Back to Journals » Journal of Inflammation Research » Volume 17
Optimal Timing for Corticosteroid Therapy in Idiopathic Granulomatous Mastitis: A Retrospective Analysis Highlighting Early Intervention Efficacy
Authors Wang P , Sun JZ , Fang HY, Yang DJ, Ren GS
Received 26 September 2024
Accepted for publication 19 November 2024
Published 25 November 2024 Volume 2024:17 Pages 9617—9624
DOI https://doi.org/10.2147/JIR.S498018
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Pin Wang,1– 4 Jia-Zheng Sun,3,4 Hui-Ying Fang,3,4 De-Juan Yang,3,4 Guo-Sheng Ren3,4
1Department of General Surgery, the Third People’s Hospital of Chengdu, Chengdu, People’s Republic of China; 2Center of Breast and Thyroid Surgery, the Third People’s Hospital of Chengdu, Chengdu, People’s Republic of China; 3Chongqing Key Laboratory of Molecular Oncology and Epigenetics, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 4Department of Breast and Thyroid Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Pin Wang, Email [email protected]
Background: Idiopathic granulomatous mastitis (IGM) is a chronic breast condition known for its aggressive nature and tendency for persistence and recurrence. Steroids are commonly used as the first-line treatment for IGM, but issues such as the optimal timing, and duration of treatment remain debated.
Methods: We retrospectively analyzed 343 IGM cases treated at the Third People’s Hospital of Chengdu from September 2012 to September 2023. Based on inclusion and exclusion criteria, a total of 188 patients were included in the study. Patients were categorized into lump (78 cases), abscess (81 cases), and sinus tract stages (29 cases) according to their initial diagnosis upon admission. Prednisolone was initiated at 0.75 mg/kg/day, and after effective treatment, the dosage was adjusted by 5– 10 mg weekly, followed by a maintenance dose of 2.5– 5 mg/day. Clinical characteristics, treatment responses, adverse effects, recurrence rates, and follow-up data were assessed.
Results: The median duration of prednisone treatment in our study was 87 days (range, 21– 281 days). Positive response rates to prednisolone were 78.2% in the lump stage, 60.5% in the abscess stage, and 62.1% in the sinus tract stage. Continuing low-dose prednisone for 3 months post-effective treatment reduced recurrence rates and side effect risks. Weight gain was the most common side effect (39.36%).
Conclusion: Early steroid therapy, especially in the lump stage, demonstrated superior efficacy. Following a regimen of starting with a full dose, tapering slowly, and maintaining a low dose for around 3 months steroids treatment is recommended to minimize recurrence rate and adverse effects.
Keywords: Idiopathic Granulomatous Mastitis (IGM), Steroids, Breast, Prednisolone
Introduction
Idiopathic Granulomatous Mastitis (GM) is a chronic inflammatory breast disease characterized by the presence of non-caseating granulomas and inflammatory cell infiltration in breast tissue.1 Despite its low global incidence, the disease is more prevalent in developing countries than in developed nations. The complex disease course and higher recurrence rates pose challenges in clinical practice.2,3
In the early stages, GM typically presents as a rapidly enlarging breast lump accompanied by breast swelling, redness, and pain. As the disease progresses, most patients enter the abscess stage. During this phase, the breast lump begins to suppurate, and abscess rupture may occur. Without effective treatment, the disease may advance to the sinus tract stage. In the sinus tract stage, the abscess is not completely cleared, forming a sinus tract connected to the skin of the breast, resulting in persistent discharge of pus from the sinus, which is difficult to heal.1
Currently, corticosteroids are widely used as one of the first-line treatments for Idiopathic Granulomatous Mastitis.4–7 However, there is significant variation in the efficacy and administration of corticosteroids among different studies, and research on their effectiveness and safety remains limited.2,8 Therefore, we aim to further investigate the optimal timing, and duration of steroid treatment. This study retrospectively analyzed patients treated with prednisone over the past 10 years, exploring the efficacy of steroid treatment at different stages of the disease. Through this research, we hope to provide more precise treatment recommendations and guidance for patients with granulomatous mastitis, while minimizing disease recurrence and adverse drug events.
