher ratio of MMP-12/TIMP1 in VDD mice may account for the higher susceptibility to lung remodeling.38 Upregulated MMP-3, MMP-8, MMP-12, TNF-α, IFN-γ, IL-1α, IL-1β, and IL-6 mRNA expression was detected in the brains of Trypanosoma brucei-infected mice, which can be reduced by minocycline treatment at 30 days.40 Furthermore, Nelson et al found that the MMP-12 mRNA expression in whole lungs and alveolar macrophages was induced by murine Pneumocystis, which is CD4+ T cell-dependent.41 MMP-12−/− mice exhibit impaired bacterial clearance and increased mortality when challenged with both Gram-negative and Gram-positive bacteria in the peritoneum and lung where macrophages are enriched, suggesting an antimicrobial role of MMP-12.42 In conclusion, microbial infection affects the MMP-12 expression. On the other hand, macrophage-derived MMP-12 exerts an antibacterial effect, which is manifested as MMP-12 stored in macrophages enters phagolysosomes containing pathogens and adheres to the bacterial cell wall, damaging the cell membrane and causing cell death42 (Figure 1).
MMP-12 and Macrophage in Oral Epithelial Integrity
Based on these facts, we hypothesized that macrophages act as the first line of defense against the invasion of periodontal pathogens through phagocytosis and the antibacterial effect of endogenous MMP-12. However, when the pathogenic factors are too virulent or the host is in hypo-immunity, the overactivated immune response damages oral epithelium integrity, where secreted MMP-12 may act as a key catalyst for periodontal epithelial barrier destruction. In other words, macrophages may play different roles in different stages of inflammation, with protective roles in the early stage and destructive roles in the late stage, resulting in different outcomes. Periodontitis is a multifactorial disease that correlates with dysbiosis and an imbalance in immune defense. However, little is known about the specific role of MMP-12 in interacting with oral microbiota and oral epithelial integrity. Table 1 summarizes studies involving the role of MMP-12 in oral microbiome-induced inflammation in epithelial barrier function.
 |
Table 1 Role of MMP-12 in Oral Microbiome-Induced Inflammation in Epithelial Barrier Function |
Interaction of MMP-12 and Immune Cells in Periodontal Inflammation
The pathogenesis of periodontitis involves a complex immune/inflammatory cascade initiated by oral bacterial biofilms formed on the tooth surface.43,44 Periodontal immunity includes the overlapping parts of innate and adaptive cellular and humoral responses. However, the dysregulation of innate and adaptive immune systems may be attributed to the etiology of periodontal disease.45 Neutrophils, macrophages, dendritic cells (DCs), and other types of innate immune cells are recruited to the affected sites and together with innate immune molecules that act as the first line of defense against pathogenic microorganism challenges in periodontitis.43,46
MMP-12 and Macrophages
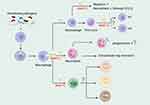
Monocytes and macrophages are the major cellular components of innate immunity that can infiltrate the injured site within a short time.47 Macrophages polarize into different subtypes according to environmental changes: classically activated M1 macrophages and alternatively activated M2 macrophages. M1 macrophages are activated under the stimulation of IFN-γ, TNF-α, or LPS and secrete proinflammatory factors that modulate the pro-inflammatory response. In contrast, M2 macrophages are polarized under the stimulation of IL-4 and IL-13, and secrete anti-inflammatory factors that modulate anti-inflammatory responses.43,48 Periodontitis is a chronic infectious disease in which M1 and M2 macrophages are involved in the destructive and reparative stages.49 The M1/M2 ratio is correlated with periodontal inflammation and reflects periodontal health.50
MMP-12 was considered as the mediator of macrophage functioning. A previous study confirmed that IL-6 from tumor cells stimulates macrophages to upregulate MMP-12 in vitro.51,52 MMP-12−/− mice showed a higher level of macrophages in the corneas compared with wild-type mice after chemical corneal injury but can be reversed by bone marrow transplantation, confirming that MMP-12 inhibits macrophage recruitment. Subsequent studies demonstrated this inhibitory effect, which is mediated by CCL2.49,51,53 In the studies of Yan et al, similar to the reduction of migrating cells, levels of MMP-12 decreased in RAW264.7 macrophages after trimethylamine N-oxide (TMAO) treatment, but the addition of recombinant mouse MMP-12 rescued the reduced cell migration.54 Moreover, MMP-12 deficiency could change the immune cell composition and reduce the plasma monocyte chemoattractant protein-1, thereby ameliorated the systemic inflammation.55 However, a case report of two cases of breast carcinoma with osteoclastic giant cells in different tumor histological backgrounds showed strong expression of VEGF and MMP-12, two factors promoting macrophage migration and angiogenesis, in both tumor and non-tumor tissues.56 Similarly, high level of MMP-12 may lead to the increased macrophage infiltration through the generation of elastin peptide, which could induce monocyte chemotaxis.57,58
In addition, MMP-12 could regulate macrophage phenotype. Vitro studies indicated a higher level of MMP-12 expression in M2 macrophages than that of M1 or bone marrow-derived macrophages, which is consistent with an in vivo model that M2-like macrophages isolated from fat-fed mice expressed higher levels of MMP-12.59 MMP-12 knockout causes increased M2 macrophage infiltration in intestinal tumors and with the same trend of M2-related biomarker mRNA and serum cytokines IL-4 and IL-13 levels that induced M2 macrophage production.60 MMP-12 deficiency alters macrophage recruitment and polarization. Yi et al found that MMP-12 regulates macrophage phenotype by driving M2 differentiation and promotes macrophage-to-myofibroblast transition, which may involve in the development of subretinal fibrosis.61 Exogenous IL-10 increased MMP-12 expression in wild-type M1 macrophages but reduced MMP-12 expression in wild-type M2 macrophages.62 Taken together, these data indicate the possible effects of MMP-12 on orchestrating macrophage infiltration. However, whether MMP-12 or other MMPs and other inflammatory factors alter the M1/M2 ratio and regulate the immune microenvironment remains unclear.
