Back to Journals » Journal of Pain Research » Volume 17
Novel Ultrasound-Guided Cervical Sympathetic Chain Pulsed Radiofrequency for Subacute Herpes Zoster Neuralgia
Authors Lin S , Lin M, Wang F, Zhuo Y, Lin K, Wang J
Received 26 March 2024
Accepted for publication 29 October 2024
Published 7 November 2024 Volume 2024:17 Pages 3627—3637
DOI https://doi.org/10.2147/JPR.S470758
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Shenshen Lin,1,2 Minyi Lin,2 Fengchai Wang,2 Yanmei Zhuo,2 Kaixing Lin,2 Jingui Wang1,2
1The Graduate School of Fujian Medical University, Fuzhou City, Fujian Province, People’s Republic of China; 2Department of Pain Management, the First Hospital of Putian City, Putian City, Fujian Province, People’s Republic of China
Correspondence: Jingui Wang, The Graduate School of Fujian Medical University, No. 1 Xuefu North Road, Minhou County, Fuzhou City, Fujian Province, People’s Republic of China, Email [email protected]
Objective: To evaluate the efficacy and safety of the novel ultrasound-guided cervical sympathetic chain pulsed radiofrequency (PRF) for subacute herpes zoster neuralgia.
Materials and Methods: Sixty patients with subacute herpes zoster neuralgia (one month until the third month after the rash onset) on the maxillofacial, head, and neck regions were enrolled in our study. Patients were randomized into two groups: sham-operated (sham) group (n = 30) and radiofrequency (RF) group (n = 30). In the RF group, the affected side cervical sympathetic chain was treated with bipolar high voltage long-duration PRF. In the sham group, the RF cannula was placed at the same position as in the RF group, but without RF energy application. The visual analog scale (VAS), Pittsburgh Sleep Quality Index (PSQI), 36-item Short Form Health Survey (SF-36), analgesic drug usage, incidence of postherpetic neuralgia, and adverse effects were recorded in both groups.
Results: In both groups, compared with preoperative, VAS and PSQI scores decreased, while SF-36 scores improved after the treatment (p < 0.05). The VAS and PSQI scores were significantly lower, whereas the SF-36 scores were significantly higher in the RF group than in the sham group at 1, 30, 90, and 180 days after the treatment (all p < 0.05), and the amount of analgesic drugs consumption was also lower in the RF group than in the sham group (p < 0.05). The incidence of postherpetic neuralgia was lower in the RF group than in the sham group (p < 0.05). No noticeable complications and side effects were observed in either group.
Conclusion: The novel ultrasound-guided cervical sympathetic chain pulsed radiofrequency could effectively relieved subacute herpes zoster neuralgia (one month until the third month after the rash onset) on the maxillofacial, head, and neck regions, and reduced the incidence of postherpetic neuralgia.
Keywords: ultrasound-guided, cervical sympathetic chain, pulsed radiofrequency, postherpetic neuralgia, subacute herpes zoster
Introduction
Herpes zoster (HZ), commonly known as shingles, is a painful, blistering skin eruption caused by the reactivation of varicella-zoster virus.1,2 Depending on the disease course, HZ was divided into three stages: acute herpes zoster (AHZ), subacute herpes zoster (SHZ), and postherpetic neuralgia (PHN). The AHZ was defined as the disease within one month after the rash onset, and the SHZ was defined as one month until the third month after the rash onset, while the PHN was defined as pain persists for more than three months after the rash onset.3,4
Postherpetic neuralgia (PHN) is a classic form of neuropathic pain that significantly affects patients’ quality of life. Many patients develop severe occupational, physical, and psychosocial disabilities, placing a substantial economic burden on both families and society.3–5 But due to the complexity of mechanism and the exact pathophysiology is still unknown, PHN is refractory to pharmacological therapies and various interventional therapies.6–8
Therefore, relieving pain intensity and preventing the occurrence of PHN in AHZ/SHZ is of great clinical significance. Many studies have shown that sympathetic nerves are closely associated with the pathology of herpetic neuralgia.3,9 Cervical sympathetic chain (CSC) is an important sympathetic nerve, which has always gained great clinical interest. It typically located posteromedial to the carotid sheath and passes over the longus colli muscle, contains two to four ganglia, including the superior, middle, inferior cervical sympathetic ganglia, and vertebral ganglia.10 Pulsed radiofrequency (PRF) is an advanced neuromodulation technique for neuralgic pain.11,12 Previous study has demonstrated that PRF on the CSC could alleviate herpetic neuralgia intensity and reduce the incidence of PHN in AHZ patients.13 But, to the best of our knowledge, to date, no study has been carried out to assess the therapeutic effect of PRF on the CSC for SHZ neuralgia.