Methods
Disease Management and Standardized Data Collection
Between September 2012 and September 2023, a total of 343 patients were pathologically diagnosed with Idiopathic Granulomatous Mastitis through biopsy. After screening based on inclusion and exclusion criteria, 188 patients were included in this study. In this study, the staging of Idiopathic granulomatous mastitis is based on the clinical manifestations and imaging characteristics at the patient’s initial diagnosis. Patients in the mass stage are characterized by the presence of one or more hard lumps in the breast detected during physical examination. Breast ultrasound may show one or more hypoechoic or isoechoic masses with clear or unclear borders, without accompanying fluid-filled dark areas. When the lumps begin to suppurate, this stage is defined as the abscess stage. Ultrasound may reveal fluid-filled dark areas within the lumps, indicating the presence of an abscess. The condition further progresses to the sinus stage when the abscess ruptures and fails to heal, forming a sinus tract connected to the skin with continuous pus discharge. Ultrasound may reveal the path and depth of the sinus tract. Patients exhibiting characteristics of both the mass and abscess stages are classified in the abscess stage; those showing features of the mass, abscess, and sinus stages are classified in the sinus stage. This retrospective study was conducted in strict accordance with the ethical principles of the “Declaration of Helsinki” (2013 revision) and received formal approval from the Ethics Committee of the Third People’s Hospital of Chengdu (Approval No.: 2021-S-75).
Inclusion and exclusion criteria are as follows:
Inclusion criteria: female, pathologically diagnosed with IGM through biopsy; age over 18 years old.
Exclusion criteria: Patients with tuberculosis, other types of granulomatous diseases, or autoimmune diseases (n=9); male patients (n=2); pregnant or lactating women (n=1); history of corticosteroid treatment in the past three months (n=42); contraindications to corticosteroids9 (n=1); patients with severe mental illness (n=1); patients who abandoned treatment midway due to intolerance of prednisone side effects or other reasons, or continued treatment at another hospital. (n=45); patients who did not agree to receive prednisone treatment (n=54).
In our research, Patients were initially given oral prednisone at a dose of 0.75 mg/kg/day. Once symptoms reached remission, the dosage was adjusted weekly by 5–10 mg based on the patient’s condition. After the disease stabilized, the treatment was maintained at 2.5–5 mg/day. Remission criteria included pain and redness relief, reduced sinus discharge, and lesion size reduction after treatment.
For patients in the mass phase, we administered prednisone treatment. For patients in the abscess phase, we performed incision and drainage of the abscess along with oral steroid hormone therapy. For those in the sinus phase, sinus tract debridement, wound dressing changes, and oral steroid hormone therapy were utilized. If no improvement was seen after 3 weeks of steroid therapy or if the condition worsened, steroid hormone therapy was discontinued, and alternative treatments like fistulectomy or local wide excision were considered. All patients are subjected to a complete blood count (CBC) examination. In the event of an increased leukocyte count, neutrophil percentage, or elevated levels of C-reactive protein (CRP), antibiotic therapy is initiated and continued until the CBC parameters revert to within the reference range.
During steroid treatment, patients visited the clinic every two weeks to monitor their condition and side effects. When calculating recurrence rates and side effects, treatments lasting over 15 days in a month are counted in the next month, while those under 15 days are counted in the previous month. Post-treatment, regular six-month follow-ups were conducted via clinic visits or phone calls to track prognosis and recurrence. Recurrence criteria are defined as the reappearance of the disease after symptoms have alleviated or disappeared following treatment, either after stopping treatment or during the follow-up period.
Statistical Analysis
Statistical analysis was conducted R (Version 4.2.1), and GraphPad Prism (9.2.0) was utilized for statistical analysis and graphing. A P value of less than 0.05 was considered statistically significant.
Results
From September 2012 to September 2023, a total of 343 patients diagnosed with idiopathic granulomatous mastitis through pathological biopsy at the Third People’s Hospital of Chengdu were screened according to strict inclusion and exclusion criteria. Among them, 188 patients were included in this study. Patients were categorized into the mass stage group, abscess stage group, and sinus stage group based on their initial diagnosis. There were 78 patients in the mass stage group, 81 patients in the abscess stage group, and 29 patients in the sinus stage group. The baseline characteristics of the study participants were analyzed and presented in Table 1. During the mass phase, 50% of patients seek medical attention within a week of symptom onset. In contrast, the abscess phase sees most patients visiting within a month, while those in the sinus phase often delay their consultations even further. This pattern of delayed medical engagement correlates with the progressive nature of the disease.