Based on the above studies, we speculate that MMP-12 may inhibit or promote the recruitment of macrophages to the specific situation. We hypothesized that in periodontitis, the release of MMP-12 may enhance macrophage recruitment, which forms a vicious inflammatory cycle. The release of proinflammatory cytokines results in periodontal ligament degradation and osteoclast differentiation, which lead to bone destruction in periodontitis. In addition, the presence of MMP-12 inhibits the polarization of macrophages towards M2-type cells and the increased M1/M2 ratio in gingiva may lead to the progression of periodontitis. Although, studies on the relationship among MMP-12, macrophages, and periodontitis are limited. Holmström et al demonstrated that gingival mucosa tissue from periodontitis patients has increased levels of MMP-12 which undermined gingival tissue integrity and reduced basal cell proliferation.23 This study also showed that monocyte-derived cells in the oral mucosa model respond to inflammatory stimuli by producing MMP-12 and promoting tissue inflammation.23
MMP-12 and Neutrophils
Traditionally, neutrophils have been acknowledged as the first immune cells that are recruited to inflamed tissues and have mainly been considered in the context of acute inflammation.63 Once recruited into tissues, neutrophils engage in complex bidirectional interactions with macrophages, mesenchymal stem cells, dendritic cells, natural killer cells, B cells, and T cells.64 Neutrophils deploy at least three effector mechanisms: phagocytosis, degranulation, and formation of neutrophil extracellular traps. Phagocytosis is the physical uptake of a microbe by forming a phagosome in which neutrophils can focus on antimicrobial activity.65 Moreover, neutrophils contribute to the activation, orientation, and expression of adaptive immune responses.64 Given their role as a component of innate and adaptive response, it is not surprising that neutrophils act as a player in the pathogenesis of numerous disorders including infection, autoimmunity, and chronic inflammatory conditions, such as periodontitis, neurodegenerative diseases, atherosclerosis,63 and cancer.64
After injury to both the epithelial and stromal layers of the cornea, neutrophil infiltration was decreased in MMP-12−/− mice, which could be explained by the reduced expression of the chemokine CXCL1.51,66 These studies demonstrated the positive role of MMP-12 in recruiting neutrophils and played an important role in corneal epithelial repair. Interestingly, recent studies have found that neutrophil depletion alters the alveolar macrophage phenotype, which may reveal the interactions between innate immune cells. The level of MMP-12 mRNA was further increased in alveolar macrophages isolated from neutrophil-depleted mice after cigarette smoking exposure compared with the control group. However, downregulation of MMP-12 mRNA was observed after the neutralization of IL-1α.67 MMP-12 and neutrophils may interact in the process of inducing inflammation. A study concerning the development of pulmonary fibrosis demonstrated that the mRNA of MMP-12 was found overexpressed in neutrophil-depleted treatment mice.68 However, another study showed that MMP-12−/− mice displayed markedly reduced neutrophilic inflammation in both BALF and airways with decreased production of inflammatory cytokines and mucus.11
Neutrophils are the most abundant leukocytes in periodontal pockets, the gingival crevices, and inflamed periodontal tissues. They play an essential role in preventing microbial invasion, maintaining the symbiosis of the bacterial community in healthy periodontal tissues and exerting an antibacterial effect in periodontitis.69 Neutrophils play a dual role in the pathological process of periodontitis. On one hand, neutrophils are recruited to the bacterial invasive sites to engulf bacteria and form intracellular vacuoles to activate intracellular antimicrobial responses, which depends on reactive oxygen species (ROS) and antimicrobial agents. The antimicrobial mechanism of neutrophils also includes oxidative burst response, degranulation, and net formation. Since MMPs are one of the antimicrobial peptides contained in the granules, we speculate that MMP-12 may play an antibacterial role in this stage. On the other hand, research showed that excessive neutrophil extracellular trap formation can exist when the formation of periodontal pockets interferes with the clearance of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which results in tissue damage, ECM degradation, bone resorption, and the progression of periodontitis.43,69
Innate immunity is the first step in the immune response to pathobionts, but when the inflammation is overactive, innate immune cells fail to remove the pathogenic bacteria, and destruction of the periodontium occurs, adaptive immunity is then activated, which leads to further destruction of the periodontium.31
MMP-12 and T Cells
Previous studies have established the contribution of immune-inflammatory response regulated by the activity of a subset of CD4+ T helper cells including Th1, Th2, Th17, and regulatory T cells (Treg).46 Several studies have summarized the role of these cells and the cytokines produced in the pathogenesis of periodontitis. Th1 cell-produced proinflammatory cytokines are usually associated with periodontal tissue damage. Whereas Th2 cell-produced cytokines are often related to tissue repair and homeostasis maintaining while some argued their role in disease progression. However, the inhibitory effect on osteoclast differentiation of both Th1 cells and Th2 cells via the production of IFN-γ and IL-4, respectively, made it difficult to ascribe the periodontal bone loss in osteoimmulogical spectrum.1 The imbalance between proinflammatory cytokines secreted by Th1 cells and the anti-inflammatory cytokines secreted by Th2 cells also causes the occurrence of the disease.43 Nevertheless, the