Back to Journals » Clinical Ophthalmology » Volume 19
Outcomes and Complications of Minimally Invasive Glaucoma Surgeries (MIGS) in Primary Angle Closure and Primary Angle Closure Glaucoma: A Systematic Review and Meta-Analysis
Authors Paik B, Chua CH, Yip LW , Yip VC
Received 26 November 2024
Accepted for publication 30 January 2025
Published 11 February 2025 Volume 2025:19 Pages 483—506
DOI https://doi.org/10.2147/OPTH.S505856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Benjamin Paik,1 Chun Hau Chua,2 Leonard W Yip,1,2 Vivien CH Yip1,2
1Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore; 2Department of Ophthalmology, National Healthcare Group Eye Institute, Tan Tock Seng Hospital, Singapore
Correspondence: Vivien CH Yip, TTSH Medical Centre, 11 Jalan Tan Tock Seng, Level 1, Singapore, 308433, Singapore, Email [email protected]
Topic: To analyse the safety and efficacy of minimally invasive glaucoma surgeries (MIGS) in lowering IOP and medication burden in primary angle closure (PAC) or primary angle closure glaucoma (PACG).
Clinical Relevance: MIGS is off-label in angle closure, as implantation of the device is difficult when there is iridotrabecular contact. However, access to the trabecular meshwork may be established with cataract removal or goniosynechiolysis. While MIGS can be performed in the same setting as phacoemulsification (MIGS-phaco), it remains controversial if the combined procedure confers a significant advantage over standalone phacoemulsification.
Methods: We performed a systematic search of MEDLINE, EMBASE, and Cochrane library for studies reporting outcomes of MIGS in PAC or PACG. Our main outcome measures were IOP-lowering from baseline and medication reduction, at 1 year. We performed meta-analyses of these outcomes using weighted mean differences (WMD) under random-effects models. Our secondary outcome was complication rate.
Results: Twenty-three studies comprising 875 patients were included in our review, from which 15 (590 patients) were included in meta-analysis. MIGS (with and without phacoemulsification) demonstrated a weighted mean reduction in IOP of 7.71 mmHg (95% CI: 5.16– 10.26), and in medication of 1.57 (95% CI: 1.17– 1.96), both at 1 year. Subgroup analyses revealed the superiority of AIT over endoCPG and iStent in both IOP-lowering (p< 0.02) and medication burden (p< 0.01). Comparison analysis between MIGS-phaco vs standalone phacoemulsification revealed superiority of MIGS-phaco in reducing medication burden (WMD 0.59, 95% CI: − 0.04– 1.22) and to a limited extent, IOP-lowering (WMD 1.22; 95% CI: − 0.96– 3.39) up till 1 year. Overall complication rate was 141/875 (16%) after excluding transient self-resolving hyphema.
Conclusion: MIGS brings about sustained reduction in IOP and medication burden with favourable side-effect profile in angle closure eyes. MIGS-phaco may be superior to standalone phacoemulsification. MIGS should be thoroughly considered for mild-moderate PACG with coexisting cataract before pursuing more invasive surgical approaches.
Keywords: MIGS, glaucoma, angle closure, surgery
Introduction
Glaucoma is a permanent optic neuropathy with characteristic optic disc changes and corresponding visual field abnormalities, where intraocular pressure (IOP) is a major modifiable risk factor.1 It is the leading cause of irreversible blindness worldwide2 and can be categorized into open vs closed angle or primary vs secondary. Treatment usually starts with medical therapy to lower IOP, followed by surgery (trabeculectomy or filtering shunt). Laser peripheral iridotomy (LPI) is also used in the treatment of primary angle closure glaucoma (PACG). Indications for surgery include failure of medical treatment and LPI, side effects of treatment such as severe ocular surface disease, or noncompliance with medical treatment.1
Minimally invasive glaucoma surgery (MIGS) is a group of techniques which aims to lower IOP and medication burden while minimizing scleral dissection and conjunctival manipulation via an ab interno approach.3 Therefore, MIGS are associated with a shorter surgical time, high safety profile, and faster recovery compared to traditional filtration surgery (trabeculectomy and tube shunts4). MIGS techniques can either aim to promote aqueous outflow or reduce aqueous production. Techniques to promote aqueous outflow include trabecular bypass surgery, which may involve implants (eg, iStent or Hydrus) or other tools to excise trabecular tissue (eg, Trabectome) or subconjunctival bleb-forming devices (eg, XEN).3 MIGS techniques to reduce aqueous production include endoscopic cyclophotocoagulation (endoCPG) which enters through the limbus, visualizes the ciliary body via flexible endoscopy, and ablates ciliary processes using a diode laser.
Conventionally, MIGS has only been approved for usage in open-angle glaucoma to promote trabecular filtration. Angle closure has been a contraindication of MIGS for various reasons: difficult surgical access to the trabecular meshwork (TM) and a predilection to fibrosis, synechiae formation and descemetization which may obstruct any implanted MIGS device or the angle itself,5 necessitating further procedures like laser iridoplasty or even stent revision surgery.6 However, such a view is falling out of favour, because it precludes many glaucoma patients from benefitting from MIGS; for instance, angle closure glaucoma accounts for 87% of cases in Asians and 70% of cases in females.7 Furthermore, in chronic PACG, prolonged irido-trabecular contact leads to damage to the TM such that IOP is raised even when the angles are re-opened, such as after a cataract surgery.8 This shows that PACG can benefit from improved trabecular drainage after MIGS. For these reasons, MIGS have been used off-label for the treatment of PACG in selected patients.
Furthermore, most patients with glaucoma tend to be elderly with concomitant cataract for which they will eventually opt to undergo surgery. As phacoemulsification is one of the most common eye surgical procedures, MIGS may be performed in the same setting (phaco-MIGS) for selected patients with the intent of improving IOP control and hence reducing medication burden. A recent meta-analysis of POAG patients showed that MIGS with cataract surgery showed higher IOP and glaucoma medication reduction compared to cataract surgery alone,9 but this difference failed to reach statistical significance. A different situation exists in PACG: since cataract surgery involves converting a narrower phakic angle to a more open pseudophakic angle, the extent of IOP reduction post-phacoemulsification is expected to be greater in angle closure glaucoma compared to open-angles.10,11 Therefore, it remains controversial if phacoemulsification alone delivers comparable outcomes (in terms of IOP and medication reduction) compared to a combination of MIGS and phaco. In particular, if the combined procedure does not offer a significant advantage over standalone cataract surgery, then there would be less justification to add to the MIGS procedure, whose complications as described above cannot be overlooked. In addition, the cost-effectiveness of implanting these MIGS devices in angle closure eyes has to be considered as well.
Therefore, the aim of this meta-analysis is to summarize the outcomes of and complications from the various types of MIGS procedures in PAC and PACG and evaluate if combined phacoemulsification with MIGS offers any advantage over standalone phacoemulsification.
Methodology
We performed this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines. This study protocol was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42023409,649) (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=409649).
