Back to Journals » Journal of Inflammation Research » Volume 17
Overproduction of Mitochondrial Fission Proteins and Mitochondrial Fission in Podocytes of Lupus Nephritis Patients
Authors Wei Q , Wu X, Chen Z, Lin H, Xiong L, Wang N
Received 25 September 2024
Accepted for publication 21 November 2024
Published 10 December 2024 Volume 2024:17 Pages 10807—10818
DOI https://doi.org/10.2147/JIR.S497813
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Qijiao Wei,1 Xinchen Wu,2 Zhihan Chen,3 He Lin,3 Lei Xiong,2 Na Wang4
1Department of Rheumatology, Children’s Hospital of Fudan University, Shanghai, 201102, People’s Republic of China; 2The First School of Clinical Medicine, Yunnan University of Chinese Medicine, Kunming, Yunnan, 650500, People’s Republic of China; 3Department of Rheumatology, Fujian Provincial Hospital, Fuzhou, 355000, People’s Republic of China; 4Department of Traditional Chinese Medicine, Children’s Hospital of Fudan University, Shanghai, 201102, People’s Republic of China
Correspondence: Na Wang, Children’s Hospital of Fudan University, No. 399 Wanyuan, Shanghai, People’s Republic of China, Email [email protected] Lei Xiong, The First School of Clinical Medicine, Yunnan University of Chinese Medicine, No. 1 huachen, Xishan District, Kunming, Yunnan Province, People’s Republic of China, Email [email protected]
Background: The glomerular injury is associated with different pathogeneses, and podocyte damage is common in various ISN/RPS class lupus nephritis (LN). In podocyte, mitochondrial morphological changes are observed in lupus nephritis (LN) in our previous study. This study aimed to explore mitochondrial fission proteins expression in podocytes using bioinformatics analysis and further to investigate the associations between mitochondrial fission proteins and laboratory features in LN.
Methods: To determine the differentially expressed genes (DEGs) between LN and normal controls, we downloaded and analyzed microarray datasets. Then download the mitochondrial genes list from the MitoMiner 4.0 database, then take the genes that are common with the DEGs. Functional enrichment analyses were then performed. Then mitochondrial fission was observed through electron microscope. We performed immunofluorescence staining to explore the expression of mitochondrial fission proteins in LN patients.
Results: Among these 658 DEGs, 5 DEGs related to mitochondrial dynamics were identified. Mitochondrial dynamics proteins were involved in mitophagy. Mitochondrial fission proteins Drp1 and Fis1 staining were significantly enhanced compared to that in the controls. 24h-UTP are positively correlated with mitochondrial fission proteins expression.
Conclusion: Mitochondrial fission was observed in LN patients’ podocytes. Mitochondrial fission proteins Drp1 and Fis1 were overproduced in podocytes, and then they can lead to mitochondrial fission, which may aggravate podocyte damage and proteinuria. While the mechanism still needs to be explored.
Keywords: lupus nephritis, podocyte injury, mitochondria fission, Drp1, Fis1
Introduction
Lupus nephritis (LN) accounts for approximately 50% of systemic lupus erythematosus (SLE) patients.1 The glomerular injury is associated with different pathogeneses, and podocyte damage is common in various ISN/RPS class LN.2 In podocyte, mitochondrial morphological changes are observed in lupus nephritis (LN). Different sized mitochondria increased and mitochondrial cristae disappeared in podocyte.3 Some studies have found increased mitochondrial mass contributed to aberrant activation and enhanced necrosis of T cells in SLE.4,5 Blocking Rab4 with 3-PEHPC results in the restoration of Drp1 and reverses mitochondrial accumulation, as well as the production of antinuclear antibodies, proteinuria, and lupus nephritis.6 Inhibiting mitochondrial fission that can protect glomerular podocytes was found in diabetic kidney disease.7 Aldosterone induced mitochondrial dysfunction and podocyte injury mediated by p53/Drp1-dependent mitochondrial fission.8 Also, one study found mitochondrial fission promoted renal fibroblast activation and fibrogenesis in chronic kidney disease.9 Whereas the relationship between mitochondrial fission and podocyte injury in LN still needs further experiments to explore. Bioinformatics analysis was used to identify DEGs related to mitochondrial dynamics in this study. Then we observed the mitochondrial fission through electron microscope. To explore the expression of mitochondrial fission proteins Drp1 and Fis1, immunofluorescence staining and semi-quantitative analysis were performed. The relationship between glomerular expression of mitochondrial fission proteins and clinical data was explored.