role of Th17 cells in periodontitis is controversial. On one hand, Th17 cells induce the epithelial cells to secrete anti-microbial peptides, which can defend against extracellular pathogens and promote neutrophil recruitment and local inflammation.1,43 On the other hand, Th17 cells can act on osteoblasts and upregulate the expression of receptor activator of the nuclear factor kappa B ligand (RANKL), which results in the maturation and differentiation of osteoclasts and ultimately, bone resorption.43,46 Tregs can be transdifferentiated from Th17 cells in some situations.1 Tregs can produce immunosuppressive cytokines and contact-dependent modulation, which play a crucial role in maintaining homeostasis and self-tolerance, in other words, that is having an inhibitory effect on the pathogenesis of periodontitis.43,46 More recently, the function of natural killer T (NKT) cells and regulatory B cells in the involvement in periodontitis have been explored and are being investigated.46,70
So far, the available data on the relation between MMP-12 and T cells in the pathological process in human diseases is still limited. A recent clinical study has demonstrated a decrease in mRNA level of T cell, Th2, and Th17 markers including MMP-12 after Ustekinumab treatment compared with the placebo-control group.71 But the interaction of MMP-12 and Th1/Th2 cells has not been clarified. However, an earlier study indicated the protective role of MMP-12 in experimental autoimmune encephalomyelitis (EAE), which is a result of an alteration of the Th1/Th2 ratio.72 MMP-12 plays a crucial role in various diseases including cancer development and progression. Immunohistochemistry (IHC) staining was performed in human hepatocellular carcinoma (HCC) tissue and the result indicated a significantly positive correlation between MMP-12 expression and FOXP3+ T cell infiltration, which was speculated that MMP-12 may serve important roles in affecting prognosis by influencing the immune system in HCC patients.73 MMP-12 is one of the most highly expressed differentially expressed genes in Tregs during acute lung injury (ALI) resolution compared with other conditions. Mock. et al showed that mice repleted with Tregs lacking MMP-12 expression have more lung neutrophil infiltration during LPS-induced ALI resolution, which indicated the role of Treg-expressed MMP-12 in Treg-promoted resolution of the inflammatory process.74 The MMP-12 production was significantly different between single-cell cultures and co-cultures. The elevated MMP response in DCs is observed in a virulence factor of the periodontal pathogen P. gingivalis Hemagglutinin B-treated Transwell co-culture system containing DCs, gingival epithelial keratinocytes, and CD4+ T-cells, which indicate the interaction of MMP-12 and periodontal pathogens.20
Periodontitis is a bacterial inflammatory response that destroys the periodontal barrier, degrades periodontal tissue, and causes loss of attachment, and resorption of alveolar bone. As mentioned above, the interaction between MMP-12 and a variety of T cells remains ill-defined. Therefore, understanding the mechanism of MMP-12 and various T cells in periodontitis is critical to the control and treatment of periodontitis in the future.
The periodontal inflammatory response is a complex process involving the communication of the host immune response and microbe. A network of cytokines and chemokines produced by activated immune cells also plays a part in cell migration, survival, and immune cell function. Given the influence of MMP-12 on immune cells in systematic disease and the altered expression in genomic levels in an experiment or clinical trials, it is conceivable that the interaction between MMP-12 and immune cells may have a role in the pathogenesis of periodontal inflammatory diseases (Figure 2). Table 2 summarizes research relating to the interaction of MMP-12 and immune cells.
 |
Table 2 Interaction of MMP-12 and Immune Cells |
Relationship of MMP-12 and Osteoimmunology
The concept of osteoimmunology was introduced by Arron and Choi in 2000, a few years after the discovery of the RANKL-RANK-osteoprotegerin (OPG) system 75. After that, osteoimmunology was widely investigated. The dual effect of T cells in regulating osteoclastogenesis through RANKL and secretion of IFN-γ 76 and early research on the role of human monocytes-derived IL-1 in bone resorption was established 77.
MMP-12 and Osteoclasts
MMP-12 was found to be expressed in chondrocytes during fetal development and malignant transformation. Kaspiris et al reported that MMP-12 is mainly expressed at the matrix of fibrocartilage tissue in moderate osteoarthritis (OA) patients and a concurrent expression of MMP-12 was noted in the matrix adjacent to the osteoblast-like cells, the bone lining cells, and the osteoclasts in the moderate and severe stages of OA. Although statistics did not show a correlation between MMP-12 and the bone resorption rate, it was associated with the osteoclast mainly in the OA end stages 78. MMP-12 mRNA was expressed in multinucleated cells induced from NCD14+ monocytes, whereas not in those from CD14+ monocytes. While with the stimulation of LIGHT, a recently identified type 2 transmembrane glycoprotein of the TNF ligand superfamily (TNFSF14), or the combination of LIGHT and RANKL, the MMP-12 mRNA expression level of NCD14+ monocytes was significantly upregulated 79. In terms of the correlation of MMP-12 and osteoclasts, a previous study that investigated the involvement of MMP-12 in bone resorption showed osteoclasts expressed MMP-12 in some specific situations. Recombinant MMP-12 cleaves the functional domains of osteopontin and bone sialoprotein, both of which are bone matrix proteins that strongly influence osteoclast activities. The study also demonstrated other proteolytic products of MMP-12, such as TNF-α, may possibly relevant to osteoclastogenesis and osteoclast survival 80.
