Back to Journals » Clinical Ophthalmology » Volume 19
One-Year Outcomes in Subjects Developing Macular Neovascularization While Undergoing Avacincaptad Pegol Therapy for Geographic Atrophy
Authors Rush RB , Klein W , Rush SW, Reinauer R
Received 2 October 2024
Accepted for publication 7 January 2025
Published 9 January 2025 Volume 2025:19 Pages 111—118
DOI https://doi.org/10.2147/OPTH.S498985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ryan B Rush,1– 3 Westin Klein,1 Sloan W Rush,1,2 Robert Reinauer4
1Department of Ophthalmology, Panhandle Eye Group, Amarillo, TX, USA; 2Department of Surgery, Texas Tech University Health Science Center, Amarillo, TX, USA; 3Department of Ophthalmology, Southwest Retina Specialists, Amarillo, TX, USA; 4Department of Ophthalmology, New Vision Eye Center, Vero Beach, FL, USA
Correspondence: Ryan B Rush, Southwest Retina Specialists, 7411 Wallace Blvd, Amarillo, TX, 79106, USA, Tel +1806 351 1870, Email [email protected]
Purpose: To assess the 12-month outcomes in subjects developing macular neovascularization (MNV) during intravitreal avacincaptad pegol (IVA) treatment for geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
Methods: This research was conducted as a case-controlled, retrospective study of AMD subjects undergoing IVA treatment for GA from two private practice institutions. Subjects were divided into 1) a Study Group of patients who developed MNV and then underwent anti-vascular endothelial growth factor (VEGF) therapy during the study period, and 2) a Control Group of patients who were complication-free during the study period. Both cohorts had a baseline Snellen visual acuity of ≥ 20/200, a baseline GA total area of ≥ 1 mm2and ≤ 17.5 mm2, and 12 months of follow-up after initiation of IVA for GA.
Results: There were 56 patients analyzed. There were no significant differences in baseline features between cohorts. The Study Group had a greater decrease in visual acuity [− 0.22 logMAR (− 0.27 to − 0.17) versus − 0.06 logMAR (− 0.12 to 0.00); p=< 0.0001], and greater GA total lesion growth [1.78 mm2 (1.53– 2.03) versus 0.78 mm2 (0.54– 1.02); p=< 0.0001] during the 12-month study period compared to the Control Group.
Conclusion: Patients developing MNV while undergoing IVA treatment for GA secondary to AMD have worse clinical outcomes despite undergoing anti-VEGF therapy compared to patients who were complication-free at 12-months. This highlights the seriousness of MNV in this patient population and may help specialists counsel patients when considering treatment for GA secondary to AMD.
Keywords: geographic atrophy, avacincaptad pegol, macular neovascularization, izervay, choroidal neovascularization, complement inhibition, age-related macular degeneration
Introduction
Avacincaptad Pegol (Izervay; Iveric Bio, USA) (2 mg/0.1 mL) became the second treatment to achieve Food and Drug Administration (FDA) approval for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD) following a positive review of the pivotal clinical trials.1,2 Although intravitreal Avacincaptad Pegol (IVA) demonstrated significantly reduced GA total lesion growth at 12 and 18 months compared to sham, adverse reactions were reported in the IVA cohort of both trials.1,2 Adverse reactions such as ocular discomfort, vitreous floaters, and subconjunctival hemorrhaging were typically mild and self-limited. However, more serious adverse reactions such as intraocular inflammation ranging from anterior uveitis to vitritis and vasculitis to full-blown endophthalmitis (sterile or infectious) was reported to occur in about 1.5% of subjects at 18-months when IVA was administered as a 2-mg monthly dose.2 However, perhaps the most significant adverse event with IVA treatment given its relatively high occurrence is the development of macular neovascularization (MNV). After 18-months of treatment in the GATHER 1 trial, MNV occurred in about 12% of subjects treated with the 2-mg IVA dose monthly (compared to 3.6% in the sham).2 Unfortunately, subjects exited the study protocols of the GATHER 1 and GATHER 2 trials whenever MNV developed, and therefore very limited data is currently available regarding clinical outcomes in this subgroup of AMD patients developing MNV while undergoing IVA treatment for GA. In this study, the authors report real-world outcomes at 12-months in patients who developed MNV while undergoing IVA treatment for GA secondary to AMD.