Based on this, we performed PRF on the CSC of patients with SHZ neuralgia on the maxillofacial, head, and neck regions in this study. The goal was to assess whether this novel procedure could alleviate SHZ neuralgia and reduce the incidence of PHN.
Materials and Methods
Study Patients
This study adhered to the Declaration of Helsinki, written informed consent was obtained from each patient. And it was approved by the ethics committee of the First Hospital of Putian City (Approval number: 2020–089). Sixty patients with SHZ on the maxillofacial, head, and neck regions admitted to the Pain Department of the First Hospital of Putian City between January 2020 and June 2022 were included (Figure 1). Among them, 34 were male and 26 were female, postherpetic pigmentation or lesions were distributed unilaterally; included were 38 cases of lesions in the area of cervical nerve innervation and 22 cases of lesions in the area of trigeminal nerve innervation.
 |
Figure 1 Study flowchart. All 60 patients were included in the treatment. |
The inclusion criteria were as follows: i) disease duration one month until the third month after the rash onset; ii) patients with SHZ located on the maxillofacial, head, and neck regions; iii) the visual analog scale (VAS) ≥4.
Exclusion criteria were as follows: i) patients with psychiatric disorders who could not cooperate; ii) patients with severe heart, brain, liver, or kidney diseases who could not tolerate minimally invasive surgery; iii) patients with skin or tissue infection at the puncture site; iv) patients with coagulation dysfunction; v) postoperative change of analgesic drug type; vi) lost visits and incomplete information.
Equipment and Drugs
The equipment used in this study included a pain management generator (PM-230, Baylis Medical Company, Montreal, Quebec, Canada), a 20G RF cannula needle (with a 0.9-mm diameter and a 10-mm active tip, Baylis Medical Company), and a GE ultrasound instrument (GE Healthcare, Chicago, USA).
The following analgesic drugs were used: pregabalin (Pfizer, USA) and tramadol (Beijing Menti Pharmaceutical Co., Ltd., Beijing, China).
PRF Procedure
The patients were placed on the affected side in the upper lateral position. Vital signs were monitored, and a linear ultrasound transducer with a short axial view was started from the level of the para-tracheal supraclavicular and moved alongside the trachea. When the ultrasound showed an annular hypoechoic image, the ultrasound transducer was shifted outward and slid up and down to find the 7th cervical transverse process. This was marked by the absence of an anterior tubercle in the transverse process. The ultrasound transducer was then moved in a more cranial direction to find the 6th cervical transverse process, which was marked by a tall and sharp anterior tubercle (Figure 2). According to Kim et al,14 the PRF targets are located at the roots of the 6th and 7th cervical transverse processes, anterior to the long cervical muscle, and posterior to the prevertebral fascia and internal jugular vein (Figure 3). An ultrasound-guided, in-plane puncture was performed, the RF cannula was delivered to the middle cervical ganglion (C6 level of the transverse processes) and inferior cervical ganglion (C7 level of the transverse processes) of the CSC and make sure the distance between the two electrodes tip was adjusted within 1 centimeter separation. A sensory test (50 hz, 1.0 ms) and a motor test (2 hz, 1.0 ms) were applied, and the patients were checked for paresthesia or phonation to exclude electrode mispositioning. In the RF group, PRF treatment was performed using the following parameters: 42°C, 2 hz, 20 ms, 80–100 V, 900s. In the sham group, the electrodes were placed as in the RF group but did not emit current.
