Back to Journals » Patient Preference and Adherence » Volume 19
Phenylketonuria as an Adherence Disease
Authors Reach G
Received 18 December 2024
Accepted for publication 7 March 2025
Published 13 April 2025 Volume 2025:19 Pages 1059—1073
DOI https://doi.org/10.2147/PPA.S512719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Gérard Reach
Health Education and Promotion Laboratory, Sorbonne Paris Nord University, Bobigny, France
Correspondence: Gérard Reach, Health Education and Promotion Laboratory, Sorbonne Paris Nord University, 74 Rue Marcel Cachin, Bobigny, 93000, France, Tel +33 (0)6 60 84 53 25, Email [email protected]
Abstract: This narrative review explores a novel challenge to adherence in patients with phenylketonuria (PKU). Traditional barriers to maintaining the core of PKU therapy, which involves dietary restrictions, include limited palatability, high costs, difficult access to low-phenylalanine foods, time constraints, and social interference. We propose that adherence may also be hindered by elevated phenylalanine levels in nonadherent patients, leading to cognitive and executive function impairment that reduces their ability to adhere to the diet. Specifically, we hypothesize that the pathophysiological features of PKU create a vicious cycle that exacerbates adherence challenges to the phenylalanine-restricted diet. We therefore propose that PKU may be considered “an adherence disease”. The implications of this hypothesis are threefold: 1. Long-term dietary nonadherence is probable among individuals with PKU; 2. the disease itself contributes to the difficulty in adhering to dietary restrictions, potentially alleviating patient guilt; 3. while treatment options depend on individual factors such as disease severity, the most effective approach to mitigating the neurocognitive and psychosocial impacts of PKU is to replace or restore phenylalanine hydroxylase activity.
Plain Language Summary: Phenylketonuria (PKU) is the most common genetic metabolic disorder. Its management requires adhering to a challenging diet that eliminates phenylalanine to prevent its accumulation in the brain, which can cause significant neurological deficits affecting the patient’s quality of life.This review focuses on long-term adherence to this diet, as part of a broader investigation into the mechanisms of nonadherence which represents a critical issue in medicine.In this article, we propose to consider PKU as an adherence disease—a unique case where the disease’s pathophysiology negatively impacts adherence through a vicious cycle, detailed in this review.This narrative review is the first to apply general adherence concepts from other diseases to PKU in an interdisciplinary manner.Finally, the concept of “adherence disease”—analogous to terms like heart disease— suggests that adherence is a beneficial function prone to failure that can be corrected by understanding its mechanisms.This investigation provides strong support for research in gene therapy for PKU.
Keywords: executive functions, impulsivity, prospective memory, gene therapy
Introduction
Phenylketonuria (PKU) is currently the most prevalent hereditary genetic metabolic disorder, with a global incidence of approximately 1 in 10,000 newborns.1 The majority of PKU cases result from mutations in the phenylalanine hydroxylase (PAH) gene on human Chromosome 12, inherited in an autosomal recessive pattern. PAH deficiency leads to elevated phenylalanine (Phe) levels. High Phe levels can also be observed in patients with inherited deficiencies of tetrahydrobiopterin (BH4), the cofactor of PAH, or PAH disruption due to the absence of its chaperone DNAJC12.1–3
Untreated PKU can manifest in various degrees of severity, ranging from classic PKU (Phe concentration > 1200 µmol/L) to moderate PKU (Phe concentration = 900–1200 µmol/L), mild PKU (Phe concentration = 600–900 µmol/L), and mild hyperphenylalaninemia (HPA) (Phe concentration = 360–600 µMol/L).4 Elevated Phe levels in patients with untreated PKU can result in major cognitive and neurological deficits, severe intellectual disability, epilepsy, behavioral issues, and visual abnormalities.
PKU is therefore managed primarily through a diet that restricts Phe, an amino acid found in high-protein foods, to prevent harmful accumulation in the body.5 Individuals with PKU must avoid or limit foods like meat, dairy, nuts, and some grains, and rely on specially formulated low-protein products and medical foods that provide essential nutrients, including large neutral amino acids (LNAA) supplements. LNAA supplements can help to compete with phenylalanine for transport across the blood-brain barrier, potentially reducing brain phenylalanine levels. Adhering to the PKU diet is challenging due to its restrictive nature, the need for precise dietary management, the limited availability and often high cost of suitable foods and supplements, time constraints, and the social and psychological impacts of maintaining such a strict diet.6–8 In addition to dietary regimen, pharmacological approaches can be employed. Sapropterin, a synthetic analogue of tetrahydrobiopterin (BH4), enhancing the activity of PAH is one such option.9 Another alternative involves subcutaneous administration of pegvaliase, an enzyme that facilitates the metabolism of Phe.10 This can enable individuals with PKU to maintain better metabolic control and potentially allow for a more relaxed dietary regimen.11 However, its use may be limited by the existence of frequent and serious side effects.10
Early initiation of treatment with a Phe-restricted diet can mitigate symptoms, with some patients being asymptomatic,12 indicating that Phe itself is the primary neurotoxin in PKU,1,13,14 even if not the exclusive one. However, early-treated PKU patients may still experience cognitive deficits if not well managed later in life, most commonly impacting attention, processing speed, executive functions, working memory, and learning.12,15,16 Treated patients may also experience psychiatric symptoms such as depressed mood, social withdrawal, generalized anxiety, low self-esteem, and lack of autonomy.13
Adherence to diet is therefore of paramount importance in PKU. Nonadherence to long-term therapies is common in all chronic diseases,17 including PKU, in both children18 and adult patients.19 Compared to other chronic diseases, the issue of adherence among individuals with PKU is unique in several ways: first, the diet is the central component of treatment, rather than medication. Additionally, the diet for PKU is different from diets required for conditions like diabetes or obesity, as adherence must take into account factors such as palatability and the cost of replacement foods. Furthermore, PKU manifests at birth, requiring adherence to be managed initially by parents and later by the individuals themselves, including through the challenging period of adolescence. Indeed, the Phe-restricted diet is typically initiated immediately after diagnosis through newborn screening and is initially managed by parents. However, adherence to dietary restrictions often declines after the age of 10 in patients with inborn errors of metabolism on low natural protein diets.20 This decline is often attributed, at least in part, to the shift in responsibility from primary caregivers to the patients themselves, who are faced with the many challenges involved in adhering to a Phe-restricted diet. However, it has been proposed that patients with PKU may encounter specific challenges in handling their treatment due to the impact of the condition on their neuropsychological status.20,21 Thus, a significant impediment in tackling psychiatric issues in PKU is that elevated Phe levels may result in symptoms such as depression, anxiety, and attention deficits, hindering individuals’ ability to adhere to dietary restrictions, thereby worsening these symptoms.22
From these observations, a clear vicious cycle emerges within PKU: 1. Deviation from the prescribed diet leads to an increase in Phe levels; 2. Elevated Phe levels contribute to the deterioration of executive function and memory, as well as increased impulsivity; 3. These neurocognitive impairments further exacerbate nonadherence. Indeed, adherence necessitates intentional processes,23 and maintaining long-term adherence poses a significant challenge, contributing to nonadherence across various diseases.24 The presence of a vicious cycle in PKU, as proposed herein, presents a unique challenge for adherence in a chronic disease. A thorough understanding of the mechanisms that lead to nonadherence is important because it could drive the exploration of innovative treatment paradigms.25 In this narrative review, we first explore adherence to the three aspects of PKU treatment: diet, medications, and follow-up. We then present evidence supporting the three components of the adherence vicious cycle in PKU, suggesting that PKU is also an adherence disease. This leads to the general conclusion that only the restoration of PAH activity, for example through gene therapy, can prevent long-term complications of PKU.