Methods
This research was conducted as a case-controlled, retrospective study of patients who underwent IVA treatment for GA secondary to AMD from two private practice institutions in Texas and Florida. The investigations were compliant with the principles of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996. The Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-15) approved the conduct of this study. Informed consent from patients was waived because all information was collected retrospectively and identifying subject data was removed. The retrospective review was undertaken on patients who were initiated on IVA treatment for the management of GA secondary to AMD from August to September 2023.
For the purpose of this study, GA secondary to AMD was diagnosed by the appearance of well-demarcated atrophic lesions of the outer retina due to the loss of photoreceptors, retinal pigment epithelium, and underlying choriocapillaris directly observed with multi-modal imaging such as optical coherence tomography (OCT), fundus autofluorescence (FAF), fluorescein angiography (FA) and indocyanine green angiography (ICGA). The terminology utilized in this study for the classification of GA is defined according to a recent published consensus opinion of experts.3 The Heidelberg Spectralis system (Heidelberg Engineering) was employed to acquire OCT, FA, ICGA, and FAF (automatic real-time function = 15). OCT scans were captured with 97-line volume scan (20 × 20, high-resolution mode, ART = 9) and 73-line volume scan (20 × 15, high-resolution mode, ART = 9) protocols. MNV was defined as the occurrence of new exudation (eg subretinal fluid, intraretinal fluid, cystoid macular edema, and/or serous pigment epithelial detachment) secondary to a choroidal neovascular membrane (CNVM) within the macula area as observed with multi-modal imaging according to previously described techniques.4 FA and ICGA were employed to support the classification of the subtype of CNVM (eg Type I, Type II, or Type III), grade the FA leakage pattern (classic, minimally classic, occult), and measure the total size of the CNVM lesion. Masked fellowship-trained retina specialists (RBR and RMR) independently evaluated each case with its associated characteristics. RegionFinder software (Heidelberg Engineering, version 2.6.4.0) was utilized to measure the area of GA on FAF according to previously described techniques.5 Interreader concordance was established when there was < 10% difference in the total GA area measured between the two masked readers. If the total GA area was not concordant between masked readers or there was interreader divergence in any of the other categorical variables for patient inclusion/exclusion, a third investigator (SWR) arbitrated the case.
A medical records search produced a list of subjects started on IVA therapy for GA during the aforementioned period. The subjects were then divided into 1) a Study Group of subjects who developed MNV and underwent anti-VEGF therapy during the 12-month study period following IVA therapy initiation, and 2) a Control Group of subjects who were complication-free during the 12-month study period following IVA therapy initiation. Table 1 displays the Inclusion/Exclusion criteria for both cohorts. Once the Study Group was set for analysis, the number of subjects in the Control Group was further reduced to equally match the number of subjects in the Study Group. This was done by assigning all subjects in the Control Group a number, and then applying a random number-generating program to select some of the subjects for inclusion in the data analysis, thereby creating cohorts that were equal in number. When both eyes of the same subject met the study’s Inclusion/Exclusion criteria, the eye with the better visual acuity was selected for analysis.