Search Methods for Identifying Studies
We performed an electronic search using MEDLINE (via PubMed), EMBASE (via Ovid), and the Cochrane Library from inception till 19 March 2023 with no language restriction, using a combination of both MeSH and non-MeSH keywords: ((“minimally invasive glaucoma surgery” OR “minimally invasive” OR “ab interno” OR “iStent” OR “iStent inject” OR “Hydrus” OR “Trabectome” OR “Trab360” OR “Trabeculotomy” OR “Goniotomy” OR “Gonioscopy-assisted transluminal trabeculotomy” OR “GATT” OR “Omni” OR “Fugo plasma blade” OR “Kahook dual blade” OR “ab interno canaloplasty” OR “XEN” OR “XEN45” OR CyPass OR StarFlo OR SOLX OR “iStent Supra” or “MINIject” OR “cyclophotocoagulation” OR “endoscopic cyclophotocoagulation”) AND (“angle closure glaucoma” OR “angle-closure glaucoma” OR “closed angle glaucoma” OR “closed-angle glaucoma” OR “narrow angle glaucoma” OR “narrow-angle glaucoma”)). The detailed search strategies for each database are presented in the Appendix.
We included all possible relevant articles by performing backward reference searching of included articles and review articles. Foreign language articles were excluded if no native speakers were available for accurate translation.
Eligibility Criteria for Considering Studies
We included all articles (randomized controlled trials, retrospective and prospective cohort studies, case series) that reported outcomes of MIGS use (with or without goniosynechialysis) in PAC or PACG. Both single and double arm studies were included. No demographic restrictions were applied. Patients may or may not have existing cataract.
If there were articles with overlapping patient populations (same study group, identical population size and interventions) we chose the publication with the larger sample size or the study that provided the most pertinent clinical information.
Interventions
In accordance with the definition of MIGS set out by the American Glaucoma Society Position Paper, we aimed to include studies on procedures which (1) reduce aqueous production by targeting the ciliary body, such as endoscopic (transpupillary) cyclophotocoagulation (endo-CPG); (2) promote aqueous outflow through (A) trabecular bypass (implants, eg, iStent, Hydrus; non-implants, eg, Trabectome, goniotomy, Kahook dual blade (KDB) goniotomy, ab interno canaloplasty (ABiC), microhook ab interno trabeculotomy, excimer laser trabeculotomy, 360 degree suture trabeculotomy, gonioscopy assisted transluminal trabeculotomy (GATT), Visco360 cum OMNI system), (B) suprachoroidal devices (Cypass, STARFlo, SOLX, iStent SUPRA, MINInject), or (C) subconjunctival bleb-forming devices (XEN (ab interno or ab externo), ExPRESS).
Exclusion Criteria
We excluded standalone cataract surgery when performed to treat angle closure, standalone goniosynechialysis, as well as non-incisional therapies such as selective laser trabeculoplasty, trans-scleral cyclomodulation (laser and ultrasound) and modified minimally invasive/small incision trabeculectomy. Patients with previous filtration or tube shunt surgery were excluded, although we still included patients with LPI or cataract surgery as long as these were not performed within the past 6 months of the MIGS procedure. We excluded studies with less than 10 patients, on pediatric population, primary angle closure suspects, secondary glaucomas (eg, pseudoexfoliative, pigmentary), and those where data for PAC/PACG could not be isolated and extracted independently of other patient subgroups. For studies which reported <1 year of follow-up data or had greater than 20% attrition rate, we excluded them from meta-analysis but included them in the computation of intra- and post-operative complications (up until the last time point followed up).
Study Selection
Following a comprehensive search and deduplication, two authors (BP and VY) initially screened all articles by title and abstract only. Conflicts were resolved by consensus. The full text of articles included in the first stage were subsequently reviewed in their entirety for final inclusion. Next, we used standardized data sheets in Microsoft Excel to extract our outcome measures from text, tables, and figures. All studies were assessed for risk of bias using the National Institute of Health Quality Assessment Tools.
Data Collection and Risk of Bias Assessment
Across all studies, two authors (BP and VY) extracted the following data if available: patient demographics (age, ethnicity); permanent glaucomatous changes (cup-disc ratio, visual field mean deviation), IOP values (baseline and at various timepoints after MIGS), number of IOP-lowering medications (baseline and at various timepoints after MIGS), and complications (intraoperative and postoperative).
Most papers did not specify if IOP was measured before or after the ocular washout. Unless otherwise specified, we assumed the reported baseline measurement to be non-washout. IOP values were reported for various timepoints; we took the 1-year timepoint which is short- to mid-term, beyond which the condition can be considered to be fairly stable.
While complications were reported in most studies, some studies just reported sentences like “no complications encountered” or words to that effect.
Risk of bias was assessed using the National Institute of Health (NIH) Quality Assessment Tool for Observational Cohort Studies, or the NIH Tool for Before-After (Pre-Post) studies with No Control Group.
Data Syntheses and Analysis
Meta-analysis of continuous outcome data was performed using the meta package (version 6.1.0) in R version 4.1.3. Where available, the mean and standard deviation of IOP and number of glaucoma medications at baseline and 1-year follow-up were used to compute their mean reduction, respectively, unless this information was already furnished by the study authors.
We used the following formulae and definitions, as described previously in Lavia et al (2017):9
Mean reduction in IOP (pre, post)
IOPR = IOPbaseline – IOPendpoint
Correlation values (Corr) of 0.3 and 0.4 were used for IOP and medication reduction, respectively. “Corr” value is the median value from our available studies.
Results from our meta-analysis were represented as forest plots with relevant studies showing effect size and their confidence interval of the outcome reported and their respective weight (in percent) that was attributed to the random-effects model for the pooled effect size. Statistical significance was set at p <0.05. We employed a random-effects model (with Hartung-Knapp adjustment) to assign study weightages and estimate the pooled study estimates to reduce the risk of a false positive result. The I2 statistic (directly based on Cochran’s Q test) was used to quantify statistical heterogeneity between studies. This statistic indicates that the percentage of variability in the effect size in our data is not due to sampling error, with I2 values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. Whenever there was substantial statistical heterogeneity (I2 > 50%) in the pooled result, we used the dmetar package in R to evaluate for outliers and influential studies using the find.outliers and InfluenceAnalysis functions, respectively. If a meta-analysis was undertaken to compare the efficacy of phaco vs phaco-MIGS, the difference in the mean reductions from baseline for each arm was employed, to obtain the weighted mean difference (WMD), as described separately.12,13 The aggregated result was reported as weighted mean difference (WMD) which is used for comparison of 2 arms and comparison of pre vs post-MIGS.