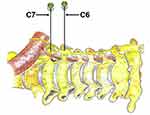 |
Figure 3 Schematic diagram of the radiofrequency cannula located on the cervical sympathetic chain. |
Analgesic Rescue
A pain assessment was performed one day after treatment, and pregabalin was administered for patients with VAS ≥4, starting with 75 mg every 12 hours. The pregabalin dosage was adjusted to ensure a VAS score ≤3, the maximum of pregabalin was 300 mg every 12 hours. If the VAS score still ≥4 while taking pregabalin up to 300 mg every 12 hours, tramadol 100 mg was administered every 12 hours for rescue.
Observation Indicators
VAS scores were evaluated before treatment and at 1, 30, 90, and 180 days after treatment. The daily dosage of pregabalin and the number of patients taking tramadol were recorded at 1, 30, 90, and 180 days after treatment. The Pittsburgh Sleep Quality Index (PSQI) was used to evaluate sleep quality of patients, and the 36-item Short Form Health Survey (SF-36) was used to assess the quality of life of patients; both were calculated before and 30, 90, and 180 days after treatment. PHN was defined as pain (VAS score ≥ 3) persists for more than three months after the rash onset. Complications and side effects such as hematoma, tachycardia, infection, and pneumothorax were recorded during the treatment and 1, 30, 90, and 180 days after the treatment.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA), and GraphPad Prism 6.0. Quantitative data are expressed as the mean ± standard deviation, and qualitative data are expressed as frequencies. A t-test was used for quantitative data comparison, and an independent-sample χ2 test or Fisher’s exact test was used for qualitative data comparison. A p-value of <0.05 was considered statistically significant.
Results
Patient Characteristics
There was no statistical difference between the two groups in terms of age, sex, disease duration, pain scores, PSQI scores, or SF-36 scores before treatment (Table 1).
 |
Table 1 Comparison of General Conditions Between the Two groups (Mean ± SD, n = 30 per Group) |
Comparison of VAS Scores
There was no statistical difference in the VAS scores between the two groups before treatment. Compared with preoperative, the VAS scores were significantly lower at each postoperative observation time point in the RF group (p < 0.05, Figure 4). Compared with the preoperative, there was no statistical difference in the sham group at 1 day after the treatment, but there was a statistical difference at 30 days, 90 days, and 180 days after the treatment (p < 0.05, Figure 4). However, the VAS score in the RF group decreased more significantly than in the sham group at the same follow-up time point (p < 0.05, Figure 4).
Comparison of Analgesic Usage
As shown in Figures 5 and 6, the daily average consumption of both pregabalin and tramadol in the RF group was lower than in the sham group at all postoperative follow-up time points (all p < 0.05 for both pregabalin and tramadol).
 |
Figure 5 The comparison of daily average pregabalin consumption between the two groups. ΔP < 0.05 compared with the Sham group. |
 |
Figure 6 Comparison of the number of patients take tramadol between the two groups. ΔP < 0.05 compared with the Sham group. |
Comparison of PSQI Scores
There was no statistical difference in PSQI scores between the two groups before treatment. The PSQI scores at 30, 90, and 180 days after treatment were significantly lower in both groups than preoperative (p < 0.05). However, compared with the sham group, PSQI scores in the RF group were lower at each postoperative follow-up time point (all p < 0.05, Figure 7).