Aspects of Nonadherence in PKU
Nonadherence to Diet
In a consensus conference, 100% of experts agreed that the sustainability of medical nutrition therapy worsens from early adolescence onward, making strict adherence to the Phe-restricted diet challenging for most patients and limiting the long-term effectiveness of this therapy in controlling blood Phe concentrations.26 In a study of 111 adults with PKU, many showed suboptimal adherence to the PKU diet, with 82% consuming natural proteins more and amino-acid supplements less than 4–5 times daily.27 Among 219 participants, 81.4% consistently adhered to their prescribed diet, while 6.9% partially adhered, and 11.8% did not adhere at all. Children demonstrated higher adherence rates compared to adults. Factors such as lack of medical food, delayed diagnosis, history of being off the diet, and age were significant predictors of psychological comorbidities, with age and history of being off the diet remaining significant in a multivariate model.28 The higher adherence observed in childhood was also supported by a systematic review focusing on treatment adherence in children and adolescents.29 Parents of children under 18 were more likely to bring low-protein food from home and request restaurants to accommodate their dietary needs than adult PKU individuals.30 The impact of age on adherence is extensively discussed in a recent review on factors influencing adherence to the PKU diet.31
Following a Phe-restricted diet requires a substantial level of understanding. A United Kingdom nationwide survey with 137 respondents revealed that 43.8% always followed a PKU diet, 28.5% returned to it, and 25.5% were off it. The overall knowledge score was 75.2%, with higher scores in general PKU knowledge than in diet-specific knowledge. Dietary adherence was linked to knowledge, with consistent dieters scoring higher.32 This highlights the importance of patient education, as individuals with PKU often need to calculate protein exchanges from food labels. A study revealed that over 90% had encountered issues with food labeling in the past six months, finding it confusing.33 A study on caregivers of children with amino acid metabolism disorders, including PKU, highlighted parent-related barriers like poor self-control, limited medical resources, the diet’s burden (restricted choices, tedious preparation, dining out challenges), and limited knowledge.34
Nonadherence to Pharmacological Treatment in PKU
Limited data are available on adherence to sapropterin. In an 86-patient survey, 41 responders were identified, with 29 continuing therapy and 12 discontinuing. There were no significant age or side effect differences between the two groups. Among continuers, 19 used sapropterin consistently, while 10 used it intermittently. Forgetfulness was reported as the primary reason for incomplete adherence by 81% of patients. The main motivation for continuation, stated by 22 patients, was the ability to tolerate more dietary protein.35 Currently, there is a notable lack of comprehensive data on adherence to pegvaliase therapy. In a recent study, a panel of 11 PKU experts reviewed 18 cases and noted challenges such as side effects and patient adherence.36 This lack of data is concerning as this treatment mitigates the necessity for protein restriction.
Nonadherence to Follow-up
Measuring Phe levels helps assess the quality of metabolic control in PKU. Additionally, Phe (or natural protein) tolerance is defined as the amount needed to maintain blood Phe concentrations within a specified therapeutic range.37 Some patients might experience excessive restrictions, as they actually could potentially elevate their protein intake without a corresponding rise in their Phe levels.38,39 This highlights the importance of regular monitoring of Phe levels in PKU management. In a survey of 44 clinics in the United States, it was observed that patient adherence to blood Phe testing decreased with age. Among patients aged 30 and older, 37% tested once a year or less. Additionally, 31% of adolescents (13–17 years) also tested only once or twice a year.19 An online survey of 64 patients with PKU in the United Kingdom revealed that 47% rarely or never conducted home blood tests for Phe levels. Only 9% tested every few months, while 31% tested monthly. The percentage of patients testing fortnightly or weekly was minimal, at 3% and 9%, respectively.40
Nonadherence in PKU: A Vicious Cycle
In the following sections, we provide evidence for three interconnected issues: (1) inadequate adherence to the prescribed diet results in elevated blood Phe levels, (2) elevated Phe levels have a detrimental effect on cognitive function, and (3) this cognitive impairment contributes to further nonadherence to both the diet and medication. These factors create a vicious cycle, suggesting that PKU can be considered an adherence disease (Figure 1). We will also explore how these mechanisms contribute to overweight and obesity, potentially offering new insights into this issue for individuals with PKU.