 |
Table 1 Macular Neovascularization and Geographic Atrophy. Baseline Criteria for Inclusion and Exclusion for Both Cohorts |
Visual acuity was measured in Snellen with pinhole approximation. The baseline examination was the appointment in which the decision to initiate IVA treatment was documented. Subjects variably underwent IVA therapy either on the same day as the examination or within 1 week of the examination in which treatment was decided. The intended IVA treatment schedule for all subjects in both cohorts was within a 29–35 day interval. The anti-VEGF treatment strategy employed for the Study Group once MNV occurred was a “treat-and-extend” schedule as previously reported by the authors.6 In brief, the protocol consisted of an initial loading dose of 3 monthly anti-VEGF injections, followed by monthly injections until the macula on OCT was dry (without intraretinal or subretinal fluid), without macular hemorrhage on biomicroscopy, and without substantive leakage of the CNVM complex on FA. The treatment interval was extended by two-week increments until a maximum inter-visit interval was achieved. When fluid (intraretinal and/or subretinal) on OCT reappeared, macular hemorrhage developed, the Snellen visual acuity dropped by 2 or more lines, or substantive leakage on FA recurred following treatment extension, the treatment interval was reduced by 1–2 weeks. The decision to stop IVA therapy in favor of anti-VEGF monotherapy in the Study Group once MNV occurred was at the discretion of the managing specialist.
Outcomes and Statistical Analyses
Change in visual acuity between cohorts during the 12-month (48–56 weeks) study period was the primary outcome. Change in total GA surface area between cohorts during the 12-month (48–56 weeks) study period was the secondary outcome. Visual acuity in Snellen was changed into logMAR units for computation. For quantitative data, the nonparametric Wilcoxon rank sum test or Wilcoxon signed-rank test (for paired data) was used to compare the 2 distributions. The JMP 11 (SAS Institute, USA) statistical software package was employed. Nominal outcome variables were assessed via contingency analysis with likelihood ratios, whereas one-way analysis of the variance assessed numerical outcomes.
Results
There were 56 subjects (28 in the Study Group and 28 in the Control Group) analyzed. All subjects in both cohorts (100%) consumed an AREDS (Age-related Eye Disease Studies) II vitamin throughout the study period. Concordance between the masked reviewers in regards to image grading was 89.3% (50/56).
The baseline characteristics of the Study and Control Groups are presented in Table 2. There were no significant differences observed between cohorts at baseline. Table 3 displays the 12-month outcomes for both cohorts. Notably, the Study Group had a greater reduction in visual acuity [−0.22 logMAR (−0.27 to −0.17) versus −0.06 logMAR (−0.12 to 0.00); p=<0.0001], and an increase in GA total lesion size [1.78 mm2 (1.53–2.03) versus 0.78 mm2 (0.54–1.02); p=<0.0001] during the 12-month study period compared to the Control Group.
 |
Table 2 Macular Neovascularization and Geographic Atrophy. Baseline Characteristics of the Study and Control Cohorts. Means with (95% Confidence Intervals) |
 |
Table 3 Macular Neovascularization and Geographic Atrophy. Outcomes of the Study and Control Cohorts at 12 Months. Means with (95% Confidence Intervals) |
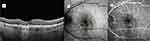
The mean onset of MNV in the Study Group was 3.2 (±1.6) months after IVA treatment initiation, and the mean visual acuity was 0.64 logMAR (±0.21) at the time of MNV diagnosis, which was significantly worse than the cohort’s baseline visual acuity (p=<0.001). The anti-VEGF agent administered for MNV treatment during the study interval was Faricimab (Vabysmo; Roche/Genentech; Basel, Switzerland)(6mg/0.05mL) in 42.9% (12/28) of subjects, aflibercept (VEGF-Trap Eye/Eylea; Regeneron, Tarrytown, NY) (2 mg/0.05mL) in 25.0% (7/28) of subjects, and bevacizumab (Avastin; Genetech, Inc)(1.25 mg/0.05mL) in 32.1% (9/28) of subjects. The mean number of anti-VEGF injections administered during the study period in the Study Group was 6.9 (±1.1), and the mean visual acuity did not significantly improve from the time of MNV diagnosis to the time of the final visual acuity at 12 months (p=0.36). All Study Group subjects underwent anti-VEGF therapy following the diagnosis of MNV, and there were only 2 subjects (7.1%) who discontinued IVA treatment once MNV developed in favor anti-VEGF monotherapy. Regarding CNVM features at the time of MNV diagnosis, 25.0% (7/28) were classified as Type 1, 32.1% (9/28) were classified as Type 2, and 42.9% (12/28) were classified as Type 3 lesions. The FA leaking patterns at the time of MNV diagnosis were as follows: 67.9% (19/28) occult, 7.1% (2/28) minimally-classic, and 21.4% (6/28) classic. The mean central macular thickness on OCT at the time of MNV diagnosis was 338.1 (±70.2) microns, which was significantly increased from the baseline value (p<0.0001). The 12-month mean central macular thickness on OCT was 250.7 (240.5–260.9) microns, which was which was significantly decreased from the value at the time of MNV diagnosis (p<0.0001). The CNVM total lesion size on ICGA was 1.5 (±1.0) mm2 at the time of MNV diagnosis, and the CNVM total lesion size on ICGA decreased to 1.31 (±1.1) mm2 at 2 months following anti-VEGF therapy (p=0.35). Figures 1–3 display one of the Study Group subject’s clinical course.