Results
Systematic Search Strategy
The initial search revealed a total of 304 publications for possible inclusion after manual deduplication, of which 91 remained for full-text review after title and abstract screening. A further 68 were excluded after applying our exclusion criteria, leaving 23 publications for qualitative synthesis (Figure 1). From this, we excluded a further 3 publications14–16due to excessively high attrition rate, 3 more due to lack of minimum 1-year follow-up for all patients,17–19 and 2 which omitted reporting of standard deviations for our outcome measures.20,21 This left 15 publications for the final meta-analysis, of which 4 were on endoCPG,22–25 5 were on ab interno trabeculotomy (AIT) such as KDB (n=2),26,27 GATT (n=1),28 trabectome (n=1),29 and goniotomy (n=1).30 Five were on trabecular bypass implants such as iStent (n=5)31–35 and 1 was on XEN45 (n=1), a subconjunctival bleb-forming device.8 These studies and their outcomes are summarized in Tables 1 and 2 respectively. We did not find any literature on suprachoroidal devices in PACG.
 |
Table 1 Baseline Characteristics of All Eligible Studies |
 |
Table 2 Outcomes of Eligible Studies |
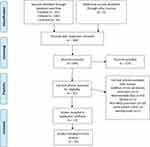 |
Figure 1 PRISMA diagram of included studies. |
Effect of MIGS on IOP Lowering
All studies reported a fall in IOP after the MIGS procedure (with or without phaco), at 1-year follow-up (Figure 2). The weight mean reduction in IOP from baseline was 7.71 (95% CI: 5.16, 10.26). Expectedly, there was a significant heterogeneity (98%) since we analyzed various MIGS techniques with or without concomitant cataract surgery. Despite the heterogeneity, which stems from a significant heterogeneity in MIGS device type, baseline glaucoma severity, and individual surgeon technical proficiency, the lower bound of 95% CI, 5.16, is still well above the line of no effect. This shows that MIGS provides a significant improvement in IOP from baseline over a wide range of efficacies. Although 4 studies (Changsapetch, Salimi (single arm), Dorairaj and Fontana) were identified as outliers, excluding these studies would mean biasing toward studies with smaller numbers; furthermore, even after excluding outliers, the pooled estimate and 95% CI remain similar (Supplementary Figure 1 and Appendix). If anything, this shows that MIGS is overall beneficial to reducing IOP despite pooling studies showing a wide range of efficacies.
 |
Figure 2 Effect of IOP reduction at 1 year across all MIGS subtypes. Mean R / MD / Mean Difference = mean reduction in IOP. Abbreviation: N, number of eyes. |
Subgroup analysis was further performed to assess the between-group differences in IOP lowering (Figure 3A). With regard to each MIGS technique’s efficacy in IOP lowering, AIT (TM cutting) had a WMD of 11.48 (95% CI 7.12, 15.83) which was superior to that of iStent which had a WMD of 4.12 (95% CI −0.39, 8.62) and this difference was statistically significant (p<0.01), as shown in Figure 3B. There was no obvious superiority of outflow targeting methods (AIT, iStent) over endo-CPG on reduction of IOP at 1 year, because even when AIT and iStent were grouped together and compared to endo-CPG, there was no significant difference between these two groups (p=0.74) (Figure 3C). However, AIT was superior to endo-CPG (Figure 3D). No significant differences in efficacy were found between iStent vs endoCPG (p=0.14) (Figure 3E).
Effect of MIGS on Reduction in Number of Glaucoma Medications
Our pooled analysis shows that MIGS delivered statistically significant reductions in medication burden through 1 year of follow-up (Figure 4). The weighted mean number of medications reduced was 1.6 (95% CI: 1.2, 2.0). Reduction in medication was reported amongst all individual studies. While studies by Changsapetch, Dorairaj and Fontana were observed to be outliers, after their exclusion, the pooled effect size is similar albeit with a lower heterogeneity (Supplementary Figure 2 and Appendix). This shows that MIGS is overall beneficial to reducing medication burden despite pooling studies showing a wide range of efficacies.
 |
Figure 4 Effect of various MIGS on glaucoma medication lowering at 1 year. |
We proceeded to perform subgroup analysis between the 3 main MIGS interventions which were represented by adequate numbers of studies each: iSTent, AIT, and endoCPG (Figure 5A).
In terms of medication reduction, AIT produced a greater reduction in medications (2.38, 95% CI 1.42, 3.35) compared to endoCPG (1.46, 95% CI 0.85, 2.07, p < 0.01) and iStent (1.10, 95% CI 0.61, 1.59, p<0.01), (Figures 5B and C respectively). That being said, there were no significant differences between iStent and endoCPG groups (p=0.17) (Figure 5D). There was no obvious superiority of outflow targeting methods (AIT, iStent) over endoCPG (p=0.57) (Figure 5E).
Comparing Standalone Phaco vs Phaco-MIGS
Five studies compared standalone phacoemulsification vs phaco-MIGS on a total of 492 patients. The weighted mean difference in IOP reduction between the 2 groups was 1.22 (95% CI: −0.96, 3.39) in favour of phaco-MIGS (Figure 6). However, the 95% CI crossed the line of no effect (0.00) and these results should be regarded with cautious optimism. The WMD in reduction of glaucoma medications was 0.59 (95% CI: −0.04, 1.22) once again in favour of phaco-MIGS (Figure 7).
 |
Figure 6 Standalone phacoemulsification vs phaco-MIGS on IOP reduction. Experimental = phaco-MIGS; Control = standalone phaco. |
 |
Figure 7 Standalone phacoemulsification vs phaco-MIGS on medication reduction. Experimental = phaco-MIGS; Control = standalone phaco. |
The authors elected not to do leave-one-out (LOO) analysis because this would further diminish the limited number of articles contributing data. However, we recognize the moderate heterogeneity in the pooled value, and hence, this result should be interpreted with caution.
Amongst studies included in our review, patients receiving standalone phaco also had an acceptable fall in IOP and number of medications from baseline (Figure 8A and B respectively). This validates the efficacy of the phaco technique in lowering IOP and medication burden in MIGS, therefore legitimizing the comparison between phaco and phaco-MIGS.
 |
Figure 8 Effect of phacoemulsification. (A) on IOP at 1 year; (B) on medication reduction. |
Adverse Outcomes
We included intra- and post-operative complications (up till time of last follow-up) from all papers in our meta-analysis, as well as other papers which we found in our literature search but could not be included in the pooled meta-analysis due to the lack of one-year data or due to excessively high dropout rate. Complication data are presented in Table 3.
 |
Table 3 Complications Reported Across Eligible Studies |
For the iStent, there were 55 adverse events reported out of 239 eyes (23.0%), consisting of transient self-resolving hyphaema (n=13); hyphaema requiring drainage (n=1); iris prolapse (n=2); iridodialysis (n=2); stent malposition (n=1); iris trauma (n=1); iStent occlusion (n=17); posterior capsular opacification (n=7); IOP spike (n=4); iris atrophy (n=3); corneal edema (n=2); rebound iritis (n=1); endothelial blood staining (n=1).
For endoCPG, there were 27 adverse events reported out of 156 eyes (17.3%), consisting of cystoid macular edema (n=4); hemorrhagic choroidal detachment (n=1); fibrinous uveitis (n=11), PCO (n=1), persistent dilated pupil (n=1) treated with pupilloplasty; IOP spike (n=3); secondary pupillary block (n=2), hyphema treated with hemostatic medicine (n=2), malignant glaucoma 5 months after surgery (n=1), zonular dehiscence (n=1) likely phaco-related.