MMP-12 and Th17/Treg in Alveolar Osteoimmunology
Osteoclasts are the main mediators of skeletal diseases that relate to bone resorption. The regulation of osteoclast formation and bone resorption is achieved by the secretion of RANKL by two subsets of T cells, helper T cells, and regulatory T cells 81. Alveolar bone resorption involves the antagonistic relationship between the activity of Th17 cells through the RANKL/RANK/OPG pathway in direct and indirect ways, altering the ratio of bone remodeling and RANKL/OPG expression and changing the secretion level of IL-17 as well as the production of inflammatory factors TNF-α, IL-1, and IL-6 which function in immunosuppression. On the other hand, Treg lymphocytes suppress the expression of RANKL and M-CSF, resulting in osteoclast formation and bone mass restoration 81. Recent studies have demonstrated that gingivitis and periodontitis have some correlation with an imbalance between Th17 cells and Tregs 82. Glycosphingolipids, one of the important components contained in the outer membrane of major oral pathogenetic bacteria Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Tannerella forsythia, and Treponema denticola activates NKT cells followed by systemic inflammation, periodontal RANKL production, osteoclastogenesis, and resulting in finally bone resorption 70.
A present study has shown a gradual increase of the Th17 subset followed by a significant reduction of the Treg/Th17 ratio in advanced stages of carotid artery stenosis (CAS), which is likely related to atherosclerosis progression but not to plaque instability, whereas higher levels of MMP-12 was seen in both asymptomatic and symptomatic CAS groups which are related to carotid plaque progression 83. However, this study did not show the correlation between MMP-12 and Treg/Th17 ratio. IL-17 is a cytokine produced by Th17 cells and an inflammatory marker in several diseases, such as atopic dermatitis 84, asthma, and gangrenous appendicitis 85. But so far, the relation of expression of MMP-12 and Th17/Treg as well as the association with periodontal diseases is still unilluminated. The potential role of MMP-12 in regulating osteoimmunology was summarized in Figure 3.
MMP-12 and Periodontitis: The Possible Treatment Strategies
To improve treatment outcomes, various adjuncts to non-surgical periodontal treatments have been suggested, including the local delivery of drugs, systemic antibiotics, and host modulation agents. Periostat (doxycycline hyclate) is prescribed as an adjunct to scaling and root planing in order to promote attachment level gain and reduce pocket depth in patients with adult periodontitis 86. Clinical studies demonstrate that Periostat results in statistically significant improvements in both attachment level and pocket depth reduction for patients with moderate to severe periodontitis 87. It is the only approved adjunctive therapy designed specifically to inhibit the tissue-destroying enzymes responsible for the breakdown of the periodontium. However, the potential clinical benefits must be carefully weighed against risks, such as antibiotic resistance, adverse reactions, and drug interactions 31,86,88.
Previous research showed a lower level of colonic mucosa permeability, less infiltration of immune cells, and reduced expression of pro-inflammatory cytokines in MMP-12 knockout mice 6,34. These results hint that inhibition of MMP-12 activities may reduce the permeability of oral mucosal epithelium, infiltration of immune cells, and the expression of pro-inflammatory cytokines to alleviate gingival inflammation in periodontitis. Moreover, MMP-12 level upregulates in several systemic infectious diseases 37–41 and was considered as the therapeutic target of various diseases. MMP-12 was demonstrated to be the most probable therapeutic target of MBZM-N-IBT for its treatment of arthritis 89. Pharmacological inhibition of MMP-12 exerts protective effects and improves the survival rate in angiotensin II–induced abdominal aortic aneurysms mice 90. The activity of MMP-12 was reduced by the treatment of bestatin in the spleen and lung tissues of rats in a model of LPS-induced sepsis 91. Similarly, macrophage-derived lung MMP-12 inflammation was alleviated by Paeoniflorin in murine silicosis models 92. In terms of oral diseases, research showed that P. gingivalis supernatant triggers MMP-1, MMP-2, MMP-3, and MMP-14 expression in human gingival fibroblasts 93. Whether P. gingivalis can activate MMP-12 expression in periodontal tissue is still unknown. Macrophages play a critical role in the initiation, maintenance, and resolution of inflammation while neutrophils are also major effectors of acute inflammation, as well as contributors to chronic inflammatory conditions and adaptive immune responses 94,95. Decreasing infiltration and recruitment of macrophages and neutrophils may inhibit the inflammatory response to some extent.
MMP-12 inhibitors, such as MMP408, and RXP470.1 have been developed and are being tested in various preclinical and clinical trials in terms of respiratory diseases 96. It is meaningful to create and develop prospective MMP-12-selective inhibitors as efficient drug candidates due to the significance of MMP-12 in periodontitis 97. With those facts, we assume that theses inhibitors could be the potential strategy for treating periodontal diseases. We could assume that MMP-12 inhibition related drugs, compounds or mouthwashes may be the possible strategy of alleviating periodontal inflammation.
However, the role of MMP-12 in periodontitis development and progression is still not fully understood. At present, the relationship between MMP-12 and periodontitis is unclear. The possibility of the treatment of periodontitis targeting MMP-12 depends on the outcome of future research.
Conclusion
The story of the MMP-12 in the progression of periodontitis is far from complete. MMP-12, a member of the MMP family, is capable of degrading a wide spectrum of ECM components and could regulate inflammatory states by affecting the function of epithelial cells, and immune cells. The current review summarized the potential connection of MMP-12 with related molecular pathways in the different pathological processes of periodontitis, which uncovers the key role of MMP-12 in the development of periodontitis and provides the theory basis for making MMP-12 a potential diagnostic and therapeutic target in the management of periodontal disease.