 |
Figure 2 The optical coherence tomography image (2A), indocyanine green angiography image (2B), and the fluorescein angiography image (2C) are from the same patient as Figure 1. After receiving two avacincaptad pegol injections, the patient developed new-onset macular neovascularization. Optical coherence tomography demonstrates intraretinal edema consistent with Type 3 choroidal neovascularization and the central macular thickness increased to 371 microns. The indocyanine green angiography-measured surface area of the lesion (yellow encirclement) was 2.15 mm2.The fluorescein angiographic leakage pattern was occult and displayed a central “hot spot” (arrow). The patient’s Snellen visual acuity decreased to 20/40. He was started on bevacizumab therapy and continued with avacincaptad pegol therapy. |
 |
Figure 3 The optical coherence tomography image (3A), fundus autofluorescence image (3B), and the indocyanine green angiography image (3C) are from the same patient as Figures 1 and 2 taken 12 months after initiation with the first avacincaptad pegol injection. Since the time of Figure 2, the patient had received 7 bevacizumab injections and 9 avacincaptad pegol injections. Optical coherence tomography revealed the absence of any retinal edema and the central macular thickness had decreased to 317 microns. The total geographic atrophy surface area on fundus autofluorescence increased to 2.75 mm2 (yellow encirclement) and was now multifocal. The indocyanine green angiography-measured surface area of the lesion (yellow encirclement) had been reduced to 1.44 mm2. The patient’s Snellen visual acuity was 20/50. |
Subgroup analysis was performed with respect to MNV features at the time of diagnosis as they relate to change in visual acuity or change in GA total surface area during the 12-month study period. There were no associations in central macular thickness on OCT, CNVM subtypes, FA leakage patterns, nor ICGA-measured CNVM total surface area sizes with change in visual acuity or change in GA total surface during the study period.
Discussion
It currently is unknown why there is an increased rate of MNV in eyes with GA undergoing complement inhibition. Proposed explanations for this higher incidence include retention of healthier VEGF A-producing cells due to clinical efficacy of complement inhibition treatment,7 inhibition of C3a and C5a production may result in a switch from pro-inflammatory M1 macrophages to pro-angiogenic M2 macrophages8 and the reduction of C3 and C5 in a laser-induced CNVM mouse model resulted in increased MNV compared to controls,9 inflammasome activation in macrophages and microglial cells whose cytokines are necessary to maintain homeostasis of the choroidal vasculature is reduced by complement inhibition,10 and lastly MNV may have a protective effect in regards to slowing the progression of GA.11 Although our study was not designed to determine the root cause for MNV in eyes with GA receiving complement inhibition, it was designed to provide a description of the MNV features and report visual outcomes and GA growth patterns after 12 months from IVA treatment initiation compared to a Control Group.