 |
Table 1 Statistical Analysis of Clinical Stages in Idiopathic Granulomatous Mastitis |
In this study, the initial oral dose of prednisone was 0.75 mg/kg/day. Upon reaching remission of symptoms, the dosage was adjusted by 5–10 mg weekly or biweekly based on the patient’s condition, with subsequent maintenance at a small dose of 2.5–5 mg/day. The median duration of prednisone treatment in our study was 87 days (range, 21–281 days). Among patients who started corticosteroid therapy during the mass stage, 61 (78.2%) showed good response (including 10 patients with complete disappearance of the mass after prednisone treatment), with 12 patients experiencing mass recurrence during follow-up. Additionally, 17 patients (21.8%) who did not respond to prednisone treatment progressed to the abscess stage, of whom we performed incision and drainage for those with unsatisfactory efficacy, with 6 patients experiencing recurrence during follow-up. For patients in the abscess stage receiving prednisone treatment combined with abscess incision and drainage, 49 patients (60.5%) showed effectiveness, with 10 cases experiencing recurrence during follow-up. 32 ineffective cases, after prolonged dressing changes and wound healing, 9 patients experiencing recurrence. The efficacy rate of prednisone treatment for patients in the sinus stage was 62.1% (18 cases), with 8 cases of recurrence, and 11 ineffective cases underwent local sinus tract excision surgery, resulting in 1 recurrence (Figure 1 and Table 2).
 |
Figure 1 Statistical Chart of Treatment Effectiveness and Recurrence Rates Across Different Stages of Idiopathic Granulomatous Mastitis. |
As there is currently no unified standard for the duration of corticosteroid therapy in the treatment of idiopathic granulomatous mastitis, we calculated the duration of prednisone treatment and recurrence rate among the 128 patients who responded effectively to prednisone treatment. Additionally, considering that a longer period of steroid therapy may lead to increased occurrence of related side effects, we also recorded the side effects of patients at different treatment durations. From the graph (Figure 2), it can be observed that the recurrence rate significantly decreases with continuous oral prednisone treatment for three months compared to shorter durations. At the third month, the incidence of treatment-related side effects also begins to rise. Therefore, based on considering both the recurrence rate and side effects, we recommend limiting the total duration of prednisone treatment to approximately 3 months.
 |
Figure 2 Temporal Analysis of Adverse Event Rates and Recurrence Following Prednisone Therapy. |
In the analysis of treatment side effects during the study period, a total of 188 cases were examined. Weight gain emerged as the most prevalent side effect, affecting 74 patients, which corresponds to 39.36% of all cases. Nausea and vomiting were observed in 25 cases, amounting to 13.30% of the total. Alopecia and fatigue were each reported in 21 cases, with an incidence rate of 11.17% for both conditions. Menstrual irregularities were documented in 12 cases, representing 6.38% of the cases reviewed. Hyperglycemia was noted in 11 cases, accounting for 5.85%, while restlessness was reported in 9 cases, or 4.79%. Hypertension was also documented in 9 cases, at the same rate as restlessness. Urticaria was observed in 8 cases (4.26%), and lower limb edema in 6 cases (3.19%). Acne was less common, affecting 5 cases (2.66%), and somnolence was the least frequent, noted in 3 cases (1.60%) (Figure 3).
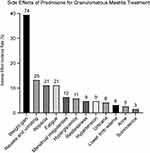 |
Figure 3 Adverse Reactions to Prednisone Treatment for Granulomatous Mastitis. |
Discussion
Idiopathic Granulomatous Mastitis (IGM) is a rare non-puerperal inflammatory breast disease characterized by lobular non-caseating granulomatous inflammation, infiltrated by lymphocytes, neutrophils, plasma cells, monocytes, and eosinophils.10 It is often associated with symptoms such as breast lumps, pain, and redness. The etiology of IGM remains unclear, with researchers suggesting it may be immune-mediated reactions, hormonal factors, or bacterial agents that contribute to its development.11–16 Due to its diverse clinical presentation, long treatment duration, prolonged course, and high recurrence rate, the diagnosis and treatment of IGM can be challenging.17,18
According to our retrospective study, we found that initiating corticosteroid therapy in the early stage of the disease, specifically during the mass stage, was more effective compared to later stages. The effectiveness rate in the mass stage could reach 78.2%, while it was only 60.5% and 62.1% in the abscess and sinus tract stages, respectively. Surprisingly, among the mass stage patients who responded well to corticosteroid treatment, 10 patients experienced complete disappearance of the mass (confirmed by physical examination and imaging), and none of these 10 patients experienced recurrence during the follow-up period. This proactive approach of using corticosteroid therapy can rapidly reduce inflammation, alleviate symptoms, shrink masses, prevent disease progression to the abscess stage, and avoid invasive procedures such as abscess incision and drainage.
Studies on corticosteroid treatment for IGM have reported varying recurrence rates, ranging from 20% to 50%.19,20 The higher recurrence rate of this condition reflects the complexity of disease management and the necessity for personalized treatment plans. Our study results are consistent with existing literature, showing a recurrence rate of approximately 24.46% (46 out of 188 patients) among all patients, with a noticeable downward trend in recurrence rates among patients receiving corticosteroid treatment for 3 months.