 |
Figure 1 The vicious cycle of nonadherence in PKU: PKU as an adherence disease. |
This hypothesis is supported by a study involving 12 patients that investigated the impact of slow-release large neutral amino acids (LNAAs; 1 g/kg/day) on adherence. The treatment notably reduced the Phe/tyrosine ratio, suggesting enhanced metabolic control. Medication adherence assessed using the Morisky questionnaire revealed a notable improvement. Initially, three patients reported medium adherence, while nine reported low adherence, with 60% achieving full adherence in the past month. By the end of the study, adherence had increased, with 96% achieving full adherence.41
First Arm of the Vicious Cycle: The Impact of Nonadherence to Diet on Phe Levels in PKU
The primary goal of the Phe-restricted diet in PKU is to reduce circulating Phe levels and mitigate its potential toxicity. Therefore, it is not unexpected that nonadherence to diet can compromise this effect. A Cochrane analysis of historical studies on children with PKU found a significant increase in Phe levels following diet termination or relaxation.42 In a recent study of 30 adult patients with PKU, adherence to the Phe-restricted diet varied. Sixteen patients adhered to the regimen, maintaining low Phe protein intake, while 14 were nonadherent, with an average of 5.4 years without adhering to the diet. Adherent patients maintained Phe concentrations within recommended ranges (120–600 µmol/L), while nonadherent patients had higher concentrations (861 vs 464 µmol/L; p = 0.002). Interestingly, nonadherent patients also had a higher body mass index than adherent ones (31.8 vs 24.9 kg/m²; p = 0.006).43 A study involving nine adult patients with PKU who had discontinued their diet showed that reintroducing the diet for one year led to an improvement in cognitive functions.44
Furthermore, elevated Phe levels may serve as a predictor for treatment discontinuation. A retrospective study involving 75 children with early-treated PKU aged 7 to 13 revealed a progressive increase in mean blood Phe concentrations with age, most notably between the first and second years. The percentage of samples exceeding recommended Phe levels escalated from 13% in infancy to 67% in early adolescence. Notably, individuals who discontinued follow-ups exhibited higher mean Phe concentrations (360 vs 220.9 μMol/L; p = 0.004), particularly during the second year. Blood Phe levels at 12–23 months and 6–8 years significantly predicted discontinuation before reaching the age of 13. In addition, the study observed a rise in the proportion of patients with insufficient annual blood Phe checks as they grew older.18
Second Arm of the Vicious Cycle: The Impact of Phe Levels on Brain Damage and Executive Functions
Individuals with PKU often exhibit abnormalities in brain white matter, which can be assessed using diffusion tensor imaging to evaluate microstructural integrity. A study involving 32 individuals with PKU and 12 healthy controls found that higher Phe levels were linked to significant changes in mean diffusivity in the brain, leading to cognitive performance decline.45 A retrospective study with 36 children with early-treated PKU confirmed that mean Phe levels, Phe exposure, and the variability of Phe exposure were the key predictors of white matter integrity.46 However, a study of 30 adult patients with PKU revealed widespread cortical thickness reductions, despite good metabolic control in childhood and adolescence. These changes did not show significant correlations with metabolic parameters or cognitive performance, possibly due to effective metabolic control during earlier years.47
There is substantial evidence linking elevated Phe levels to cognitive impairments in individuals with PKU. For instance, a study comparing preschool children with HPA (moderate increase in Phe) and those with PKU (high Phe levels) found that children with PKU had significant impairments in cognitive inhibition, verbal working memory, and visuospatial working memory compared to children with HPA.48 In a study of 57 adult patients with early-treated PKU, Phe levels increased during adolescence, likely due to relaxed dietary restrictions, showing a significant correlation between intelligence quotient, information processing abilities, and blood Phe levels during childhood and adolescence in older patients.49 Three meta-analyses have shown a correlation between Phe levels and various cognitive dysfunctions.12,50,51 A study of 13 children with PKU found significant correlations between overall executive function deficits and tyrosine levels and the Phe/tyrosine ratio, with no correlation with Phe levels due to well-controlled conditions in these children.52
It is important to consider not only Phe levels but also their systemic fluctuations, which may arise from the lack of dietary smoothing associated with hepatic PAH deficiency.53 Two studies in children with PKU suggested that variability in blood Phe levels might have a more significant impact on cognitive functioning than overall Phe exposure.54,55 Another study involving children with classic (n = 14) or mild (n = 13) PKU indicated that Phe variability was associated with impaired working memory, verbal fluency, inhibitory control, and theory of mind.56 Finally, a study involving 29 patients with classic PKU revealed a correlation between the Full Scale Intelligence Quotient and Phe levels, but not with their variations. Conversely, 21 patients with moderate PKU exhibited the opposite pattern.57 Phe levels and their variability may operate through distinct mechanisms: Phe peaks could potentially harm developing brains by affecting white matter integrity, while cumulative Phe exposure, as seen in average levels, could be more detrimental to adult brains due to impacts on neurotransmitter levels.58
Interventional studies confirm the relationship between Phe levels and executive dysfunctions. In a study of 10 adult patients with PKU and inadequate metabolic control receiving 12 months of LNAAs (0.8–1 g/kg/day), plasma Phe levels remained stable, while tyrosine levels significantly increased. Psychometric assessments showed improvements in distress, well-being, executive functions, attention, and vigilance, with no noticeable changes in hand dexterity.59 Although an association does not imply a causal relationship, a short-term (6 months) beneficial effect of BH4 on both Phe levels and white matter integrity was observed.60 Furthermore, BH4 treatment was linked to enhancements in working memory and brain activation, with neural changes becoming apparent earlier than improvements in working memory performance.61
Third Arm of the Vicious Cycle: The Role of Impaired Executive Functions in Nonadherence to Long-Term Therapies
To understand the impact of cognitive functions on adherence, it is important to acknowledge that nonadherence in chronic diseases often stems from a preference for immediate gratification over long-term health benefits.62 Therefore, adhering to long-term therapies requires consistent effort based on the patient’s logical reasoning, advanced cognitive abilities, and self-regulation. This reliance on intact executive functions enables individuals to control impulsivity and effectively utilize memory to mitigate nonadherence due to forgetfulness.63
Impulsivity and Adherence to Medication and Diet
The Concept of Intertemporal Choice
Neuroeconomics has highlighted the significance of impatience in compromising the ability to resist immediate temptations in favor of long-term goals.64 The perceived value of an object diminishes when its acquisition is delayed. Empirical data strongly supports the idea that the devaluation of value (V) can be best represented by a hyperbolic function, such as V(t) = V(t0)/(1 + kt), where “k” denotes a coefficient of impatience or impulsivity that varies among individuals.65 The decision-making process regarding adherence to chronic disease treatment can be viewed as an “intertemporal choice” between immediate satisfaction and the remote goal of maintaining health. Individuals with a high present bias (a high k value) experience shifts in preferences influenced by the hyperbolic discount function which results in an increase in the perceived value of immediate rewards as they approach. This preference reversal is illustrated in a restaurant scenario: initially, the desire to stay slim outweighs the temptation for dessert, but as time passes, the craving for dessert intensifies, ultimately making it rational to indulge in when presented, regardless of previous intentions.66 A substantial body of evidence suggests an impact of impulsivity on medication adherence across various conditions, including prediabetes, type 2 diabetes, type 1 diabetes, hypertension, heart failure, asthma, and multiple sclerosis, as well as the initiation of preventive aspirin therapy and hormonal therapy in breast cancer.67,68 Currently, there is no literature evidence linking time discounting to adherence to diet and/or medication in PKU.