More studies are needed to illuminate the specific role of MMP-12 in interaction with periodontal pathogens, and immune cells as well as the mechanism of regulating osteoimmunology of alveolar bone loss in the different stages of periodontal inflammatory microenvironment. How MMP-12 interacts with other MMPs in the progression of periodontitis should also be further elucidated. After that, further exploration of MMP-12 related diagnostic techniques may allow scholars to discover effective chair-side tests or mouth-rinse screening tests for monitoring periodontal inflammation. The meaningful long-term goal is to explore the possibility and efficiency of selective MMP-12 inhibitors, MMP-12 related natural products, novel chemical drugs, and synthetic materials in the role of inhibiting periodontal inflammation and reducing periodontal tissue destruction. Therefore, it is certain that the more medical research is done to examine MMP-12 and periodontitis, the better the prospects will be for patients’ periodontal health.
Acknowledgment
Bingpeng Lin and Yufei Fan are co-first authors for this study. This work was supported by the National Natural Science Foundation of China (82150410451), the Science and Technology Planning Projects of Guangzhou City, China (No. 202201020203,202201020117), the Special projects in key fields of Guangdong Colleges and Universities, China (No.2021ZDZX2058) and Higher Education Young Innovative Talent Program of Guangdong Province (2023KQNCX058).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Tsukasaki M. RANKL and osteoimmunology in periodontitis. J Bone Miner Metab. 2021;39(1):82–90. doi:10.1007/s00774-020-01165-3
2. Kwon T, Lamster IB, Levin L. Current concepts in the management of periodontitis. Int Dental J. 2021;71(6):462–476. doi:10.1111/idj.12630
3. Martínez-García M, Hernández-Lemus E. Periodontal inflammation and systemic diseases: an overview. Front Physiol. 2021;12.
4. Zalewska EA, Ławicka R, Grygorczuk P, et al. Importance of metalloproteinase 8 (MMP-8) in the diagnosis of periodontitis. Int J Mol Sci. 2024;25(5):2721. doi:10.3390/ijms25052721
5. Knapinska AM, Amar S, He Z, et al. Matrix metalloproteinases as reagents for cell isolation. Enzyme Microb Technol. 2016;93:29–43. doi:10.1016/j.enzmictec.2016.07.009
6. Song M, Zhang S, Tao Z, et al. MMP-12 siRNA improves the homeostasis of the small intestine and metabolic dysfunction in high-fat diet feeding-induced obese mice. Biomaterials. 2021;278:121183. doi:10.1016/j.biomaterials.2021.121183
7. Lagente V, Le Quement C, Boichot E. Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets. 2009;13(3):287–295. doi:10.1517/14728220902751632
8. Abd-Elaziz K, Jesenak M, Vasakova M, et al. Revisiting matrix metalloproteinase 12: its role in pathophysiology of asthma and related pulmonary diseases. Curr Opin Pulm Med. 2021;27(1):54–60. doi:10.1097/MCP.0000000000000743
9. Bassiouni W, Ali MAM, Schulz R. Multifunctional intracellular matrix metalloproteinases: implications in disease. FEBS J. 2021;288(24):7162–7182. doi:10.1111/febs.15701
10. Kraft L, Erdenesukh T, Sauter M, et al. Blocking the IL-1β signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res Cardio. 2019;114(2):11. doi:10.1007/s00395-019-0719-0
11. Zhou JS, Li ZY. Cigarette smoke-initiated autoimmunity facilitates sensitisation to elastin-induced COPD-like pathologies in mice. Eur Respir J. 2020;56(3).
12. Lanone S, Zheng T, Zhu Z, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and −12 in IL-13–induced inflammation and remodeling. J Clin Invest. 2002;110(4):463–474. doi:10.1172/JCI0214136
13. Chakraborty S, Sampath D, Yu Lin MO, et al. Agrin-matrix metalloproteinase-12 axis confers a mechanically competent microenvironment in skin wound healing. Nat Commun. 2021;12(1):6349. doi:10.1038/s41467-021-26717-7
14. Mouton AJ, Rivera Gonzalez OJ, Kaminski AR, et al. Matrix metalloproteinase-12 as an endogenous resolution promoting factor following myocardial infarction. Pharmacol Res. 2018;137:252–258. doi:10.1016/j.phrs.2018.10.026
15. Hendrix AY, Kheradmand F. The role of matrix metalloproteinases in development, repair, and destruction of the lungs. Prog Mol Biol Transl Sci. 2017;148:1–29.
16. Kremastiotis G, Handa I, Jackson C, et al. Disparate effects of MMP and TIMP modulation on coronary atherosclerosis and associated myocardial fibrosis. Sci Rep. 2021;11(1):23081. doi:10.1038/s41598-021-02508-4
17. Didangelos A, Yin X, Mandal K, et al. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach*. Mol Cell Proteomics. 2011;10(8):
18. Dharmasiri S, Garrido-Martin EM, Harris RJ. Human intestinal macrophages are involved in the pathology of both ulcerative colitis and crohn disease. Inflamm Bowel Dis. 2021;27(10):1641–1652. doi:10.1093/ibd/izab029
19. He L, Kang Q, Chan KI, Zhang Y, Zhong Z, Tan W The immunomodulatory role of matrix metalloproteinases in colitis-associated cancer. Front Immunol. 2023;13.