In regards to MNV features, a mix of CNVM subtypes were observed, and the FA leakage pattern was mostly occult. The CNVM total surface area measured by ICGA was similar to those previously reported by the authors12 in new-onset, treatment-naïve neovascular AMD both at baseline and after two anti-VEGF injections. However, the central macular thickness on OCT after identification of MNV but prior to anti-VEGF treatment was notably less than the values reported in new-onset, treatment-naïve neovascular AMD not associated with complement inhibition treatment.6,12–14 The authors believe this finding may be explained by the fact that all of the subjects in this study had either parafoveal or subfoveal GA, and therefore were starting with a lower central macular thickness which increased after exudation but not to the degree of neovascular AMD subjects without GA who would have had a thicker central macular thickness to start with. This study did not reveal during subgroup analysis any significant associations in MNV features as they relate to either change in visual acuity or change in GA total surface area during the 12-month study period. That is, neither central macular thickness, CNVM subtype, FA leaking pattern, nor ICGA-measured CNVM total surface area could be linked to change in visual acuity or change in GA total surface area at 12 months.
In regards to change in visual acuity and GA total surface area after 12 months from IVA treatment initiation, the Study Group with MNV notably had worse outcomes compared to the Control Group without MNV (or any other serious adverse event) during the study interval. Although subjects in GATHER 1 and GATHER 2 exited the trial protocol when MNV occurred and therefore did not report any outcomes other than the overall incidence of MNV during the study period in their publications,1,2 the OAKS/DERBY trials insisted that subjects continue receiving complement inhibition in addition to anti-VEGF therapy.15 The authors of the OAKS/DERBY trials15 did not provide any data regarding change in GA total surface area in subjects developing MNV, but they did report that the mean anti-VEGF injection frequency was about 1 injection bimonthly and that no subject treated with complement inhibition who developed MNV discontinued the trial for that reason (although one subject did discontinue complement inhibition in favor of anti-VEGF therapy monotherapy). In our “real-world” study, a high majority of the subjects who developed MNV continued IVA treatment in addition to anti-VEGF therapy and were managed with a treat-and-extend protocol. The authors of the OAKS/DERBY trials15 did report that visual acuity from the visit preceding MNV development to the visit at 24 months (with a median of follow up of 447 days) was similar between monthly, bimonthly, and sham groups (all about a net loss of 5–6 ETDRS letters). The subjects developing MNV in our study had significantly worse visual acuity at study’s end compared to the Control Group, although the median follow-up after MNV development in our study was just 255 days, thereby making a comparison with the OAKS/DERBY trials15 in this matter difficult as it remains unknown if the visual acuity would have become more similar over time between cohorts in our study if the study period was longer; in addition to this, the OAKS/DERBY trials15 used a different complement inhibitor (pegcetacoplan) than the complement inhibitor in our study, making a comparison in MNV features confounding. Since the subjects developing MNV in our study also had a significant growth in GA total surface area compared to Control subjects, it stands to reason that this may also have contributed in the worsening of visual acuity at study’s end for the Study Group. Since nearly all of the Study Group subjects continued IVA therapy in addition to anti-VEGF therapy once MNV occurred, the authors speculate that MNV may have contributed to the progression in GA total surface area in Study Group subjects compared to Control Group subjects without MNV. The fact that the central macular thickness on OCT was similar at 12-months to its baseline value prior to MNV in Study Group subjects suggests that the MNV was well-stabilized with anti-VEGF therapy by the end of study period and that persistent exudation cannot explain why Study Group subjects had worse visual acuity at 12-months. Indeed, some researchers have suggested that the anti-VEGF treatment itself may promote development or progression of GA.16 Further research is needed to clarify the exact role of MNV and its association with progression of GA in AMD subjects.