Adolescence as a Paradigm and the Dual-Brain Argument
Adolescence is a period of life characterized by a high prevalence of nonadherence in chronic diseases such as type 1 diabetes,69 asthma,70 and human immunodeficiency virus (HIV).71 In patients with PKU who receive early treatment, we have seen that Phe levels typically rise from childhood to adulthood.19,28,31 Additionally, nonadherence during adolescence is a significant predictor of disengagement from follow-up care.72 Factors contributing to high nonadherence rates include the transition to independent living, social pressures related to food and lifestyle choices, and challenges in maintaining a PKU diet in social interactions with peers.29 However, this phenomenon may also reflect broader underlying factors. Adolescents are more likely to take risks, with late adolescents showing the highest levels of recklessness, which is probably present from mid-adolescence but only expressed when opportunities arise.73
Both nonadherence to recommendations and the tendency for risk-taking observed in adolescence may be attributed to two key traits: impatience, leading to a preference for instant gratification, and disobedience. Research has shown a correlation between acts of disobedience, such as not wearing a seatbelt in the back of a car, and poor adherence to medication regimens.74 Additionally, reactance, which is the refusal to comply due to perceived restrictions on freedom, is also associated with inadequate adherence.75 The impatience and disobedience observed in adolescence may have an evolutionary basis; in ancient times, these traits may have encouraged young males to take risks and explore beyond their familial and societal boundaries in search of a mate.76
This discussion on adolescent behaviors leads to the dual-brain concept proposed by Casey76 and Steinberg in 2008.77 This argument suggests that adolescents’ inclination toward risky behavior is due to the uneven development of two brain systems: one focused on seeking rewards and the other on regulating impulses. Risk-taking peaks during adolescence because the socioemotional system matures early, driving attraction to exciting activities, while the cognitive control system develops more slowly, this disequilibrium resulting in a lack of ability to control hazardous impulses.78 The competing neurobehavioral decision systems theory by Bickel is another dual-systems model that explains self-control failures by emphasizing the interaction between impulsive and executive decision systems. The impulsive system, involving limbic and paralimbic regions, and the executive system, involving the prefrontal and parietal cortices, are interdependent and compete for control during decision-making.79 These models are similar to Daniel Kahneman’s dual theory of thinking, known as System 1 and System 2.80 Brain imaging data across different age groups support these hypotheses.81,82
Role of Impulsivity in Overweight and Obesity
Some studies suggest that PKU is often associated with overweight and obesity.83–86 In the framework of our hypothesis, it is helpful to explore how impulsivity and executive function impairments contribute to weight gain in the general population. A 2016 meta-analysis found a link between elevated delay discounting and increased consumption of unhealthy foods, as well as a higher risk of obesity.87 Subsequent research indicated that obese individuals show impairments in executive functions, particularly in inhibition and working memory.88 Individuals who successfully lost weight demonstrated lower delay discounting compared to obese controls89,90 and a 2021 meta-analysis identified baseline inhibition and delay discounting as predictors of future weight loss.91 Household food shoppers with higher delay discounting tend to choose lower-quality, higher-energy foods and beverages.92 In the NutriNet-Santé Study, impulsivity was linked to higher consumption of low-quality, high-energy foods and beverages while negatively impacting the intake of healthier options.93 Finally, studies have also shown that obese individuals exhibit greater activation in the right dorsolateral prefrontal cortex during delay discounting tasks.94
These findings suggest that impulsivity and working memory deficits contribute to overweight and the consumption of unhealthy foods. It is therefore possible that overweight and obesity in some patients with PKU are not solely due to the low-protein, high-carbohydrate diet but also related to behavioral issues that lead to nonadherence to the prescribed diet aimed at reducing circulating Phe levels. Interestingly, a meta-analysis revealed that the increased prevalence of overweight and obesity compared to that in healthy controls is only observed in classic PKU.84 Furthermore, the tendency for overweight in PKU primarily affects women.83 This aligns with a study showing high delay discounting in obese women but not in men,95 as well as data suggesting higher impulsivity in women than in men.96
Dopamine and Serotonin and Intertemporal Choice
In this section, we will explore how brain deficits in dopamine and serotonin contribute to impulsivity and other executive function deficits. This is particularly relevant in the context of PKU, where PAH deficiency results in elevated Phe levels. LNAAs, including Phe, tyrosine, and tryptophan, compete for transport across the blood-brain barrier through L-type amino acid transporters, which have a high affinity for Phe. Elevated Phe levels saturate these transporters, reducing the entry of other LNAAs into the brain. This, in turn, lowers the availability of tyrosine and tryptophan, impairing dopamine and serotonin synthesis.97
Dopamine and Intertemporal Choice