Methods
Microarray Data
NCBI-GEO10 is a public functional genomics data repository. GSE 112943, GSE 113342, GSE 32591 were selected and downloaded. Total of 14 LN and 6 normal kidney biopsy samples were contained in GSE113342 dataset.11 And 32 LN and 14 normal biopsy samples in GSE 32591.12 And 14 LN and 7 normal biopsy samples in GSE112943.13
Identification of DEGs
We used the GEO2R online tool to identify DEGs between LN and normal controls. We set log fold change (FC) >1 and P-value <0.05. Log FC <0 was considered downregulated, whereas log FC >0 was considered upregulated. Download the mitochondrial genes list from the MitoMiner 4.0 database.14 The intersections of mitochondrial genes from 3 datasets are taken, and the resulting common DEGs are then combined.
Functional Enrichment
Gene Ontology (GO) function for analyzing genes biological processes and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were executed using the online biological information database database for Annotation, Visualization and Integrated Discovery (DAVID).15–17 P <0.05 is considered statistically significant.
Patients
The study was approved by the Ethics Committee of the Fujian Provincial Hospital (K2021-04-046) and complies with the Declaration of Helsinki. This study included 30 lupus nephritis (LN) patients with a mean age of 32.70 ± 12.17 years. Six types of pathological classifications (Class II, III, IV, V, III+V, and IV+V) were used, with five patients in each group. Additionally, 15 patients with a mean age of 55.69 ± 7.27 years who underwent nephrectomization due to renal tumors were used as normal controls. Further details can be found in a previous study.18
Morphology of Mitochondria Was Observed
The kidney tissue of both LN patients and normal controls were preserved in ice-cold 1% glutaraldehyde in 0.1 M PBS for transmission electron microscopy. The samples were then sliced by laboratory staff, and the mitochondria and mitophagy were observed using a transmission electron microscopy.
Immunofluorescence Staining of Mitochondrial Fission Proteins
Renal biopsy specimens were embedded in an OCT mixture (Sakura, Hayward, CA, USA) and sliced into 5 μm frozen sections.3 The mouse anti-Drp1 and Fis1 antibody (Proteintech Group, Inc., Chicago, IL, USA) were used. While rabbit anti-synaptopodin antibody (Proteintech Group, Inc. Chicago, IL, USA) was used as a podocyte marker for double immunofluorescence staining. Images were taken with a fluorescence microscope (Nikon Eclipse C1, Japan). The integral optical density (IOD) and the area ratio (AR) of the positively stained area to the glomerular area were used as semi-quantitative values to measure the expression of Drp1 and Fis1.18,19
Correlation of Drp1, Fis1 with Clinical and Laboratory Data
Thirty LN patients and fifteen healthy controls were used for this correlation analysis. The clinical data used in this study was obtained from the cohort used for fluorescence staining of mitochondrial fission proteins.
Statistical Analysis
The t-test or Mann–Whitney U-test were used to compare the differences between the two groups using SPSS. The χ2 test was used to calculate the ratio. A p-value of less than 0.05 was considered statistically significant.