Our study’s strengths include the novelty of the information presented in a “real-world” setting, its complete data sets, its application of widely-available and standardized software for quantifying GA, and its case-controlled design. Our study’s weaknesses include its use of Snellen acuity with pinhole approximation rather than ETDRS letter scoring, its retrospective design, and the relatively small number of subjects included in the analysis. However, our study provides specialists with relevant information for counseling their patients and highlights the seriousness of MNV as it relates to the patient’s overall functional and anatomical outcomes when it develops in AMD subjects treated for GA. In conclusion, subjects undergoing IVA treatment for GA secondary to AMD who develop MNV during the course of treatment have worse vision and a greater progression of GA compared to subjects who are adverse event-free after 12-months follow-up. Additional clinical research is warranted to elucidate the specific role of MNV and its potential association with GA progression in AMD subjects.
Abbreviations
AMD, age-related macular degeneration; VEGF, vascular endothelial growth factor; CMT, central macular thickness; OCT, optical coherence tomography; VA, visual acuity; IVA, intravitreal avacincaptad pegol.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics
The study was approved by the Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-15) in accordance with the Ethical Standards laid down in the Declaration of Helsinki. Informed consent from study participants was waived because this was a retrospective study with no identifying patient information presented.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Patel SS, Lally DR, Hsu J, et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye. 2023;17:3551–3557.
2. Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, Phase 3 trial. Lancet. 2023;402(10411):1449–1458. doi:10.1016/S0140-6736(23)01583-0
3. Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of Atrophy Report 3. Ophthalmology. 2018;125:537–548. doi:10.1016/j.ophtha.2017.09.028
4. Guymer R, Wu Z. Age-related macular degeneration (AMD): more than meets the eye. The role of multimodal imaging in today’s management of AMD-A review. Clin Exp Ophthalmol. 2020;48(7):983–995. doi:10.1111/ceo.13837
5. Schmitz-Valckenberg S, Brinkmann CK, Alten F, et al. Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:7640–7646. doi:10.1167/iovs.11-7457
6. Rush RB, Simunovic MP, Vandiver L, et al. Treat-and-extend bevacizumab for neovascular age-related macular degeneration: the importance of baseline characteristics. Retina. 2014;34(5):846–852. doi:10.1097/IAE.0000000000000033
7. Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164.
8. Hong H, Tian XY. The role of macrophages in vascular repair and regeneration after ischemic injury. Int J Mol Sci. 2020;21:E6328. doi:10.3390/ijms21176328
9. Poor SH, Qiu Y, Fassbender ES, et al. Reliability of the mouse model of choroidal neovascularization induced by laser photocoagulation. Invest Ophthalmol Vis Sci. 2014;55:6525–6534. doi:10.1167/iovs.14-15067
10. Brandstetter C, Holz FG, Krohne TU. Complement component C5a primes retinal pigment epithelial cells for inflammasome activation by lipofuscin-mediated photooxidative damage. J Biol Chem. 2015;290:31189–31198. doi:10.1074/jbc.M115.671180
11. Heiferman MJ, Fawzi AA. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS One. 2019;14:e0217805. doi:10.1371/journal.pone.0217805
12. Rush RB, Rush SW, Aragon AV, Ysasaga JE. Evaluation of choroidal neovascularization with indocyanine green angiography in neovascular age-related macular degeneration subjects undergoing intravitreal bevacizumab therapy. Am J Ophthalmol. 2014;158(2):337–344. doi:10.1016/j.ajo.2014.05.007
13. CATT Research Group; Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908.
14. Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi:10.1016/S0140-6736(22)00010-1
15. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402(10411):1434–1448. doi:10.1016/S0140-6736(23)01520-9
16. Gemenetzi M, Lotery AJ, Patel PJ. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye. 2017;31(1):1–9. doi:10.1038/eye.2016.208
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Real-World Outcomes in Pre-Existing Neovascular Age-Related Macular Degeneration Subjects Undergoing Avacincaptad Therapy for Geographic Atrophy
Rush RB, Klein W, Rush SW, Reinauer RM
Clinical Ophthalmology 2024, 18:4011-4018
Published Date: 27 December 2024