20. Bates AM, Fischer CL, Abhyankar VP, et al. Matrix metalloproteinase response of dendritic cell, gingival epithelial keratinocyte, and T-Cell transwell co-cultures treated with porphyromonas gingivalis hemagglutinin-B. Int J Mol Sci. 2018;19(12).
21. Fatemi K, Rezaee SA, Banihashem SA, et al. Importance of MMP-8 in salivary and gingival crevicular fluids of periodontitis patients. Iran J Immunol. 2020;17(3):236–243. doi:10.22034/iji.2020.81170.1512
22. Buduneli N, Vardar S, Atilla G, et al. Gingival crevicular fluid matrix metalloproteinase-8 levels following adjunctive use of meloxicam and initial phase of periodontal therapy. J Periodontol. 2002;73(1):103–109. doi:10.1902/jop.2002.73.1.103
23. Björnfot Holmström S, Clark R, Zwicker S, et al. Gingival tissue inflammation promotes increased matrix metalloproteinase-12 Production by CD200Rlow monocyte-derived cells in periodontitis. J Immunol. 2017;199(12):4023–4035. doi:10.4049/jimmunol.1700672
24. Holmström SB, Lira-Junior R, Zwicker S, et al. MMP-12 and S100s in saliva reflect different aspects of periodontal inflammation. Cytokine. 2019;113:155–161. doi:10.1016/j.cyto.2018.06.036
25. Qian L, Xuedong Z, Yaping F, et al. [Analysis of salivary protease spectrum in chronic periodontitis]. Hua xi kou qiang yi xue za zhi. 2017;35(1):37–42. doi:10.7518/hxkq.2017.01.005
26. Gonçalves PF, Huang H, McAninley S, et al. Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J Periodontol. 2013;84(12):1801–1808. doi:10.1902/jop.2013.130002
27. Matsumoto S, Kobayashi T, Katoh M, et al. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol. 1998;153(1):109–119. doi:10.1016/S0002-9440(10)65551-4
28. Chen X, Lei H, Cheng Y, et al. CXCL8, MMP12, and MMP13 are common biomarkers of periodontitis and oral squamous cell carcinoma. Oral Dis. 2024;30(2):390–407. doi:10.1111/odi.14419
29. Narimiya T, Wada S, Kanzaki H, et al. Orthodontic tensile strain induces angiogenesis via type IV collagen degradation by matrix metalloproteinase-12. J Periodontal Res. 2017;52(5):842–852. doi:10.1111/jre.12453
30. Björnfot Holmström S, Clark R. Gingival tissue inflammation promotes increased matrix metalloproteinase-12 production by CD200R(low) monocyte-derived cells in periodontitis. Cell Death Discov. 2017;199(12):4023–4035.
31. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3(1):17038. doi:10.1038/nrdp.2017.38
32. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi:10.1038/s41579-018-0089-x
33. Abusleme L, Hoare A, Hong B-Y, et al. Microbial signatures of health, gingivitis, and periodontitis. Periodontology. 2021;86(1):57–78. doi:10.1111/prd.12362
34. Nighot M, Ganapathy AS, Saha K. Matrix metalloproteinase MMP-12 promotes macrophage transmigration across intestinal epithelial tight junctions and increases severity of experimental colitis. J Crohn’s amp Colitis. 2021;15(10):1751–1765. doi:10.1093/ecco-jcc/jjab064
35. Chen S-Y, Fang C-Y, Su B-H, et al. Early growth response protein 1 exacerbates murine inflammatory bowel disease by transcriptional activation of matrix metalloproteinase 12. Biomedicines. 2024;12(4):780. doi:10.3390/biomedicines12040780
36. Yu Lin MO, Sampath D, Bosykh DA, et al. YAP/TAZ drive agrin–matrix metalloproteinase 12–mediated diabetic skin wound healing. J Invest Dermatol. 2024. doi:10.1016/j.jid.2024.05.005
37. Baquero M, Vulikh K, Wong C, et al. Effects of inflammatory stimuli on responses of macrophages to mycoplasma bovis infection. Vet Microbiol. 2021;262:109235. doi:10.1016/j.vetmic.2021.109235
38. Serré J, Mathyssen C, Ajime TT, et al. Airway infection with nontypeable Haemophilus influenzae is more rapidly eradicated in vitamin D deficient mice. J Steroid Biochem Mol Biol. 2019;187:42–51. doi:10.1016/j.jsbmb.2018.10.021
39. Serré J, Tanjeko AT, Mathyssen C, et al. Effects of repeated infections with non-typeable Haemophilus influenzae on lung in vitamin D deficient and smoking mice. Respir Res. 2022;23(1):40.
40. Masocha W, Rottenberg ME, Kristensson K. Minocycline impedes African trypanosome invasion of the brain in a murine model. Antimicrob Agents Chemother. 2006;50(5):1798–1804. doi:10.1128/AAC.50.5.1798-1804.2006
41. Nelson M, Christmann BS, Dunaway CW, et al. experimental Pneumocystis lung infection promotes M2a alveolar macrophage-derived MMP12 production. Am J Physiol Lung Cell Mol Physiol. 2012;303(5):L469–75. doi:10.1152/ajplung.00158.2012
42. Houghton AM, Hartzell WO, Robbins CS, et al. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460(7255):637–641. doi:10.1038/nature08181
43. Xu XW, Liu X, Shi C, et al. Roles of immune cells and mechanisms of immune responses in periodontitis. Chin J Dental Res. 2021;24(4):219–230. doi:10.3290/j.cjdr.b2440547
44. Cekici A, Kantarci A, Hasturk H, et al. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology. 2014;64(1):57–80. doi:10.1111/prd.12002
45. Slots J. Periodontitis: facts, fallacies and the future. Periodontology. 2017;75(1):7–23. doi:10.1111/prd.12221
46. Zou J, Zeng Z, Xie W, et al. Immunotherapy with regulatory T and B cells in periodontitis. Int Immunopharmacol. 2022;109:108797. doi:10.1016/j.intimp.2022.108797
47. Almubarak A, Tanagala KK, Papapanou PN, Lalla E, Momen-Heravi F Disruption of monocyte and macrophage homeostasis in periodontitis. Front Immun. 2020; 11.