For AIT, there were 211 adverse events reported out of 393 eyes (53.7%), consisting of transient self-resolving hyphaema (n=152); hyphaema requiring surgical drainage (n=4); delayed hyphaema on POD 4 (n=1); postoperative IOP spike (n=35); cyclodialysis (n=1); persistent intraocular inflammation (n=3); posterior capsular opacification treated with YAG (n=1); AC washout (n=4); corneal edema (n=8), recurrence of PAS at region of removed TM (n=2) requiring argon laser iridoplasty. In fact, the rates of transient hyphaema in some studies (Chira-adisai, Fontana, Sharkawi, Wang) neared 100%.
For some studies16,26,35 including two on XEN,8,16 no complications could be extracted as these were reported collectively with patients from other irrelevant subgroups.
Complication rate was calculated based on the incidence of complications over the total number of patients that did MIGS (with or without concomitant phacoemulsification); patients receiving standalone phacoemulsification were excluded from this calculation. Since a successful TM incision causes reflux bleeding from the AC at a high rate,37 transient self-resolving hyphema from AIT (ie, no drainage or hemostasis required) can be disregarded as a complication. If we exclude such cases from the calculation, the overall complication rate (intraoperative and postoperative) was 141/820 (17.2%).
Quality Assessment (Risk of Bias)
On the whole, all studies were assessed to be either good or fair quality, signifying a generally low risk of bias, as shown in Table 4. Some studies did not categorically specify their eligibility criteria or discuss the statistical power of the study, hence increasing the risk of bias.
 |
Table 4 Risk of Bias Assessment for Studies Included in Meta-Analysis. NIH Quality Assessment Tool for Observational Cohort Studies |
Discussion
MIGS Delivers Long-Lasting Effects on IOP and Medication Burden Reduction
Just as glaucoma has many subtypes and severities, many are the current treatment options and increasing over time. The increasing options add further complexity and nuances to the realm of glaucoma management. While it is usually performed for OAG to promote trabecular drainage, MIGS’ utility in angle closure glaucoma is not as well established. Part of the controversy lies in attempting canal-based procedures in angle closure, which is still regarded as a contraindication due to the technical challenge of visualizing the TM in the angle to make the initial goniotomy or device implantation intraoperatively.38 Furthermore, even if a device was to be successfully implanted, its efficacy may be affected by changes in the trabecular meshwork and/or endothelial damage in the Schlemm’s canal.39 Additionally, narrow angles also leave a lesser margin for error, especially if a deployed stent gets displaced, migrates, or otherwise malpositioned (eg, buried within the TM).40 The iris can obstruct the lumen of the stent and narrow angle increases the risk of this occurring – with rates up to 27% in one study.34
This meta-analysis aimed to investigate the efficacy and safety of various MIGS with or without phacoemulsification to treat PACG. Our pooled analysis demonstrates that MIGS deliver statistically significant reductions in IOP and medication burden through 1 year of follow-up. Moreover, studies which followed patients until 2 years (Al-Habash, Dorairaj, Lai, Sharkawi) and 3 years (Salimi) reported sustained reductions in IOP and medications from baseline, most of which are statistically significant. However, almost all patients who underwent MIGS also had concomitant phaco in the same setting. Altogether, our meta-analysis shows that all classes of MIGS, when combined with lens extraction, are able to bring about persistent reductions to both IOP and medications in PACG, albeit to varying extents.
In our meta-analysis, the majority of interventions were combined MIGS-phaco. Few studies in our review provided data on standalone MIGS (all of which happen to be AIT), because studies by Chira-adisai, Sharkawi, and Gabbay had to be excluded from pooled analysis due to their high dropout rate (and risk introducing significant bias to the analysis). However, the paucity of evidence on standalone MIGS for PACG reflects that in the management of PACG, MIGS is performed as an add-on to phacoemulsification and not the other way round; this is because the bulky lens component in angle closure is the key pathology in PACG rather than impaired trabecular filtration. That being said, although MIGS per se do not reduce IOP as much as traditional incisional glaucoma surgeries as elucidated in a recent review41, MIGS appear to consistently enhance the IOP reduction that phacoemulsification provides (around 5 mmHg from unmedicated washout baseline)42,43 in POAG patients in whom trabecular dysfunction is a major contributor to elevated IOP.
While results at 1 year appear promising, further durability of such approaches can only be attested to in long-term studies, which are still lacking. Our meta-analysis presents a tentative case for the incorporation of MIGS into the therapeutic armamentarium in carefully selected PACG patients.
Combination MIGS-Phaco May Be Superior to Standalone Phaco
Our meta-analysis demonstrates that, in the treatment of PACG, combination MIGS-phaco is superior to standalone phacoemulsification in reducing medication burden. However, there was only weak evidence to support the superiority of MIGS-phaco in terms of IOP reduction, as the 95% CI crossed the line of no effect. It is possible that the majority of IOP reduction is from the lens removal per se, with minimal additional effects from the MIGS.
At times, lens removal and GSL is insufficient to reduce IOP in PACG; this is due to concurrent TM dysfunction and/or endothelial damage in Schlemm’s canal.44 This is because prolonged irido-trabecular contact is observed to cause many pathological changes, such as pigment accumulation in trabecular spaces, noninflammatory degeneration, loss of endothelial cells, and reactive repair processes, which are similar to the histopathologic changes of POAG. These changes are usually present even in areas away from PAS.39,44 However, though MIGS may augment the effects of lens removal through bypassing the trabecular meshwork, if the level of occlusion is further downstream at the Schlemm’s canal or collector channels, performing AIT or inserting iStent alone will be less effective at lowering IOP. Other MIGS approaches, such as ab interno XEN, can shunt aqueous directly into the subconjunctival space, and endoCPG, which reduces aqueous production and hence IOP in any type of glaucoma, may be effective in such instances.
Our meta-analysis only followed patients up till 1 year; limited data are present for 2 and 3 years. Given the scarcity of long-term follow-up data, it remains important to monitor IOP over time, since MIGS efficacy may wane over time: for instance, implants (iStent/Hydrus) can be occluded by peripheral anterior synechiae;45 for AIT (eg, KDB), there can be fibrovascular membrane or scarring due to inflammatory reactions after TM instrumentation.46
Complications from MIGS as a Whole
As a whole, MIGS tend to have less complications than trabeculectomy or glaucoma drainage devices. The complication rate of MIGS (with or without phaco) among all eligible papers in our review is 293/875 (33.4%), though it drops to 141/875 (16.1%) after excluding transient, self-resolving hyphaema which is non sight-threatening. Furthermore, the incidence of hyphaema is surgeon-dependent as the anterior chamber can be pressurized to prevent reflux of blood from the episcleral veins. Even by only considering complications from papers in our meta-analysis, the complication rate remained relatively constant at 89/590 (15.0%). Across the MIGS, the most common complications were hyphaema (including transient and self-resolving types) (24.9%), IOP spike (4.9%), and postoperative inflammation (3.31%) comprising corneal edema (n=10), uveitis (n=12), cystoid macula edema (n=4), and persistent intraocular inflammation not otherwise specified (n=3). This is in comparison to the Tube Versus Trabeculectomy Study which reported early postoperative complications of 22% in GDD and 39% in Trabeculectomy (excluding intraoperative and complications occurring after 1 month).47 These suggest how all types of MIGS have a more favorable side effect profile compared to traditional surgeries. Of note, there were no reported complications of hypotony, diplopia, and infection, which are feared to be common complications of trabeculectomy and GDD, as elucidated in the Tube Versus Trabeculectomy Study.47 In particular, MIGS poses less risk of postoperative hypotony since MIGS devices are designed to drain aqueous against the episcleral venous pressure. Diplopia (usually binocular) complicates up to 20–23% of GDD surgeries48,49 and is often a result of GDD placement in the orbital causing oculomotor maladjustment, while MIGS does not involve orbital surgery. Infections like blebitis can complicate trabeculectomy and GDD, and the risk increases with hypotony50 and use of antiproliferative agents51,52 which are associated with these traditional surgeries. Therefore, MIGS offers an option for patients in whom medication compliance is an issue, have severe ocular surface disease from topical medications, and/or have uncontrolled IOP even on medications, yet their glaucoma is not nearly severe enough to warrant trabeculectomy and its attendant risks.