For patients experiencing a relapse, we implement a stage-specific management strategy, re-administering the initial treatment dosage appropriate to their current phase. In cases where patients suffer significant side effects from prednisone during the initial treatment or show poor response upon readministration, we adjust the treatment plan to fit their individual circumstances, which may include lump excision for those in the lump stage, sinus tract removal following drainage and pus clearance for those with an abscess, contingent on patient preference, and for those in the sinus tract stage, we may opt for an extended resection of the affected tissue post-debridement.
While corticosteroid therapy, including prednisone, is a cornerstone in the treatment of Idiopathic Granulomatous Mastitis (IGM), the potential adverse effects associated with long-term oral corticosteroid treatment are of significant concern. The treatment approach should balance the anti-inflammatory benefits with the risk of systemic side effects. According to the literature, the initial dosage of prednisone typically ranges from 0.5 mg/d to 0.8 mg/(kg·d),1,21,22 with subsequent adjustments based on the patient’s response and disease progression. However, the specific guidelines for prednisone use in IGM are not uniform across studies.
In our clinical experience, we follow a principle of “start early, taper slowly, and maintain a low dose” to optimize therapeutic outcomes while minimizing adverse effects. We initiate prednisone at an effective dose (0.75 mg/kg/day) to control inflammation and then gradually reduce the dosage. Our study indicates that once a therapeutic effect is achieved, a tapering strategy is employed, with the dosage eventually maintained at 2.5–5 mg/day. This approach has shown a relatively low recurrence rate after more than three months of prednisone use, while the incidence of drug-related adverse reactions begins to escalate. Therefore, we propose that the total duration of corticosteroid therapy should be limited to approximately three months. This recommendation aims to balance the effectiveness of treatment with the reduction of potential side effects, allowing for clinical remission while mitigating the risk of long-term corticosteroid-related complications.
Conclusions
Our study underscores the importance of early corticosteroid therapy and demonstrates the significant efficacy of this strategy in the mass stage. While the treatment process may carry risks of recurrence and adverse reactions, we recommend cautiously controlling the duration of corticosteroid treatment to approximately three months to reduce the recurrence rate and minimize drug toxicity and side effects.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
This retrospective study was conducted in strict accordance with the ethical principles of the “Declaration of Helsinki” (2013 revision) and received formal approval from the Ethics Committee of the Third People’s Hospital of Chengdu (Approval No.: 2021-S-75). The research involved the analysis of historical medical records, and we adhered strictly to the ethical guidelines of the Third People’s Hospital of Chengdu, with a particular emphasis on the protection of patient privacy and data confidentiality. To safeguard the privacy rights of all participants, we implemented anonymization of all personal information involved, ensuring that individual privacy is fully respected and protected throughout the research process and is not subject to any form of infringement.
Acknowledgments
The authors express their gratitude to the physicians who participated in the project by contributing patient data and assisting with clinical follow-ups. The views presented in this article are solely those of the authors and do not necessarily reflect the views of their affiliated institutions, the publisher, the editors, or the reviewers. Furthermore, any product evaluated in this article or any claims made by its manufacturer are not endorsed or guaranteed by the publisher.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Sichuan Province Medical Youth Innovation Research Project Plan (Grant No. Q23009), which aims to recognize and nurture outstanding young scholars, and was awarded to Dr. Wang Pin.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Yuan QQ, Xiao SY, Farouk O, et al. Management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition). Mil Med Res. 2022;9(1):20. doi:10.1186/s40779-022-00380-5