A study using monetary choice tasks and functional magnetic resonance imaging investigated the Val158Met polymorphism of the catechol-O-methyl transferase gene, which affects dopamine degradation. The study found that this polymorphism is associated with impulsive choice behavior and neural activity in the dorsal prefrontal cortex and posterior parietal cortex. Individuals with the 158Met allele, associated with higher dopamine levels, exhibited less bias toward immediate rewards than those with the 158Val allele.98 Another study by the same research group99 investigated the impact of acute dopamine depletion in healthy adult males. This depletion was induced by consuming a beverage lacking dopamine precursors. The results showed an increased bias toward immediate rewards in participants with the val/val genotype, indicating individuals with high catechol-O-methyl transferase activity and lower baseline brain dopamine levels. Conversely, a single dose of haloperidol was found to mitigate delay discounting by elevating extra-synaptic striatal dopamine levels through blocking presynaptic D2 receptors.100 In a separate study, striatal dopamine D2/3 receptor (D2/3R) availability and plasma monoamine metabolite levels were assessed in 18 adults with PKU compared to 12 healthy controls. The PKU group exhibited lower levels of homovanillic acid (a dopamine metabolite) and 3-methoxy-4-hydroxy-phenylglycol (a norepinephrine metabolite) along with higher impulsivity levels. Additionally, the PKU group showed higher mean D2/3R availability, suggesting reduced synaptic dopamine levels. Within the PKU group, D2/3R availability positively correlated with impulsivity and error rates on a cognitive flexibility task.101
These data provide strong evidence supporting the role of dopamine in delay discounting. Lower dopamine reactivity in the nucleus accumbens core is associated with greater temporal discounting, and studies have shown that lesions in this area lead to higher levels of delay discounting.102 Expanding on the positive effects of dopamine on patience, researchers have investigated the impacts of tyrosine administration, the precursor of dopamine, on cognitive functions. A meta-analysis103 has shown that tyrosine supplementation can prevent declines in cognitive task performance, particularly under physically or mentally demanding conditions. This includes tasks such as working memory tasks, multitasking, and convergent thinking, which involves generating a single solution to multiple problems.
Serotonin and Cognitive Functions
In the brain, L-tryptophan is converted to 5-hydroxytryptophan by tryptophan hydroxylase types 1 and 2 (TPH1 and TPH2). Subsequent decarboxylation via L-aromatic acid decarboxylase converts 5-hydroxytryptophan to serotonin. Several studies reviewed by Aquili104 examined the effects of genetic variations in the TPH1 and TPH2 genes on brain activity and performance in inhibitory control tasks. One study using the Go/Nogo task found that individuals with the risk allele for TPH1 had reduced medial prefrontal cortex activity during response inhibition, with no behavioral differences noted.105 Another study on the 5-choice serial reaction time task revealed that the TPH2 risk allele (T/T homozygous at the 703 polymorphism) was associated with more premature responses, suggesting inhibitory control deficits linked to reduced input from the ventromedial prefrontal cortex and increased nucleus accumbens response in impulsive T allele carriers.106 Aquili’s review also discussed acute tryptophan depletion, presenting contradictory findings, and concluded that tryptophan and the prefrontal cortex may modulate certain types of response inhibition, cognitive interference, and selective attention, but not reversal learning.
Memory and Adherence to Medication
The implementation of a long-term treatment regimen involving repetitive actions (such as taking medication) necessitates the engagement of prospective memory, which refers to the ability to remember to perform a task at a later time.107 This cognitive function relies on the concept of “implementation intention”, where individuals set specific cues to trigger actions, such as deciding to take medication. A conceptual model of prospective memory108 involves several key stages. First, an intention is formed and linked to a specific cue, which can be time-based (eg, “take medication every four hours”) or event-based (eg, “take medication after dinner”). Subsequently, an action plan is created, and the cue-intention pairing is established. The second stage includes a delay between intention formation and execution, which may last from minutes to weeks, during which attention may shift to other activities, limiting rehearsal of the cue-intention pairing. In the third stage, the ability to detect and recognize the cue becomes crucial, requiring self-initiated retrieval of the intention. The final stages involve recalling the intention from retrospective memory, which is essential for prospective memory success, and executing the intention, typically an automatic process unless significant neurological deficits like apraxia or aphasia are present.
A 2012 literature review highlighted the role of prospective memory in treatment adherence for various conditions, including HIV, rheumatoid arthritis, and diabetes.108 A study involving 309 patients with heart failure revealed significant associations between higher attention, executive function, and memory scores and improved medication adherence in unadjusted regression analysis. However, after adjusting for age and minority status, the associations between attention and adherence, as well as executive function and adherence, lost significance. In contrast, the relationship between reduced memory and lower adherence remained significant in the adjusted analysis (p = 0.008).109
The case of HIV is interesting due to its association, as in the case of PKU, with cognitive impairments affecting memory, processing speed, attention, and concentration.110 A study using the Memory for Intentions Screening Test and other executive function assessments in patients with HIV infection identified a factor linked to treatment adherence.111 A 2018 literature review112 indicated moderate prospective memory deficits in HIV patients, largely independent of common comorbid factors (eg, depression, hepatitis C co-infection) and unrelated to traditional clinical markers of disease severity. These deficits suggest dysregulated strategic processes, such as cue monitoring, consistent with frontal systems pathology and executive dysfunction in HIV-related neural injury. Conversely, more spontaneous and automatic prospective memory processes, relying on less affected posterior brain regions, appeared relatively preserved.