Despite having its own complications, MIGS confer two important benefits: first, they preserve the health of the conjunctiva, via the ab interno conjunctiva-sparing approach, preserving the option for tube shunt or filtration surgery down the line without compromising its efficacy.53 Second, as shown in our review, MIGS bring about a long-lasting significant reduction in topical medications, hence sparing the health of the ocular surface. Ocular surface disease may predispose to (sub)conjunctival fibrosis54 and hence cause late bleb filtration failure if trabeculectomy is ever performed.
However, since the majority of studies did combination MIGS-phaco, we cannot tell if these are complications of MIGS or the phacoemulsification.
Efficacy, Benefits and Pitfalls of Various MIGS Techniques
MIGS represent a very heterogenous group of techniques and each glaucoma subtype is expected to respond differently to different MIGS. Currently, the favored MIGS for OAG is iStent (43.7%).55 Although MIGS remain rarely used for PACG, the most common techniques used are endoCPG (13.3%) followed by iStent (10.3%), although these proportions are much smaller than standard surgery (GDD at 34.1% and trabeculectomy at 33.9%).55 So far, few studies have directly compared one type of MIGS vs another. This is important because while MIGS all share similar benefits of minimal conjunctival manipulation and scleral dissection, their reported results are different. Our subgroup analysis shows that AIT was superior to endoCPG and iStent in reducing IOP and IOP-lowering medications through 1 year of follow-up; these differences were statistically significant. EndoCPG was superior to iStent in reducing IOP and medication burden, but only the former outcome reached statistical significance. It was not meaningful to compare between devices of the same class (eg, Trabectome vs KDB) due to each being represented by too little studies.
The inherent differences in surgical technique may explain the different efficacies. Comparing AIT and iStent, AIT has a greater likelihood of involving collector channels en bloc with the incised/resected TM.37 Conversely, iStent is placed comparatively more proximal, hence efficacy may be limited by resistance if present – which might explain its underperformance relative to AIT. As with all Schlemm’s canal devices, IOP cannot be lowered beyond episcleral venous pressure; if this is high to begin with, then the IOP reduction is likely to be modest. This will be insufficient for patients needing significant IOP reduction (eg, advanced glaucoma with severe pattern deviation), in which cyclophotocoagulation or bypassing the canal directly (eg, XEN) might be a better option. By the same token, glaucoma conditions with high episcleral venous pressure (eg, uveitic glaucoma, cavernous sinus thrombosis, SVC syndrome, neovascular glaucoma) should not be considered for iStent. Additionally, younger patients may benefit more from Schlemm’s canal-based MIGS devices as they preserve anatomy for future surgeries.
While endoCPG is generally thought to be the most effective due to its dual action of reducing aqueous production and widening the angle recess (even in synechial angle closure),21 compared to other techniques (iStent or AIT) which only target aqueous outflow.24 However, endoCPG underperformed AIT in our meta-analysis. This might be because the treatment range across the studies was inadequate. In the 4 studies included in subgroup analysis, only 1 study treated up to 360°; the other studies treated up to 180° or even 150°. However, treatment of at least 270° is required to have significant IOP reduction.56,57 Treatment of down to 180° can still be clinically meaningful, albeit less effective. In practice, it is understandable why surgeons adopt a conservative approach, since overtreatment can cause significant tissue damage and severe complications such as traumatic iris injury, ciliary block glaucoma, and phthisis. As seen, there is a fine line to tread in balancing efficacy and complications when using endoCPG to treat PACG.
That being said, choosing a MIGS necessitates thorough consideration of pros and cons of all options, rather than a blanket adoption of the technique with the highest IOP-lowering effect, or a one-size-fits-all approach. AIT might be most efficacious but has complications that cannot be overlooked. First, hyphema is the most common complication of AIT as elucidated in our review with its incidence nearing 100% in 4 out of the 6 papers that reported it. While hyphema is expected after TM incision, this is supposed to be transient and self-limiting; failure of it to re-absorb after 4 days with medical management (topical steroids) will warrant surgical drainage. Second, it causes relatively more anterior chamber inflammation (than iStent) as shown in a separate study in POAG: AC flare returned to normal within 1 week for iStent but took a month for AIT.58 This is likely due to higher amounts of energy or tissue disruption delivered to the TM using AIT.59 Third, AIT has severe IOP spike more so than other MIGS; while well-reported (even in POAG), the underlying mechanisms remain poorly understood.60,61 Fourth, the ideal TM incision range remains unclear. The study, which treated a greater angle (360°) such as Fontana, reported a greater IOP and medication reduction compared to those settling for smaller incision ranges like Dorairaj (110–140°) and Wang (90–120°); conversely, excessively wide incisions may increase bleeding, cause transient IOP elevation, and impede performance of subsequent incisions if IOP increases again in the future.37 Furthermore, while the complication rate for AIT (excluding hyphema) was lower than iStent, a lot of studies (especially those on AIT) did not categorically state that there were complications; rather, they did not address complications at all. In any case, after excluding transient hyphema for both AIT and iStent, their percentage of remaining adverse events is 17.0% (59/348) and 17.6% (42/239), respectively. While we assumed all complications that occurred would have been reported, the complication rate may be higher than calculated in our review.
Implantable devices like iStent, despite having the least IOP-lowering effect, cause less tissue disruption62 and hence lesser postoperative inflammation and IOP spikes. However, device associated adverse events such as implant migration, extrusion, dislocation, endothelial cell loss, conjunctival erosion, and device obstruction, can lead to inadequate IOP reduction.
Given the various pros and cons of each MIGS technique, surgeons may also consider a combination of various MIGS that target various components of glaucoma pathophysiology. Just like how some medications may reduce aqueous production, while others increase aqueous outflow; similarly, MIGS inflow and outflow procedures can be amalgamated by either done together or at an interval (eg, ECP combined with iStent). Although we lack the level of data resolution needed to evaluate the efficacy of each type of MIGS in different patient populations (furthermore, we excluded secondary glaucomas from this meta-analysis), the principles outlined above – when extrapolated to disease pathophysiology – can serve as general guidelines for patient selection.