48. Yunna C, Mengru H, Lei W, et al. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi:10.1016/j.ejphar.2020.173090
49. Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R Polarized macrophages in periodontitis: characteristics, function, and molecular signaling. Front Immunol. 2021;12.
50. Yang J, Zhu Y, Duan D, et al. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Arch Oral Biol. 2018;96:234–242. doi:10.1016/j.archoralbio.2017.03.006
51. Chan MF, Li J, Bertrand A, et al. Protective effects of matrix metalloproteinase-12 following corneal injury. J Cell Sci. 2013;126(17):3948–3960. doi:10.1242/jcs.128033
52. Jiang L, Yang M, He S, et al. MMP12 knockout prevents weight and muscle loss in tumor-bearing mice. BMC Cancer. 2021;21(1):1297. doi:10.1186/s12885-021-09004-y
53. Wolf M, Clay SM, Zheng S, et al. MMP12 inhibits corneal neovascularization and inflammation through Regulation of CCL2. Sci Rep. 2019;9(1):11579. doi:10.1038/s41598-019-47831-z
54. Yan M, Zhao C, Lu S et al. Trimethylamine N‐oxide exacerbates acetaminophen‐induced liver injury by interfering with macrophage‐mediated liver regeneration. J Cell Physiol. 2021;237.
55. Amor M, Bianco V, Buerger M, et al. Genetic deletion of MMP12 ameliorates cardiometabolic disease by improving insulin sensitivity, systemic inflammation, and atherosclerotic features in mice. Cardiovasc Diabetol. 2023;22(1):327. doi:10.1186/s12933-023-02064-3
56. Shishido-Hara Y, Kurata A, Fujiwara M, et al. Two cases of breast carcinoma with osteoclastic giant cells: are the osteoclastic giant cells pro-tumoural differentiation of macrophages? Diagn Pathol. 2010;5(1):55. doi:10.1186/1746-1596-5-55
57. Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66(4):859–862. doi:10.1172/JCI109926
58. Chen Y, Yang X, Kitajima S et al. Macrophage elastase derived from adventitial macrophages modulates aortic remodeling. Front Cell Develop Biol. 2023;10.
59. Lee J-T, Pamir N, Liu N-C, et al. Macrophage metalloelastase (MMP12) regulates adipose tissue expansion, insulin sensitivity, and expression of inducible nitric oxide synthase. Endocrinology. 2014;155(9):3409–3420. doi:10.1210/en.2014-1037
60. Yang M, Zhang X, Liu Q, et al. Knocking out matrix metalloproteinase 12 causes the accumulation of M2 macrophages in intestinal tumor microenvironment of mice. Cancer Immu Immun. 2020;69(8):1409–1421. doi:10.1007/s00262-020-02538-3
61. Yi C, Liu J, Deng W, et al. Macrophage elastase (MMP12) critically contributes to the development of subretinal fibrosis. J Neuroinflammation. 2022;19(1):78. doi:10.1186/s12974-022-02433-x
62. Malinina A, Dikeman D, Westbrook R, et al. IL10 deficiency promotes alveolar enlargement and lymphoid dysmorphogenesis in the aged murine lung. Aging Cell. 2020;19(4):e13130. doi:10.1111/acel.13130
63. Soehnlein O, Steffens S, Hidalgo A, et al. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017;17(4):248–261. doi:10.1038/nri.2017.10
64. Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi:10.1038/nri3024
65. Burn GL, Foti A, Marsman G, et al. The Neutrophil. Immunity. 2021;54(7):1377–1391. doi:10.1016/j.immuni.2021.06.006
66. Wolf M, Maltseva I, Clay SM, et al. Effects of MMP12 on cell motility and inflammation during corneal epithelial repair. Exp Eye Res. 2017;160:11–20. doi:10.1016/j.exer.2017.04.007
67. Milad N, Pineault M, Lechasseur A, et al. Neutrophils and IL-1α regulate surfactant homeostasis during cigarette smoking. J Immunol. 2021;206(8):1923–1931. doi:10.4049/jimmunol.2001182
68. Puerta-Arias JD, Pino-Tamayo PA, Arango JC, et al. Depletion of neutrophils promotes the resolution of pulmonary inflammation and fibrosis in mice infected with paracoccidioides brasiliensis. PLoS One. 2016;11(9):e0163985. doi:10.1371/journal.pone.0163985
69. Jiang Q, Zhao Y, Shui Y et al. Interactions between neutrophils and periodontal pathogens in late-onset periodontitis. Front Cell Infect Microbiol. 2021;11.