Currently, there is no literature evidence on specific prospective memory in patients with PKU. Several studies have documented deficits in working memory13,16,113–116 while others have not found differences between clinical and control group in this domain.51,117
Summary: The Impact of Executive Functions, Impulsivity, and Memory on Adherence
Finally, three studies published in a single article aimed to identify common cognitive factors affecting medication adherence across different conditions such as hyperlipidemia, diabetes, and early breast cancer. The studies consistently demonstrated that cognitive function significantly predicted nonadherence, with attention and psychomotor speed being the most reliable predictors. In individuals with hyperlipidemia, mental flexibility and working memory were linked to adherence to the correct dose and timing over 24 weeks. For patients with diabetes, attention and psychomotor speed were the primary predictors of adherence to prescribed doses. In patients with early-stage breast cancer, attention and executive function were linked to adherence to dosing frequency and inter-dose intervals, while verbal learning and memory were related to adherence over 24 weeks. These findings underscore the consistent influence of specific cognitive functions on medication adherence across diverse medical conditions, irrespective of treatment complexity.118
Altogether, these findings support the proposed model in Figure 2, illustrating the relationship between executive functions and adherence to therapies (both diet and medications) in patients with PKU.
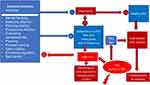 |
Figure 2 PKU, Executive functions, impulsivity and adherence in PKU. |
This figure illustrates various executive functions that may influence the mental mechanisms underlying nonadherence in patients with phenylketonuria (PKU). The deficiency of PAH characteristic of PKU leads to elevated Phe levels, which in turn has a detrimental effect on executive functions, particularly inhibiting impulsivity control. When impulsivity is not adequately controlled, it hinders adherence to specific treatments for PKU, including dietary restrictions, medication adherence, follow-up appointments, and overall healthy eating habits. This model suggests that impulsivity may also contribute to overweight and obesity in these patients, especially when combined with a high-carbohydrate PKU diet. Inadequate adherence to the PKU diet after starting therapy can result in increased Phe levels, further compromising executive functions and perpetuating a vicious cycle.
Discussion
A first criticism of the argument presented here is that some of the arguments used are more about medication adherence than dietary adherence. However, adherence extends beyond just medications to include lifestyle changes and any medical prescriptions.119 Moreover, medication and lifestyle nonadherence are often interconnected. For instance, we demonstrated in a study involving patients with gout that nonadherence to urate-lowering medications was associated with nonadherence to specific gout dietary recommendations and a lack of monitoring uric acid levels, suggesting that nonadherence is a syndrome.120 Lastly, the role of prospective memory in adhering to a diet is questionable, but it may be involved when taking LNAA supplements, using drugs such as sapropterin or when monitoring Phe levels.
Secondly, the argument for a vicious cycle in PKU presented herein is a hypothesis that requires empirical validation, as it may be no more than a conceptual construct. This hypothesis is based on a narrative review, which is inherently less structured and more susceptible to subjective interpretation than systematic reviews, even though efforts have been made to support each statement with references. Nevertheless, this approach may introduce potential selection biases.
However, this type of review has the advantage of providing a broad understanding of an issue by integrating findings from different disciplines (eg reference to neuroeconomics, comparisons with other diseases) and applying current conceptual frameworks on adherence to a specific disease, PKU, which is novel. It also highlights a number of gaps in the research and the need for further investigation: collection of data on adherence to sapropterin and pegvaliase, specific investigation of time discounting and prospective memory in people with PKU, and consideration of the fact that adherence in the current PKU literature is often assessed by circulating Phe levels (an indirect measure) rather than dietary adherence questionnaires, which could allow real comparisons of adherence levels based on Phe concentrations.
Filling in these gaps can be a first step in the validation of the hypothesis. If the hypothesis is correct, appropriate statistical techniques should be able to demonstrate in a cohort of patients with PKU exhibiting a large range of Phe control the presence of cluster associations121 between Phe and tyrosine levels and variability, their ratio, BMI, adherence to PKU diet, LNNA, sapropterin and medication for other diseases, impulsivity, prospective memory, and executive functions.
Conclusion
This novel conceptual framework presents PKU as an adherence disease, with three key implications. First, the existence of a vicious cycle makes dietary adherence a formidable challenge for patients with PKU. Long-term dietary nonadherence is therefore probable among individuals with PKU. Second, the inability to adhere to dietary restrictions is partly a consequence of the disease itself, which could help patients feel less guilty. Lastly, if nonadherence to the diet is a consequence of the underlying PAH deficiency, complete avoidance of neurological and psychological complications in PKU may require restoration of PAH activity (Figure 3).
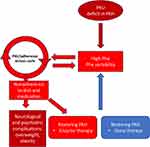 |
Figure 3 Restoring Phenylalanine Hydroxylase (PAH) as a treatment for PKU. |
There are two main approaches to restoration of PAH activity. The first method involves enzyme therapy, such as pegvaliase, which can help manage the condition. However, pegvaliase may pose challenges due to nonadherence issues and severe side effects. The second approach is gene therapy, which holds promise for long-term intervention in PKU. A review published in 2024122, outlined various methods considered for gene therapy in PKU, including gene addition, delivery of therapeutic mRNA via lipid nanoparticles, gene editing/correction, or gene insertion. A detailed description of these methods is beyond the scope of this article.
Gene therapy in PKU may be the only way to achieve long-term effective intervention in a disease that can potentially lead to a number of neurocognitive deficits altering the quality of life and requires a challenging dietary regimen, with nonadherence causing distress and guilt. The concepts presented here may influence inclusion criteria for these innovative therapies, such as complete PAH deficiency (classic PKU), poor tolerance to phenylalanine leading to high Phe levels with significant variability, uncontrolled disease even with well-conducted treatment, including sapropterin when indicated, documented low adherence to diet and medication and confirmed executive function impairment.
Finally, the concept of “adherence disease”—similar to how we refer to heart disease— proposed herein holds significant implications in the field of adherence. It suggests that adherence can be perceived as a beneficial function which develops in adulthood and is susceptible to failure, but that this failure can potentially be remedied, once its mechanisms are understood. We suggest that PKU might serve as a model for such failure due to the biochemical abnormalities associated with the condition. The pathophysiological insights discussed here could provide valuable understanding of the mechanisms underlying nonadherence in chronic diseases more broadly.