Results
DEG Identification
A total of 12376 DEGs were identified from the GSE112943 dataset, including 9434 upregulated and 2942 downregulated genes. In the GSE113342 dataset, 68 DEGs were identified, including 42 upregulated and 26 downregulated genes. In the GSE32591 dataset, 171 DEGs were observed, consisting of 28 upregulated and 143 downregulated genes. After combining the three datasets, a total of 10045 DEGs were found, with 658 DEGs being common between the datasets and mitochondrial genes, as shown in the Venn diagram (Figure 1a). The 658 DEGs consisted of 142 downregulated genes and 516 upregulated genes. These DEGs associated with mitochondrial dynamics are plotted in Figure 1b, and the basic information of 5 DEGs related to mitochondrial dynamics are shown in Table 1.
 |
Table 1 Basic Information on the 5 DEGs Related to Mitochondrial Dynamics |
GO Annotation and KEGG Pathway Enrichment Analyses
The findings regarding GO and KEGG are presented in Table 2 and Figure 2. GO biological process analysis revealed that the 658 DEGs were significantly linked to apoptotic process and translation. The top three significant changes in cell component were observed in mitochondrion, peroxisome and ribosome. The primary changes in molecular function were associated with RNA binding. KEGG pathway analysis indicated that the DEGs were mainly associated with metabolism and biosynthesis. We also found mitochondrial dynamics proteins are involved in mitophagy.
 |
Table 2 KEGG Pathway Enrichment Analysis of DEGs in LN Glomerulus (Partial) |
 |
Figure 2 GO annotation analyses. The DEGs enrichment of BP, MF, CC (The top ten, P < 0.05). |
Mitochondrial Fission
Mitochondria in the podocytes from health control showed ellipsoid shape. And cristae could be seen clearly in mitochondria. While, different sized mitochondria were observed in podocytes of LN patients, mitochondrial cristae were disappeared. Through the electron microscope, we found that mitochondrial ridge structure is damaged (Figure 3).
 |
Figure 3 Mitochondrial fission. Red arrows represented the fission mitochondria. |
Expression of Drp1 and Fis1 in Various ISN/RPS Class LN Patients
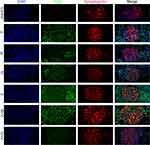
Immunofluorescence microscopy revealed that the glomerular staining of Drp1 in health controls was negative, while the staining in patients with class IV+V was weak. In Class III+V, the Drp1 proteins were found to colocalize with the podocyte marker synaptopodin (Figure 4). The staining of Fis1 in health control was very weak, but it was significantly enhanced in Class III and Fis1 was also found to colocalize with the synaptopodin marker (Figure 5). The semi-quantitative analysis of the results is presented in Table 3 and Figure 6.
 |
Table 3 Glomerular Expression of Drp1 and Fis1 in Various ISN/RPS Class LN Patients |
 |
Figure 6 Glomerular expression of Drp1 and Fis1 in various ISN/RPS class LN patients. Note: *P < 0.05, **P < 0.01, *** P < 0.001. |
Correlation of Drp1 and Fis1 with Laboratory Data
The IOD of Drp1 was found to be positively correlated with C3, C4 and 24h-UTP. The AR of Fis1 was positively correlated with BUN and 24h-UTP, while the IOD of Fis1 was negatively correlated with 24h-UTP. However, no correlation was observed between the AR of Drp1 and laboratory data. We analyzed the association between Drp1 and Fis1 and clinical measures in patients with different pathological types of lupus nephritis. And we can see that there is no significant correlation between the clinical indicators of patients with type IV lupus nephritis and DRP1 and Fis1. The results are presented in Figures 7 and 8.
 |
Figure 7 The correlation of mitochondrial fission proteins and laboratory data. Note: *P < 0.05, **P < 0.01, *** P < 0.001, ****P < 0.0001. |
Discussion
Bioinformatics analysis was used to identify 5 DEGs related to mitochondrial dynamics, including 4 mitochondrial fission genes (DNM1L, FIS1, MFF and MIEF2) and 1 mitochondrial fusion gene (OPA1).