70. Melgar-Rodríguez S, Cafferata EA, Díaz NI, et al. Natural killer T (NKT) cells and periodontitis: potential regulatory role of NKT10 cells. Med Inflam. 2021;2021:5573937. doi:10.1155/2021/5573937
71. Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2017;26(1):28–35. doi:10.1111/exd.13112
72. Weaver A, Da Silva AG, Nuttall RK, et al. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB j. 2005;19(12):1668–1670. doi:10.1096/fj.04-2030fje
73. He MK, Le Y, Zhang Y-F, et al. Matrix metalloproteinase 12 expression is associated with tumor FOXP3+ regulatory T cell infiltration and poor prognosis in hepatocellular carcinoma. Oncol Lett. 2018;16(1):475–482. doi:10.3892/ol.2018.8642
74. Mock JR, Dial CF, Tune MK, et al. Transcriptional analysis of Foxp3+ Tregs and functions of two identified molecules during resolution of ALI. JCI Insight. 2019;4(6). doi:10.1172/jci.insight.124958.
75. Gruber R. Osteoimmunology: inflammatory osteolysis and regeneration of the alveolar bone. J Clin Periodontol. 2019;46(S21):52–69. doi:10.1111/jcpe.13056
76. Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408(6812):600–605. doi:10.1038/35046102
77. Gowen M, Meikle MC, Reynolds JJ. Stimulation of bone resorption in vitro by a non-prostanoid factor released by human monocytes in culture. Biochim Biophys Acta, Mol Cell Res. 1983;762(3):471–474. doi:10.1016/0167-4889(83)90014-9
78. Kaspiris A, Khaldi L, Chronopoulos E, et al. Macrophage-specific metalloelastase (MMP-12) immunoexpression in the osteochondral unit in osteoarthritis correlates with BMI and disease severity. Pathophysiology. 2015;22(3):143–151. doi:10.1016/j.pathophys.2015.06.001
79. Ishida S, Yamane S, Nakano S, et al. The interaction of monocytes with rheumatoid synovial cells is a key step in LIGHT-mediated inflammatory bone destruction. Immunology. 2009;128(1 Suppl):e315–24. doi:10.1111/j.1365-2567.2008.02965.x
80. Hou P, Troen T, Ovejero MC, et al. Matrix metalloproteinase-12 (MMP-12) in osteoclasts: new lesson on the involvement of MMPs in bone resorption. Bone. 2004;34(1):37–47. doi:10.1016/j.bone.2003.08.011
81. Huang F, Wong P, Li J, et al. Osteoimmunology: the correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J Cell & Mol Med. 2022;26(13):3591–3597. doi:10.1111/jcmm.17399
82. Wang W, Wang X, Lu S et al. Metabolic disturbance and Th17/Treg imbalance are associated with progression of gingivitis. Front Immunol. 2021;12.
83. Del Porto F, Cifani N, Proietta M, et al. Regulatory T CD4 + CD25+ lymphocytes increase in symptomatic carotid artery stenosis. Ann Med. 2017;49(4):283–290. doi:10.1080/07853890.2016.1241427
84. Zhou X, Suárez-Fariñas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Eur Respir J. 2017;7(1):8707.
85. Rubér M, Andersson M, Petersson BF, et al. Systemic Th17-like cytokine pattern in gangrenous appendicitis but not in phlegmonous appendicitis. Surgery. 2010;147(3):366–372. doi:10.1016/j.surg.2009.09.039
86. Treatment of chronic periodontitis. Br Dent J. 2006;201(4):237. doi:10.1038/sj.bdj.4813957
87. Periostat (doxycycline 20mg). Br Dent J. 2006;200(2):115. doi:10.1038/sj.bdj.4813235
88. Matesanz-Pérez P, et al. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol. 2012;40.
89. Moharana Alok K, Gaur M, Mohapatra TK, Dash RN, Subudhi BB Network pharmacology, molecular docking and in vivo-based analysis on the effects of the MBZM-N-IBT for arthritis. Curr Comput Aided Drug Des. 2024;20:1–17.
90. Di Gregoli K, Atkinson G, Williams H, et al. Pharmacological inhibition of MMP-12 exerts protective effects on angiotensin II-induced abdominal aortic aneurysms in apolipoprotein E-deficient mice. Int J Mol Sci. 2024;25(11):5809. doi:10.3390/ijms25115809
91. Nieradko-Iwanicka B, Piasecki J, Borzęcki A. Treatment with bestatin (the exogenous synthetic inhibitor of metalloproteinases) reduces the activity of metalloproteinase 2 and 12 in the spleen and lung tissues of rats in a model of lipopolysaccharide-induced sepsis. Biomed Pharmacother. 2024;174:116480. doi:10.1016/j.biopha.2024.116480
92. Li T, Mao N, Xie Z, et al. Paeoniflorin mitigates MMP-12 inflammation in silicosis via Yang-Yin-Qing-Fei Decoction in murine models. Phytomedicine. 2024;129:155616. doi:10.1016/j.phymed.2024.155616
93. Zhou J, Windsor LJ. Porphyromonas gingivalis affects host collagen degradation by affecting expression, activation, and inhibition of matrix metalloproteinases. J Periodontal Res. 2006;41(1):47–54. doi:10.1111/j.1600-0765.2005.00835.x
94. Fujiwara N, Kobayashi K. Macrophages in Inflammation. Cur Drug Tar Inflamm Allergy. 2005;4(3):281–286. doi:10.2174/1568010054022024
95. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
96. Chelluboina B, Nalamolu KR, Klopfenstein JD, et al. MMP-12, a promising therapeutic target for neurological diseases. Mol Neurobiol. 2018;55(2):1405–1409. doi:10.1007/s12035-017-0418-5
97. Jha T. Modeling Inhibitors of Matrix Metalloproteinases.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.