Limitations of Meta-Analysis
The results of our meta-analysis need to be interpreted in the context of known limitations. First, in the majority of studies, multiple surgical techniques in the same setting made it difficult to ascertain which was the main driver of reductions to IOP and medication burden. For example, some studies used varying combinations of phacoemulsification, goniosynechialysis, and viscogoniosynechialysis alongside the MIGS procedure. Second, there were also heterogeneity among the studies in terms of study type (RCT, prospective and retrospective studies), with further heterogeneity contributed by the type of MIGS, sample size, and the baseline patient profile: demographics, glaucoma severity, baseline medications, and past surgical history, all of which have a bearing on the outcome measures of interest. For instance, the underlying severity of glaucoma or an initial high IOP at outset can speak for downstream TM insufficiency. However, this heterogeneity attests to the generalizability of the results among a wide variety of patients of different severities as well as the benefits common to all MIGS procedures. Third, our conclusion of MIGS-phaco being superior to phaco is supported only by the five studies which compared phacoemulsification to phaco-MIGS, among which only iStent and endoCPG were represented. However, since AIT was evaluated to be superior to iStent and endoCPG in terms of reducing IOP and medication burden, it is postulated that phaco-AIT is likely to remain superior to standalone phacoemulsification. Lastly, these results do not generalize for all types of glaucoma. Patients with advanced glaucoma will require lower IOP targets, which MIGS cannot deliver.
Suggestions for Future Work
In assembling this review, we have uncovered several knowledge gaps in the evidence base for MIGS in PACG. First, published ophthalmic literature does not include RCTs comparing trabeculectomy or GDD versus MIGS in the PACG context. Second, where possible, washed-out IOPs should be reported to permit meaningful comparison across studies and MIGS techniques. Third, long-term outcomes should expand beyond IOP and medication burden reduction, to functional and structural markers of glaucoma progression, such as mean visual field defect and nerve fibre layer/ganglion cell loss. Fourth, since baseline demographics were collected from patients, authors should assess if any of these are risk factors for developing complications from MIGS. Fifth, cost-effectiveness studies of MIGS vis-à-vis traditional approaches should be conducted. This is because MIGS devices are comparatively expensive to traditional surgeries: GDD can cost as little as USD 50, but MIGS devices can cost >400 USD – not taking into account the extra costs of surgical goniolenses, or steep learning curve required for MIGS.63 However, reduced rates of early postoperative visits, interventions, and re-interventions (eg, trabeculectomy) might make MIGS more cost-effective. Sixth, studies directly comparing different MIGS techniques will be useful in guiding clinical decision-making. Lastly, collecting patient-reported outcomes such as quality of life or visual recovery would make for a more holistic assessment of MIGS.
Conclusion
MIGS, with or without concomitant phacoemulsification in the same setting, brings about a reduction in IOP and medication burden for the short to intermediate term, but it remains to be seen if the results are durable in the long term. Analysis of a limited number of studies shows the superiority of MIGS-phaco over standalone phacoemulsification. Given their favourable side effect profile compared to traditional (ab externo) trabeculectomy or GDDs, the additional benefit of adding MIGS procedure potentially outweighs its attendant risks. Given the large proportion of mild-moderate cases included in this analysis, clinicians should adopt a lower threshold to consider MIGS than traditional approaches, to minimize disease progression and medication side effects. Though traditional approaches remain the gold standard for patients with advanced PACG, MIGS should be thoroughly considered for mild-moderate PACG with coexisting cataract before pursuing more invasive surgical approaches.
Acknowledgments
The abstract of this paper was presented at the NHG Eye Institute 9th International Ophthalmology Congress as a poster presentation with interim findings.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Spaeth GL. European glaucoma society terminology and guidelines for glaucoma, 5th Edition. Br J Ophthalmol. 2021;105(Suppl 1):1–169. doi:10.1136/bjophthalmol-2021-egsguidelines
2. Kang JM, Tanna AP. Glaucoma. Med Clin North Am. 2021;105(3):493–510. doi:10.1016/j.mcna.2021.01.004
3. Fellman RL, Mattox C, Singh K, et al. American glaucoma society position paper: microinvasive glaucoma surgery. Ophthalmol Glaucoma. 2020;3(1):1–6. doi:10.1016/j.ogla.2019.12.003
4. Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi:10.1097/ICU.0b013e32834ff1e7
5. Liu J, Jung J, Francis BA. Ab interno trabeculotomy: trabectome™ surgical treatment for open-angle glaucoma. Expert Rev Ophthalmol. 2009;4(2):119–128. doi:10.1586/eop.09.8
6. Wellik SR, Dale EA. A review of the iStent(®) trabecular micro-bypass stent: safety and efficacy. Clin Ophthalmol. 2015;9:677–684. doi:10.2147/OPTH.S57217
7. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262. doi:10.1136/bjo.2005.081224
8. Sng CCA, Chew PTK, Htoon HM, Lun K, Jeyabal P, Ang M. Case series of combined XEN implantation and phacoemulsification in Chinese eyes: one-year outcomes. Adv Ther. 2019;36(12):3519–3529. doi:10.1007/s12325-019-01127-w
9. Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi:10.1371/journal.pone.0183142
10. Lai JS, Tham CC, Chan JC, Lam DS. Phacotrabeculectomy in treatment of primary angle-closure glaucoma and primary open-angle glaucoma. Jpn J Ophthalmol. 2004;48(4):408–411. doi:10.1007/s10384-003-0075-2
11. Man X, Chan NC, Baig N, et al. Anatomical effects of clear lens extraction by phacoemulsification versus trabeculectomy on anterior chamber drainage angle in primary angle-closure glaucoma (PACG) patients. Graefes Arch Clin Exp Ophthalmol. 2015;253(5):773–778. doi:10.1007/s00417-015-2936-z
12. Friedrich JO, Adhikari NKJ, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Method. 2008;8(1):32. doi:10.1186/1471-2288-8-32
13. Higgins JPT, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27(29):6072–6092. doi:10.1002/sim.3427
14. Chira-Adisai T, Mori K, Kobayashi A, et al. Outcomes of combined gonioscopy-assisted transluminal trabeculotomy and goniosynechialysis in primary angle closure: a retrospective case series. Int Ophthalmol. 2021;41(4):1223–1231. doi:10.1007/s10792-020-01676-y
15. Sharkawi E, Artes PH, Lindegger DJ, et al. Gonioscopy-assisted transluminal trabeculotomy in primary angle-closure glaucoma. Graefes Arch Clin Exp Ophthalmol. 2021;259(10):3019–3026. doi:10.1007/s00417-021-05244-7
16. Gabbay IE, Goldberg M, Allen F, et al. Efficacy and safety data for the Ab interno XEN45 gel stent implant at 3 Years: a retrospective analysis. Eur J Ophthalmol;2021. 11206721211014381. doi:10.1177/11206721211014381
17. Shokoohi-Rad S, Karimi F, Zarei-Ghanavati S, Tireh H. Phacoemulsification, visco-goniosynechialysis, and goniotomy in patients with primary angle-closure glaucoma: a comparative study. Eur J Ophthalmol. 2021;31(1):88–95. doi:10.1177/1120672119879331
18. Villavicencio JCI, Arbelaez NA, Lastra BR, et al. Primary outcomes of patients with chronic angle-closure glaucoma treated with combined phacoemulsification, viscogoniosynechialysis, and endocyclophotocoagulation. J Ophthalmol. 2019;2019:1.