Enrichment analysis revealed that mitochondrial dynamics proteins were involved in mitophagy. Electron microscopy showed that mitochondrial fission and mitochondrial autophagy were present, suggesting that mitochondrial dynamics and mitophagy may be involved in the pathogenesis of proteinuria in LN. Literature suggests that the regulation of mitochondrial dynamics is linked to the initiation of mitophagy.20 It is widely accepted that mitochondrial fission plays a crucial role in facilitating mitophagy in various types of mammalian cells. The primary regulator responsible for mitochondrial fission is Drp1. It is a cytosolic protein, undergoes translocation to the mitochondrial outer membrane to initiate the fission process. In the absence of Drp1-mediated mitochondrial division, mitochondria exhibit increased connectivity and size, leading to impaired mitophagy in the heart and brain of mice.21 Meanwhile, one study found that Drp1 translocated to mitochondria and was phosphorylated at S616 in response to ischemia/reperfusion injury (IRI). Inhibiting Drp1 phosphorylation significantly suppressed without affecting general autophagy suggesting that Drp1 was involved the process of mitochondrial fragmentation and downregulation of mitophagy significantly aggravated kidney dysfunction indicating that mitophagy was activated via Drp1-dependent pathway to protect cells from IRI-induced apoptosis.22 And the role of mitochondrial fission on mitophagy remains to be determined.
We then proceeded to further investigate the expression of Drp1 and Fis1 in the glomerulus. Our findings showed that the staining of Drp1 and Fis1 in Lupus patients was significantly increased and they colocalized with the podocyte marker synaptopodin. Moreover, 24h-UTP was positively correlated with Drp1 and Fis1. Mitochondria are dynamic organelles that change their morphology through fission and fusion in order to maintain their function. The majority of viewpoints suggest that excessive mitochondrial fission leading to mitochondrial fragmentation is an inevitable stage of cell apoptosis. Mitochondrial fragmentation results in an increased permeability of the mitochondrial outer membrane, leading to the release of cytochrome c from the mitochondria. Cytochrome c is a crucial apoptotic trigger that can activate caspase enzymes, thus promoting apoptosis.23
Our previous study found that in the doxorubicin rat kidney disease model, the expression of Drp1 and Fis1 increased in glomerular podocytes. We overexpressed Drp1 in podocytes and found that mitochondrial fission increased and podocyte apoptosis increased.19 Downregulation of Drp1 and Fis1 can inhibit mitochondrial fission and prevent high glucose-induced apoptosis in retinal endothelial cells.24 Our findings suggest that increased expression of Drp1 and Fis1 leads to mitochondrial fission, which aggravates podocyte damage and the occurrence of proteinuria. However, the specific mechanism still needs to be explored.
Conclusions
Among the 658 DEGs identified, 5 DEGs related to mitochondrial dynamics were identified. Enrichment analysis revealed that mitochondrial dynamics proteins were involved in mitophagy. 24h-UTP was found to be positively correlated with the expression of mitochondrial fission proteins. We hypothesize that increased expression of mitochondrial fission proteins leads to mitochondrial fission, which aggravates podocyte damage and proteinuria. However, the underlying mechanism still needs to be further explored.
Ethics Approval and Consent to Participate
The studies involving human participants were reviewed and approved by the ethics committee of Fujian Provincial Hospital, and all participants provided their written informed consent prior to their involvement.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
First author: Wei Qijiao.
Funding
This work was supported by a grant from Shanghai Pujiang Young Rheumatologists Training Program (SPROG2403), National Natural Science Foundation of China (grant no. 82205189),Qihuang Scholars - National Traditional Chinese Medicine Leading Talents Support Program (No.2022-6), National Famous Traditional Chinese Medicine Expert Inheritance Studio Construction Project (No.2022-75).