Comparison of SF-36 Scores
There was no statistical difference in the SF-36 scores between the two groups before treatment. Compared with preoperative, the SF-36 scores improved significantly in both groups after the treatment (p < 0.05). However, the SF-36 scores improved significantly greater in the RF group than in the sham group at each postoperative follow-up time point (all p < 0.05, Figure 8).
Incidence of PHN
At 3 months after rash onset, the VAS scores of 17 patients were <3 and without taking analgesic drugs in the RF group, the incidence of PHN was 43.3%. While, there were only eight patients VAS < 3 and without taking analgesic drugs in the sham group, the incidence of PHN was 73.3%. The difference was statistically significant between the two groups (p < 0.05).
Complications
No complications such as infection, pneumothorax, local hematoma, spinal nerve injury, or vocal cord dysfunction were observed in either groups during the study.
Discussion
Immune system dysfunction results in reactivation of latent HZ virus and causes severe inflammation in the primary sensory neurons together with cutaneous inflammation, subsequently induced neuralgia.15,16 HZ neuralgia is a classic neuropathic pain, its characterized by persistent or paroxysmal burning, stabbing, or lancinating pain, often associated with allodynia and hyperalgesia. This greatly affects individuals, resulting in poor sleep quality and quality of life, increased economic burden and productivity loss.3–5
Sympathetic nerve blockade (SNB) was widely used for HZ neuralgia in clinic.3 The stellate ganglion (SG) is formed by the fusion of the inferior cervical ganglion and the first thoracic sympathetic ganglion, the dominant area of SG is the maxillofacial, head, neck, and upper limbs.10,17 Numerous studies have reported the efficacy of stellate ganglion block (SGB) for neurovascular diseases in the responsible area.17 Makharita et al3 reported that SGB could dramatically decrease neuralgia and incidence of PHN. In SGB, local anesthetics are injected at the level of the C6 transverse process. Thereafter, the liquid could diffuse along the cervical sympathetic chain to the stellate ganglion, and even to the superior cervical ganglion, so the SGB should be considered as a cervical sympathetic chain block.14 However, a single SGB often provides only short-term effects, and multiple puncture may result in patient suffering, poor compliance, infection, tissue, or nerve injury.14 To overcome the shortage of single SGB, Pain Physicians are trying to figure out a new way. PRF is a well-received neuromodulation technique, first proposed by Sluijter in 1997 and has widely used for neuropathic pain.18 PRF delivers short bursts of radiofrequency currents to the nervous tissue without causing nerve damage, selectively affects small diameter C and Aδ nociceptive fiber,19 and it has a higher effect on C fibers than Aδ fibers.20 The biological effects of PRF were associated with the electromagnetic field disrupting or somehow modulating neuronal transmission.13 Research has showed that applied PRF to the SG/CSC could achieve prolonged pain relief than a single nerve blockade for neuropathic pain.13,14,21,22 Inspired by this, we presented PRF to the CSC for patients with SHZ neuralgia.
In this study, the majority of patients received positive results in the RF group, and pain decreased overtime after the procedure. Compared with the sham group, VAS scores decreased more significant at every follow-up time point. Ding et al21 presented SG pulsed radiofrequency for patients with HZ neuralgia (the natural course of the disease exceeded 1 month) on facial and upper limb regions, and VAS decreased after the operation during 6 months follow-up, this is consistent with our results. Lin et al13 reported PRF on the CSC of patients with AHZ neuralgia (the natural course of the disease within 1 month) on the oral, maxillofacial, neck, and upper limb regions; the results showed that it could relieve acute herpetic neuralgia and reduce PHN incidence. The incidence of PHN was 16.7%, which was superior to this study (which was 43.3%), mainly because of the different inclusion criteria. Kim et al23 found that patients with SHZ neuralgia received more significant pain relief than those with a disease course more than three months after PRF. Makharita et al24 reported that early sympathetic ganglion modulation could dramatically shorten disease duration, decrease pain intensity, and reduce the incidence of PHN, which was similar to our results.