Disclosure
The author reports personal fees as a speaker at symposia organized by Novo-Nordisk, Abbott-Diagnostics, Lifescan, Bayer-Diagnostics, Mylan-Viatris, Astra-Zeneca, Timkl (lectures on patients’ adherence, doctors’ clinical inertia, patient education, and hospitality in the hospital); and personal fees as a participant in the scientific boards of Sanofi-Aventis, Targedys and Timkl.
References
1. van Spronsen FJ, Blau N, Harding C, Burlina A, Longo N, Bosch AM. Phenylketonuria. Nat Rev Dis Primers. 2021;7:36. doi:10.1038/s41572-021-00267-0
2. Chen A, Pan Y, Chen J. Clinical, genetic, and experimental research of hyperphenylalaninemia. Front Genet. 2023;13:1051153. doi:10.3389/fgene.2022.1051153
3. Elhawary NA, AlJahdali IA, Abumansour IS, et al. Genetic etiology and clinical challenges of phenylketonuria. Hum Genomics. 2022;16:22. doi:10.1186/s40246-022-00398-9
4. Regier DS, Greene CL. Phenylalanine Hydroxylase Deficiency. In: Adam MP, Feldman J, Mirzaa GM, editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 2000:1993–2024.
5. van Wegberg AMJ, MacDonald A, Ahring K, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12:162. doi:10.1186/s13023-017-0685-2
6. Ford S, O’Driscoll M, MacDonald A. Living with Phenylketonuria: lessons from the PKU community. Mol Genet Metab Rep. 2018;17:57–63. doi:10.1016/j.ymgmr.2018.10.002
7. Evans S, Daly A, Wildgoose J, et al. Mealtime anxiety and coping behaviour in parents and children during weaning in PKU: a case-control study. Nutrients. 2019;11:2857. doi:10.3390/nu11122857
8. Wood G, Pinto A, Evans S, et al. Special low protein foods prescribed in England for pku patients: an analysis of prescribing patterns and cost. Nutrients. 2021;13:3977. doi:10.3390/nu13113977
9. Longo N, Arnold GL, Pridjian G, et al. Long-term safety and efficacy of sapropterin: the PKUDOS registry experience. Mol Genet Metab. 2015;114:557–563. doi:10.1016/j.ymgme.2015.02.003
10. Rohr F, Burton B, Dee A, et al. Evaluating change in diet with pegvaliase treatment in adults with phenylketonuria: analysis of Phase 3 clinical trial data. Mol Genet Metab. 2024;14:108122. doi:10.1016/j.ymgme.2023.108122
11. Cunningham A, Rohr F, Splett P, et al. Nutrition management of PKU with pegvaliase therapy: update of the web-based PKU nutrition management guideline recommendations. Orphanet J Rare Dis. 2023;18:155. doi:10.1186/s13023-023-02751-0
12. Thomas L, Olson A, Romani C. The impact of metabolic control on cognition, neurophysiology, and well-being in PKU: a systematic review and meta-analysis of the within-participant literature. Mol Genet Metab. 2023;138:106969. doi:10.1016/j.ymgme.2022.106969
13. Ashe K, Kelso W, Farrand S, et al. Psychiatric and cognitive aspects of phenylketonuria: the limitations of diet and promise of new treatments. Front Psychiatry. 2019;10:561. doi:10.3389/fpsyt.2019.00561
14. van Vliet D, van Wegberg AMJ, Ahring K, et al. Can untreated PKU patients escape from intellectual disability? A systematic review. Orphanet J Rare Dis. 2018;13:149. doi:10.1186/s13023-018-0890-7
15. Aitkenhead L, Krishna G, Ellerton C, et al. Long-term cognitive and psychosocial outcomes in adults with phenylketonuria. J Inherit Metab Dis. 2021;44:1353–1368. doi:10.1002/jimd.12413
16. Abgottspon S, Muri R, Christ SE, et al. Neural correlates of working memory and its association with metabolic parameters in early-treated adults with phenylketonuria. Neuroimage Clin. 2022;34:102974. doi:10.1016/j.nicl.2022.102974
17. Sabaté E, editor; WHO publication. Adherence to Long-Term Therapies, Evidence for Action. Geneva: World Health Organization;2003.
18. García MI, Araya G, Coo S, Waisbren SE, de la Parra A. Treatment adherence during childhood in individuals with phenylketonuria: early signs of treatment discontinuation. Mol Genet Metab Rep. 2017;11:54–58. doi:10.1016/j.ymgmr.2017.04.006
19. Jurecki ER, Cederbaum S, Kopesky J, et al. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol Genet Metab. 2017;120:190–197. doi:10.1016/j.ymgme.2017.01.001
20. MacDdonald A, van Rijn M, Feillet F, et al. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann Nutr Metab. 2012;61:289–295. doi:10.1159/000342256
21. Jahja R, Huijbregts SC, de Sonneville LM, van der Meere JJ, van Spronsen FJ. Neurocognitive evidence for revision of treatment targets and guidelines for phenylketonuria. J Pediatr. 2014;164:895–899.e2. doi:10.1016/j.jpeds.2013.12.015
22. Brumm VL, Bilder D, Waisbren SE. Psychiatric symptoms and disorders in phenylketonuria. Mol Genet Metab. 2010;99(Suppl 1):S59–63. doi:10.1016/j.ymgme.2009.10.182