19. Gupta S, Sethi A, Yadav S, et al. Safety and efficacy of incisional goniotomy as an adjunct with phacoemulsification in primary angle-closure glaucoma. J Cataract Refract Surg. 2021;47(4):504–511. doi:10.1097/j.jcrs.0000000000000481
20. Pathak-Ray V. Intermediate results of phaco-endocycloplasty in an exclusive cohort of angle closure glaucoma: potential for change. Intl Ophthalmol. 2019;39(10):2257–2265. doi:10.1007/s10792-018-01062-9
21. Pathak-Ray V, Choudhari N. Phaco-endocycloplasty versus phacotrabeculectomy in primary angle-closure glaucoma: a prospective randomized study. Ophthalmol Glaucoma. 2020;3(6):434–442. doi:10.1016/j.ogla.2020.06.006
22. Lai ISW, Chan NCY, Ling A, et al. Combined phacoemulsification-endoscopic cyclophotocoagulation versus phacoemulsification alone in primary angle-closure glaucoma: a pilot randomized controlled trial. Ophthalmol Glaucoma. 2021;4(6):589–596. doi:10.1016/j.ogla.2021.03.007
23. Zhou WS, Lin WX, Geng YY, Wang T. Combined phacoemulsification and goniosynechialysis with or without endoscopic cyclophotocoagulation in the treatment of PACG with cataract. Int J Ophthalmol. 2020;13(9):1385–1390. doi:10.18240/ijo.2020.09.08
24. Lin MM, Rageh A, Turalba AV, et al. Differential efficacy of combined phacoemulsification and endocyclophotocoagulation in open-angle glaucoma versus angle-closure glaucoma. J Glaucoma. 2019;28(5):473–480. doi:10.1097/IJG.0000000000001225
25. Panse K, Le C, Hubbell M, Ayyala RS. Surgical outcomes of phacoemulsification/goniosynechialysis with and without endocyclophotocoagulation in patients with chronic angle closure glaucoma. Ind J Ophthalmol. 2019;67(3):366–370. doi:10.4103/ijo.IJO_895_18
26. Al Habash A, Albuainain A. Long term outcome of combined phacoemulsification and excisional goniotomy with the Kahook Dual Blade in different subtypes of glaucoma. Sci Rep. 2021;11(1):10660. doi:10.1038/s41598-021-90223-5
27. Dorairaj S, Tam MD, Balasubramani GK. Two-year clinical outcomes of combined phacoemulsification, goniosynechialysis, and excisional goniotomy for angle-closure glaucoma. Asia-Pac J Ophthalmol. 2021;10(2):183–187. doi:10.1097/APO.0000000000000321
28. Fontana L, De Maria M, Iannetta D, Moramarco A. Gonioscopy-assisted transluminal trabeculotomy for chronic angle-closure glaucoma: preliminary results. Graefes Arch Clin Exp Ophthalmol. 2022;260(2):545–551. doi:10.1007/s00417-021-05400-z
29. Wang Y, Liang ZQ, Zhang Y, Hennein L, Han Y, Wu HJ. Efficacy and safety of phacoemulsification plus goniosynechialysis and trabectome in patients with primary angle-closure glaucoma. Sci Rep. 2021;11(1):13921. doi:10.1038/s41598-021-92972-9
30. Song Y, Zhang Y, Li F, et al. One-year results of a multicenter study: intraocular pressure–lowering effect of combined phacoemulsification, goniosynechialysis, and goniotomy for cases of advanced primary angle-closure glaucoma with cataract. Asia-Pac J Ophthalmol. 2022;11(6). doi:10.1097/APO.0000000000000579.
31. Chansangpetch S, Lau K, Perez CI, Nguyen N, Porco TC, Lin SC. Efficacy of cataract surgery with trabecular microbypass stent implantation in combined-mechanism angle closure glaucoma patients. Am J Ophthalmol. 2018;195:191–198. doi:10.1016/j.ajo.2018.08.003
32. Chen DZ, Sng CCA, Sangtam T, et al. Phacoemulsification vs phacoemulsification with micro-bypass stent implantation in primary angle closure and primary angle closure glaucoma: a randomized single-masked clinical study. Clin Exp Ophthalmol. 2020;48(4):450–461. doi:10.1111/ceo.13721
33. Salimi A, Abu-Nada M, Harasymowycz P. Matched cohort study of cataract surgery with and without trabecular microbypass stent implantation in primary angle-closure glaucoma. Am J Ophthalmol. 2021;224:310–320. doi:10.1016/j.ajo.2020.12.032
34. Hernstadt DJ, Cheng J, Htoon HM, Sangtam T, Thomas A, Sng CCA. Case series of combined istent implantation and phacoemulsification in eyes with primary angle closure disease: one-year outcomes. Adv Ther. 2019;36(4):976–986. doi:10.1007/s12325-019-00899-5
35. Salimi A, watt H, Harasymowycz P. Three-year outcomes of second-generation trabecular micro-bypass stents (istent inject) with phacoemulsification in various glaucoma subtypes and severities. J Glaucoma. 2021;30(3):266–275. doi:10.1097/IJG.0000000000001716
36. Gabbay IE, Allen F, Morley C, Pearsall T, Bowes OM, Ruben S. Efficacy and safety data for the XEN45 implant at 2 years: a retrospective analysis. Br J Ophthalmol. 2020;104(8):1125–1130. doi:10.1136/bjophthalmol-2019-313870
37. Kasahara M, Shoji N. Effectiveness and limitations of minimally invasive glaucoma surgery targeting Schlemm’s canal. Jpn J Ophthalmol. 2021;65(1):6–22. doi:10.1007/s10384-020-00781-w
38. Khaimi MA, Harvey BJ, Hsueh J, Leal C, Baykal A. Canaloplasty via an ab-interno surgical technique in patients with primary angle closure glaucoma. Intl Ophthalmol. 2024;44(1):401. doi:10.1007/s10792-024-03322-3
39. Hamanaka T, Kasahara K, Takemura T. Histopathology of the trabecular meshwork and Schlemm’s canal in primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2011;52(12):8849–8861. doi:10.1167/iovs.11-7591
40. Gillmann K, Mansouri K, Ambresin A, Bravetti GE, Mermoud A. A prospective analysis of istent inject microstent implantation: surgical outcomes, endothelial cell density, and device position at 12 months. J Glaucoma. 2020;29(8):639–647. doi:10.1097/IJG.0000000000001546
41. Birnbaum FA, Neeson C, Solá-Del Valle D. Microinvasive Glaucoma Surgery: an Evidence-Based Review. Seminars Ophthalmol. 2021;36(8):772–786. doi:10.1080/08820538.2021.1903513
42. Samuelson TW, Chang DF, Ahmed IIK, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. doi:10.1016/j.ophtha.2018.05.012
43. Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. doi:10.1016/j.ophtha.2019.03.006
44. Sihota R, Lakshmaiah NC, Walia KB, Sharma S, Pailoor J, Agarwal HC. The trabecular meshwork in acute and chronic angle closure glaucoma. Indian J Ophthalmol. 2001;49(4):255–259.