The analgesic mechanism of sympathetic nerve modulation is still not clear. The sympathetic nerve could regulate the functions of vasculature, glandular secretion, and pain transmission, blocking it could dilate blood vessels, increasing blood flow.25,26 HZ viral reactivation in ganglion and spread through the affected nerve to peripheral dermatomal, which results in severe ganglionitis and neuritis, induced a profound sympathetic stimulation, leading to vasospasm of the endoneural arterioles, decreasing blood flow in the intraneural capillary bed, resulting in nerve ischemia and edema.27,28 At early stage of HZ, by intervening sympathetic nerve, it could attenuate vasospasm and prevent the irreversible nerve damage.9 Based on previous studies, we postulated that PRF on the CSC on patients with SHZ neuralgia could reduce repetitive inflammatory stimuli and alleviate endoneurial arterioles vasospasm. Hence, attenuating nerve ischemia and edema prevent nerve damage and finally account for pain relief and PHN prevention.
While the exact analgesic mechanism of PRF is still unclear, it is currently believed that the analgesia was produced by neuromodulation,29 and eliciting an anti-inflammatory response.4 PRF causes nerve ultrastructural change, such as disorganization of microtubules and microfilaments, abnormal of morphology and membranes in mitochondria.20 Several researches found that bipolar, high-voltage, long-duration PRF was superior than standed PRF,30,31 The longest PRF therapy duration has been reported was 900 seconds.12 Based on the above, in order to achieve superior outcome we set the PRF parameters as follows: bipolar high voltage (80–100 V) and long duration of 900 seconds.
In the present study, we used ultrasound guided during the procedure, for it allows real-time visualization of the vessels and nerves, thus the RF cannula could be easily advanced to the target to avoid injury to these structures. Thus, it could minimize complications and procedure time, as in this study no serious complications occurred in either of the groups. The CSC includes superior, middle, and inferior cervical ganglions, and the ultrasound image of the cervical sympathetic chain ganglion appears as a longitudinally elongated hypoechoic nodule with a hypoechoic linear structure attached to its upper and lower ends.10 For the small structures of ganglion, the detection rate of the cervical sympathetic ganglion under ultrasound was reported to be 41.35%.10 Due to the anatomical variability of the cervical sympathetic ganglion, we localized the PRF target at the transverse processes of C6 and C7, anterior to the longus colli muscle and posterior to the prevertebral fascia and the internal jugular vein, according to Kim et al,14 and then looked for ovoid or rhombic-shaped hypoechoic node ultrasound images near this localization point. We found that for patients who could identify the ovoid or rhombic hypoechoic nodes under ultrasound during the procedure, pain relief was more significant. Furthermore, patients may experience warmth and abnormal sensations in the lesion area of herpes zoster during the PRF procedure. However, some patients were dissatisfied with their pain relief in the RF group, we speculated it may be due to the anatomical variations, the oval or rhombic hypoechoic nodule ultrasound images could not be found during the procedure.
HZ neuralgia is usually accompanied by anxiety, depression, and insomnia, which significantly affect patient’s quality of life. The goal of HZ neuralgia treatment is to relieve pain, improve sleep quality, and enhance the quality of life. Therefore, we used VAS to evaluate pain intensity, PSQI to evaluate sleep quality and SF-36 to assess the quality of life. These three indicators turned out to be better than preoperative, and the RF group was superior to the sham group. Complications such as procedural suffering, nausea, vomiting, tachycardia, and hypertension were milder and resolved rapidly. No serious complications occurred in either of the groups.
Our study had several limitations. First, this is a single-center study with a limited sample size. Second, the follow-up period may not be long enough. Third, due to the COVID-19 pandemic some patients received home isolation and centralized isolation during the follow-up period, which had a certain impact on the assessment of the pain intensity, sleep quality and quality of life. In the study, we failed to carry out an effective intervention on this influencing factor, which may have impacted the research results. However, a randomized prospective controlled study with larger sample sizes is needed in future study.