2. Freeman CM, Xia BT, Wilson GC, et al. Idiopathic granulomatous mastitis: a diagnostic and therapeutic challenge. Am J Surg. 2017;214(4):701–706. doi:10.1016/j.amjsurg.2017.07.002
3. Coombe RF, Hamed H. An update on granulomatous mastitis: a rare and complex condition. Br J Hosp Med. 2021;82(5):1–7. doi:10.12968/hmed.2020.0718
4. Azzam MI, Alnaimat F, Al-Nazer MW, et al. Idiopathic granulomatous mastitis: clinical, histopathological, and radiological characteristics and management approaches. Rheumatol Int. 2023;43(10):1859–1869. doi:10.1007/s00296-023-05375-6
5. Akin M, Karabacak H, Esendagli G, et al. Coexistence of idiopathic granulomatous mastitis and erythemanodosum: successful treatment with corticosteroids. Turk J Med Sci. 2017;47(5):1590–1592. doi:10.3906/sag-1611-100
6. Boufettal H, Mahdaoui S, Noun M, et al. Idiopathic granulomatous mastitis with favorable outcome with medical treatment. Rev Med Interne. 2011;32(2):e26–28. doi:10.1016/j.revmed.2009.12.018
7. Nakamura T, Yoshioka K, Miyashita T, et al. Granulomatous mastitis complicated by arthralgia and erythema nodosum successfully treated with prednisolone and methotrexate. Intern Med. 2012;51(20):2957–2960. doi:10.2169/internalmedicine.51.7846
8. Wolfrum A, Kummel S, Theuerkauf I, Pelz E, Reinisch M. Granulomatous Mastitis: a Therapeutic and Diagnostic Challenge. Breast Care. 2018;13(6):413–418. doi:10.1159/000495146
9. Faggiano A, Mazzilli R, Natalicchio A, et al. Corticosteroids in oncology: use, overuse, indications, contraindications. An Italian Association of Medical Oncology (AIOM)/ Italian Association of Medical Diabetologists (AMD)/ Italian Society of Endocrinology (SIE)/ Italian Society of Pharmacology (SIF) multidisciplinary consensus position paper. Crit Rev Oncol Hematol. 2022;180:103826. doi:10.1016/j.critrevonc.2022.103826
10. Cui L, Sun C, Guo J, Zhang X, Liu S. Pathological manifestations of granulomatous lobular mastitis. Front Med Lausanne. 2024;11:1326587. doi:10.3389/fmed.2024.1326587
11. Wang L, Jorns JM. Cystic neutrophilic granulomatous mastitis: corynebacterium species-associated infection with distinct histology. Clin Microbiol Infect. 2021;27(2):236–237. doi:10.1016/j.cmi.2020.06.037
12. Wang J, Xu H, Li Z, et al. Pathogens in patients with granulomatous lobular mastitis. Int J Infect Dis Apr. 2019;81:123–127. doi:10.1016/j.ijid.2019.01.034
13. Cakir C, Nayci AE, Ferlengez E, Guler M, Idiz UO. Cytokines the Etiology of Idiopathic Granulomatous Mastitis. J Coll Physicians Surg Pak. 2022;32(7):869–873.
14. Li S, Huang Q, Song P, et al. Clinical characteristics and therapeutic strategy of granulomatous mastitis accompanied by Corynebacterium kroppenstedtii: a retrospective cohort study. BMC Womens Health. 2023;23(1):388. doi:10.1186/s12905-023-02509-7
15. Bi J, Li Z, Lin X, et al. Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. Int J Infect Dis. 2021;113:243–250. doi:10.1016/j.ijid.2021.10.019
16. Wang X, He X, Liu J, et al. Immune pathogenesis of idiopathic granulomatous mastitis: from etiology toward therapeutic approaches. Front Immunol. 2024;15:1295759. doi:10.3389/fimmu.2024.1295759
17. Steuer AB, Stern MJ, Cobos G, et al. Clinical Characteristics and Medical Management of Idiopathic Granulomatous Mastitis. JAMA Dermatol. 2020;156(4):460–464. doi:10.1001/jamadermatol.2019.4516
18. Tang ELS, CSB H, Chan PMY, Chen JJC, Goh MH, Tan EY. The therapeutic dilemma of idiopathic granulomatous mastitis. Ann Acad Med Singap. 2021;50(8):598–605. doi:10.47102/annals-acadmedsg.2020645
19. Toman D, Prokop J, Kubala O, Kepicova M, Jelinek P, Vavra P. Granulomatous mastitis treatment options and our experience. Rozhl Chir. 2021;100(4):192. doi:10.33699/PIS.2021.100.4.
20. Al Awfi MM, Al rahbi SK. Idiopathic Granulomatous Mastitis: six years of experience and the current evidence in literature. Sultan Qaboos Univ Med J. 2023;23(1):36–41. doi:10.18295/squmj.4.2022.030
21. Kehribar DY, Duran TI, Polat AK, Ozgen M. Effectiveness of Methotrexate in Idiopathic Granulomatous Mastitis Treatment. Am J Med Sci. 2020;360(5):560–565. doi:10.1016/j.amjms.2020.05.029
22. Montazer M, Dadashzadeh M, Moosavi Toomatari SE. Comparison of the Outcome of Low Dose and High-Dose Corticosteroid in the Treatment of Idiopathic Granulomatous Mastitis. Asian Pac J Cancer Prev. 2020;21(4):993–996. doi:10.31557/APJCP.2020.21.4.993
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