Disclosure
The authors declare that there are no commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Anders HJ, Saxena R, Zhao MH, et al. Lupus nephritis. Nat Rev Dis Primers. 2020;6(1):7. doi:10.1038/s41572-019-0141-9
2. Wang Y, Yu F, Song D, et al. Podocyte involvement in lupus nephritis based on the 2003 ISN/RPS system: a large cohort study from a single centre. Rheumatology. 2014;53(7):1235–1244. doi:10.1093/rheumatology/ket491
3. QiJiao W, Han X, Na G, et al. Overproduction of mitochondrial fission. proteins in membranous nephropathy in children. Kidney Blood Press Res. 2018;43:1927–1934. doi:10.1159/000496006
4. Caza TN, Fernandez DR, Talaber G, et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. 2014;73(10):1888–1897. doi:10.1136/annrheumdis-2013-203794
5. Leishangthem BD, Sharma A, Bhatnagar A. Role of altered mitochondria functions in the pathogenesis of systemic lupus erythematosus. Lupus. 2016;25(3):272–281. doi:10.1177/0961203315605370
6. Fonseca TB, Á S-G, Milosevic I, Raimundo N. Mitochondrial fission requires DRP1 but not dynamins. Nature. 2019;570(7761):E34–E42. PMID: 31217603. doi:10.1038/s41586-019-1296-y
7. Qin X, Zhao Y, Gong J, et al. Berberine protects glomerular podocytes via inhibiting Drp1-mediated mitochondrial fission and dysfunction. Theranostics. 2019;9(6):1698–1713. PMID: 31037132; PMCID: PMC6485199. doi:10.7150/thno.30640
8. Yuan Y, Zhang A, Qi J, et al. p53/Drp1-dependent mitochondrial fission mediates aldosterone-induced podocyte injury and mitochondrial dysfunction. Am J Physiol Renal Physiol. 2018;314(5):F798–F808. PMID: 28659272. doi:10.1152/ajprenal.00055.2017
9. Wang Y, Lu M, Xiong L, et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020;11(1):29. doi:10.1038/s41419-019-2218-5
10. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991–5. doi:10.1093/nar/gks1193
11. Mejia-Vilet JM, Parikh SV, Song H, et al. Immune gene expression in kidney biopsies of lupus nephritis patients at diagnosis and at renal flare. Nephrol Dial Transplant. 2019;34(7):1197–1206. doi:10.1093/ndt/gfy125
12. Berthier CC, Bethunaickan R, Gonzalez-Rivera T, et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J Immunol. 2012;189(2):988–1001. doi:10.4049/jimmunol.1103031
13. Ko WC, Li L, Young TR, et al. Gene expression profiling in the skin reveals strong similarities between subacute and chronic cutaneous lupus that are distinct from lupus nephritis. J Invest Dermatol. 2021;141(12):2808–2819. doi:10.1016/j.jid.2021.04.030
14. Wu B, Tang X, Ke H, et al. Gene regulation network of prognostic biomarker YAP1 in human cancers: an integrated bioinformatics study. Pathol Oncol Res. 2021;27:1609768. doi:10.3389/pore.2021.1609768
15. G D Jr, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. doi:10.1186/gb-2003-4-5-p3
16. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi:10.1093/nar/28.1.27
17. Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43(Database issue):D1049–56. doi:10.1093/nar/gku1179
18. Qijiao W, Zhihan C, Makota P, et al. Glomerular expression of S100a8 in lupus nephritis: an integrated bioinformatics analysis. Front Immunol. 2022;13:843576. doi:10.3389/fimmu.2022.843576
19. QiJiao W, Xiaoya L, Guohong W, et al. Mitochondrial fission proteins are involved in proteinuria in Adriamycin-induced rat nephropathy. J Nephrol Dialy Transplant. 2017;26(1):37–43.
20. Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi:10.1016/j.redox.2014.11.006
21. Kageyama Y, Hoshijima M, Seo K, et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi:10.15252/embj.201488658
22. Li N, Wang H, Jiang C, et al. Renal ischemia/reperfusion-induced mitophagy protects against renal dysfunction via Drp1-dependent-pathway. Exp Cell Res. 2018;369(1):27–33. doi:10.1016/j.yexcr.2018.04.025
23. Elgass K, Pakay J, Ryan MT, et al. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta. 2013;1833(1):150–161. doi:10.1016/j.bbamcr.2012.05.002
24. Kim D, Sankaramoorthy A, Roy S. Downregulation of Drp1 and Fis1 inhibits mitochondrial fission and prevents high glucose-induced apoptosis in retinal endothelial cells. Cells. 2020;9(7):1662. doi:10.3390/cells9071662
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.