In conclusion, ultrasound-guided bipolar high-voltage long-duration PRF on the CSC for patients with SHZ neuralgia in the maxillofacial, head, and neck regions could achieve conspicuous pain relief and reduce the incidence of PHN. The efficacy and safety of this procedure is worthy of recommendation for clinical application.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Project supported by the Natural Science Foundation of Fujian Province, China (Grant No. 2023J011723); Putian College Scientific Research Project (Grant No. 2019068).
Disclosure
Each author certifies that he or she, or a member of his or her immediate family, has no commercial association (ie, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript.
References
1. Forstenpointner J, Rice A, Finnerup N, Baron R. Up-date on clinical management of postherpetic neuralgia and mechanism-based treatment: new options in therapy. J Infect Dis. 2018;218:S120–S126. doi:10.1093/infdis/jiy381
2. Schutzer-Weissmann J, Farquhar-Smith P. Post-herpetic neuralgia - a review of current management and future directions. Expert Opinion Pharmacother. 2017;18:1739–1750. doi:10.1080/14656566.2017.1392508
3. Makharita M. Prevention of post-herpetic neuralgia from dream to reality: a ten-step model. Pain Physician. 2017;20:E209–E220. doi:10.36076/ppj.2017.E220
4. Fan X, Ren H, Xu F, et al. Comparison of the efficacy of short-term peripheral nerve stimulation and pulsed radiofrequency for treating herpes zoster ophthalmicus neuralgia. Clin J Pain. 2022;38:686–692. doi:10.1097/AJP.0000000000001074
5. Gater A, Uhart M, McCool R, Préaud E. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15:193. doi:10.1186/s12889-015-1514-y
6. Zhao P, Mei L. A clinical study of paraspinal nerve block on treatment of herpes zoster under ultrasonic guidance. Neuro-Chirurgie. 2019;65:382–386. doi:10.1016/j.neuchi.2019.06.007
7. Kurklinsky S, Palmer S, Arroliga M, Ghazi S. Neuromodulation in postherpetic neuralgia: case reports and review of the literature. Pain Med. 2018;19:1237–1244. doi:10.1093/pm/pnx175
8. Han Z, Hong T, Ding Y, Wang S, Yao P. CT-guided pulsed radiofrequency at different voltages in the treatment of postherpetic neuralgia. Front Neurosci. 2020;14:579486. doi:10.3389/fnins.2020.579486
9. SD W. Acute herpes zoster. In: SD W, editors. Common Pain Syndrome. Philadelphia: WB Saunders; 2002:171.
10. Park C, Suh C, Shin J, Baek J. Characteristics of the middle cervical sympathetic ganglion: a systematic review and meta-analysis. Pain Physician. 2018;21:9–18.
11. Ding Y, Hong T, Li H, Yao P, Zhao G. Efficacy of CT guided pulsed radiofrequency treatment for trigeminal postherpetic neuralgia. Front Neurosci. 2019;13:708. doi:10.3389/fnins.2019.00708
12. Wan C, Dong D, High-Voltage ST. Long-duration pulsed radiofrequency on gasserian ganglion improves acute/subacute zoster-related trigeminal neuralgia: a randomized, double-blinded, controlled trial. Pain Physician. 2019;22:361–368.