45. Hu R, Guo D, Hong N, Xuan X, Wang X. Comparison of Hydrus and iStent microinvasive glaucoma surgery implants in combination with phacoemulsification for treatment of open-angle glaucoma: systematic review and network meta-analysis. BMJ Open. 2022;12(6):e051496. doi:10.1136/bmjopen-2021-051496
46. Kornmann HL, Fellman RL, Feuer WJ, et al. Early results of goniotomy with the kahook dual blade, a novel device for the treatment of glaucoma. Clin Ophthalmol. 2019;13:2369–2376. doi:10.2147/OPTH.S224643
47. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–14.e1. doi:10.1016/j.ajo.2011.10.024
48. Islamaj E, Jordaan-Kuip CP, Vermeer KA, Lemij HG, de Waard PWT. Motility changes and diplopia after baerveldt glaucoma drainage device implantation or after trabeculectomy. Trans Vision Sci Technol. 2018;7(5):7. doi:10.1167/tvst.7.5.7
49. Kilgore KP, Wang F, Stern NC, et al. Rates of diplopia in Ahmed FP7, Baerveldt 250, and 350 glaucoma patients compared with medical controls. J Glaucoma. 2021;30(7):579–584. doi:10.1097/IJG.0000000000001886
50. Song A, Scott IU, Flynn HW Jr, Budenz DL. Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. Ophthalmology. 2002;109(5):985–991. doi:10.1016/S0161-6420(02)00965-X
51. Lehmann OJ, Bunce C, Matheson MM, et al. Risk factors for development of post-trabeculectomy endophthalmitis. Br J Ophthalmol. 2000;84(12):1349–1353. doi:10.1136/bjo.84.12.1349
52. Jampel HD, Quigley HA, Kerrigan-Baumrind LA, Melia BM, Friedman D, Barron Y. Risk factors for late-onset infection following glaucoma filtration surgery. Arch Ophthalmol. 2001;119(7):1001–1008. doi:10.1001/archopht.119.7.1001
53. Jea SY, Mosaed S, Vold SD, Rhee DJ. Effect of a failed trabectome on subsequent trabeculectomy. J Glaucoma. 2012;21(2):71–75. doi:10.1097/IJG.0b013e31820bcfda
54. Baudouin C. Ocular surface and external filtration surgery: mutual relationships. Dev Ophthalmol. 2012;50:64–78.
55. Yang SA, Mitchell WG, Hall N, et al. Usage patterns of minimally invasive glaucoma surgery (MIGS) differ by glaucoma type: IRIS registry analysis 2013-2018. Ophthalmic Epidemiol. 2021;29:1–9.
56. Kahook MY, Lathrop KL, Noecker RJ. One-site versus two-site endoscopic cyclophotocoagulation. J Glaucoma. 2007;16(6):527–530. doi:10.1097/IJG.0b013e3180575215
57. Huang J-Y. Endoscopic Cyclophotocoagulation. Glaucoma Today; March 2009.
58. Tokuda N, Kitaoka Y, Tsukamoto A, et al. Comparison of minimally invasive glaucoma surgery with trabecular micro-bypass stent and microhook ab interno trabeculotomy performed in conjunction with cataract surgery. Int J Ophthalmol. 2022;15(7):1082–1088. doi:10.18240/ijo.2022.07.07
59. Kurji K, Rudnisky CJ, Rayat JS, et al. Phaco-trabectome versus phaco-iStent in patients with open-angle glaucoma. Can J Ophthalmol. 2017;52(1):99–106. doi:10.1016/j.jcjo.2016.06.018
60. Murakami-Kojima S, Takahashi E, Eguchi-Matsumoto M, Saruwatari J, Nakashima K-I, Inoue T. Risk factors for intraocular pressure elevation in a six-month period after ab interno trabeculotomy using a Kahook Dual Blade. BMC Ophthalmol. 2022;22(1):327. doi:10.1186/s12886-022-02545-1
61. Shi Y, Wang H, Oatts JT, et al. A prospective study of intraocular pressure spike and failure after gonioscopy-assisted transluminal trabeculotomy in juvenile open-angle glaucoma: a prospective study of GATT in JOAG. Am J Ophthalmol. 2022;236:79–88. doi:10.1016/j.ajo.2021.10.009
62. Schweitzer JA, Hauser WH, Ibach M, et al. Prospective interventional cohort study of ocular surface disease changes in eyes after trabecular micro-bypass stent(s) implantation (iStent or iStent inject) with phacoemulsification. Ophthalmol Ther. 2020;9(4):941–953. doi:10.1007/s40123-020-00290-6
63. Otárola F, Pooley F. Minimally invasive glaucoma surgery (MIGS) devices: risks, benefits and suitability. Community Eye Health. 2021;34(112):59–60.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Interim Analysis of STREAMLINE® Surgical System Clinical Outcomes in Eyes with Glaucoma
Lazcano-Gomez G, Garg SJ, Yeu E, Kahook MY
Clinical Ophthalmology 2022, 16:1313-1320
Published Date: 27 April 2022
Short-Term Efficacy of Combined ab Interno Canaloplasty and Trabeculotomy in Pseudophakic Eyes with Open-Angle Glaucoma
Bleeker AR, Litchfield WR, Ibach MJ, Greenwood MD, Ristvedt D, Berdahl JP, Terveen DC
Clinical Ophthalmology 2022, 16:2295-2303
Published Date: 21 July 2022
Canaloplasty and Trabeculotomy with the OMNI System in Patients with Open-Angle Glaucoma: Two-Year Results from the ROMEO Study
Williamson BK, Vold SD, Campbell A, Hirsch L, Selvadurai D, Aminlari AE, Cotliar J, Dickerson Jnr JE
Clinical Ophthalmology 2023, 17:1057-1066
Published Date: 6 April 2023
Long-Term Efficacy of Successful Excisional Goniotomy with the Kahook Dual Blade
Wagner IV, Boopathiraj N, Lentz C, Dorairaj EA, Draper C, Kumar D, Checo L, Miller DD, Krambeer C, Dorairaj S
Clinical Ophthalmology 2024, 18:713-721
Published Date: 7 March 2024
Interventional Glaucoma: Improving the Patient-Provider Educational Exchange
Katz LJ, Myers JS, Herndon Jnr LW, Kresch YS, Hengerer FH
Clinical Ophthalmology 2024, 18:3365-3374
Published Date: 21 November 2024