13. Lin S, Lin M, Dai Z, Wang F, Lin K, Liu R. Novel bipolar high-voltage pulsed radiofrequency targeting the cervical sympathetic chain for treating acute herpetic neuralgia. Neuromodulation. 2023;26:1808–1816. doi:10.1016/j.neurom.2021.12.003
14. Kim E, Yoo W, Kim Y, Park H. Ultrasound-guided pulsed radiofrequency treatment of the cervical sympathetic chain for complex regional pain syndrome: a retrospective observational study. Medicine. 2017;96:e5856. doi:10.1097/MD.0000000000005856
15. Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82:3971. doi:10.1128/JVI.02592-07
16. Steain M, Sutherland J, Rodriguez M, Cunningham A, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88:2704–2716. doi:10.1128/JVI.03445-13
17. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dental Anesthesia Pain Med. 2016;16:159–163. doi:10.17245/jdapm.2016.16.3.159
18. Sluijter ME: Non-thermal radiofrequency procedures in the treatment spinal pain. In:
19. Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. European J Pain. 2006;10:171–176. doi:10.1016/j.ejpain.2005.03.001
20. Erdine S, Bilir A, Cosman E, Cosman E. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9:407–417. doi:10.1111/j.1533-2500.2009.00317.x
21. Ding Y, Yao P, Li H, et al. CT-guided stellate ganglion pulsed radiofrequency stimulation for facial and upper limb postherpetic neuralgia. Front Neurosci. 2019;13:170. doi:10.3389/fnins.2019.00170
22. Kumar A, Bhandari B, Mahiswar A, Sharma R, Purohit G. Pulsed radiofrequency to stellate ganglion for brachial plexus injury-induced complex regional pain syndrome: a case series. Saudi J Anaesthesia. 2023;17:423–426. doi:10.4103/sja.sja_20_23
23. Kim KH, Jo DH, Kim ED. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician. 2017;20:E411–E418. doi:10.36076/ppj.2017.E418
24. Makharita M, Amr Y, El-Bayoumy Y. Effect of early stellate ganglion blockade for facial pain from acute herpes zoster and incidence of postherpetic neuralgia. Pain Physician. 2012;15:467–474.
25. Kang C, Oh S, Chung R, et al. Effect of stellate ganglion block on the cerebrovascular system: magnetic resonance angiography study. Anesthesiology. 2010;113:936–944. doi:10.1097/ALN.0b013e3181ec63f5
26. Kim M, Yi M, Park P, Kang H, Lee J, Shin H. Effect of stellate ganglion block on the regional hemodynamics of the upper extremity: a randomized controlled trial. Anesthesia Analg. 2018;126:1705–1711. doi:10.1213/ANE.0000000000002528
27. Gopal D, Singh N, Jagadeesh A, Ture A, Thimmarayappa A. Comparison of left internal mammary artery diameter before and after left stellate ganglion block. Annals Cardiac Anaesthesia. 2013;16:238–242. doi:10.4103/0971-9784.119161
28. Kim E, Yoon K, Lee J, Yoon D, Kim D. The effect of oxygen administration on regional cerebral oxygen saturation after stellate ganglion block on the non-blocked side. Pain Physician. 2013;16:117–124. doi:10.36076/ppj.2013/16/117
29. Rehman S, Khan M, Hussain R, Jamshed A. Pulsed radiofrequency modulation for lingual neuralgia. British Jf Oral Maxillofacial Surg. 2012;50:e4–5. doi:10.1016/j.bjoms.2011.06.001
30. Chang MC, Cho YW, Ahn SH. Comparison between bipolar pulsed radiofrequency and monopolar pulsed radiofrequency in chronic lumbosacral radicular pain: a randomized controlled trial. Medicine. 2017;96:e6236. doi:10.1097/MD.0000000000006236
31. Yang S, Chang M. Effect of bipolar pulsed radiofrequency on chronic cervical radicular pain refractory to monopolar pulsed radiofrequency. Annals Palliative Med. 2020;9:169–174. doi:10.21037/apm.2020.02.19
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effect of Preoperative Level of Glycemic Control with Pulsed Radiofrequency on the Incidence of Postherpetic Neuralgia in Patients with Herpes Zoster Combined with Type 2 Diabetes Mellitus: A Cohort Study
Hua B, An M, Chen L, Ni H, Ni C, Yao M
Diabetes, Metabolic Syndrome and Obesity 2024, 17:3975-3987
Published Date: 24 October 2024