23. Reach G. The Mental Mechanisms of Adherence to Long-Term Therapies, Mind and Care. Springer; 2015.
24. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–443. doi:10.1592/phco.28.4.437
25. Baryakova TH, Pogostin BH, Langer R, McHugh KJ. Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems. Nat Rev Drug Discov. 2023;22:387–409. doi:10.1038/s41573-023-00670-0
26. Rocha JC, Ahring KK, Bausell H, et al. Expert consensus on the long-term effectiveness of medical nutrition therapy and its impact on the outcomes of adults with phenylketonuria. Nutrients. 2023;15:3940. doi:10.3390/nu15183940
27. Cazzorla C, Bensi G, Biasucci G, et al. Living with phenylketonuria in adulthood: the PKU ATTITUDE study. Mol Genet Metab Rep. 2018;16:39–45. doi:10.1016/j.ymgmr.2018.06.007
28. Kenneson A, Singh RH. Natural history of children and adults with phenylketonuria in the NBS-PKU Connect registry. Mol Genet Metab. 2021;134:243–249. doi:10.1016/j.ymgme.2021.10.001
29. Medford E, Hare DJ, Wittkowski A. Demographic and psychosocial influences on treatment adherence for children and adolescents with PKU: a systematic review. JIMD Rep. 2018;39:107–116. doi:10.1007/8904_2017_52
30. Poole G, Pinto A, Evans S, et al. Hungry for change: the experiences of people with PKU, and their caregivers, when eating out. Nutrients. 2022;14:626. doi:10.3390/nu14030626
31. Yagudina R, Kulikov A, Serpik V, Protsenko M, Kopeyka K. Factors affecting adherence to a low phenylalanine diet in patients with phenylketonuria: a systematic review. Nutrients. 2024;16:3119. doi:10.3390/nu16183119
32. Firman SJ, Ramachandran R, Whelan K. Knowledge, perceptions and behaviours regarding dietary management of adults living with phenylketonuria. J Hum Nutr Diet. 2022;35:1016–1029. doi:10.1111/jhn.13015
33. Hall I, Pinto A, Evans S, et al. The challenges and dilemmas of interpreting protein labelling of prepackaged foods encountered by the PKU community. Nutrients. 2022;14:1355. doi:10.3390/nu14071355
34. Lim JY, Rajikan R, Amit N, et al. Exploring the barriers and motivators to dietary adherence among caregivers of children with disorders of amino acid metabolism (AAMDs): a qualitative study. Nutrients. 2022;14:2535. doi:10.3390/nu14122535
35. Rohr F, Wessel A, Brown M, Charette K, Levy HL. Adherence to tetrahydrobiopterin therapy in patients with phenylketonuria. Mol Genet Metab. 2015;114:25–28. doi:10.1016/j.ymgme.2014.10.013
36. Scala I, Brodosi L, Gueraldi D, et al. Pegvaliase therapy for phenylketonuria: real-world case series and clinical insights. Mol Genet Metab. 2024;142:108151. doi:10.1016/j.ymgme.2024.108151
37. Pinto A, Ilgaz F, Evans S, et al. Phenylalanine tolerance over time in phenylketonuria: a systematic review and meta-analysis. Nutrients. 2023;15:3506. doi:10.3390/nu15163506
38. Pinto A, Almeida MF, MacDonald A, et al. Over restriction of dietary protein allowance: the importance of ongoing reassessment of natural protein tolerance in phenylketonuria. Nutrients. 2019;11:995. doi:10.3390/nu11050995
39. McWhorter N, Ndugga-Kabuye MK, Puurunen M, Ernst SL. Complications of the low phenylalanine diet for patients with phenylketonuria and the benefits of increased natural protein. Nutrients. 2022;14:4960. doi:10.3390/nu14234960
40. Ilgaz F, Ford S, O’Driscoll MF, MacDonald A. Adult PKU clinics in the UK-users’ experiences and perspectives. Nutrients. 2023;15:4352. doi:10.3390/nu15204352
41. Burlina AP, Cazzorla C, Massa P, Loro C, Gueraldi D, Burlina AB. The impact of a slow-release large neutral amino acids supplement on treatment adherence in adult patients with phenylketonuria. Nutrients. 2020;12:2078. doi:10.3390/nu12072078
42. Jameson E, Remmington T. Dietary interventions for phenylketonuria. Cochrane Database Syst Rev. 2020;7:CD001304. doi:10.1002/14651858.CD001304.pub3
43. Green B, Browne R, Firman S, et al. Nutritional and metabolic characteristics of UK adult phenylketonuria patients with varying dietary adherence. Nutrients. 2019;11:2459. doi:10.3390/nu11102459
44. Burgess NM, Kelso W, Malpas CB, et al. The effect of improved dietary control on cognitive and psychiatric functioning in adults with phenylketonuria: the ReDAPT study. Orphanet J Rare Dis. 2021;16:35. doi:10.1186/s13023-020-01668-2
45. Antenor-Dorsey JA, Hershey T, Rutlin J, et al. White matter integrity and executive abilities in individuals with phenylketonuria. Mol Genet Metab. 2013;109:125–131. doi:10.1016/j.ymgme.2013.03.020
46. Hood A, Antenor-Dorsey JA, Rutlin J, et al. Prolonged exposure to high and variable phenylalanine levels over the lifetime predicts brain white matter integrity in children with phenylketonuria. Mol Genet Metab. 2015;114:19–24. doi:10.1016/j.ymgme.2014.11.007
47. Muri R, Maissen-Abgottspon S, Rummel C, et al. Cortical thickness and its relationship to cognitive performance and metabolic control in adults with phenylketonuria. J Inherit Metab Dis. 2022;45:1082–1093. doi:10.1002/jimd.12561
48. Paermentier L, Cano A, Chabrol B, Roy A. Executive functions in preschool children with moderate hyperphenylalaninemia and phenylketonuria: a prospective study. Orphanet J Rare Dis. 2023;18:175. doi:10.1186/s13023-023-02764-9
49. Weglage J, Fromm J, van Teeffelen-Heithoff A, et al. Neurocognitive functioning in adults with phenylketonuria: results of a long term study. Mol Genet Metab. 2013;110:S44–8. doi:10.1016/j.ymgme.2013.08.013
50. Romani C, Olson A, Aitkenhead L, et al. Meta-analyses of cognitive functions in early-treated adults with phenylketonuria. Neurosci Biobehav Rev. 2022;143:104925. doi:10.1016/j.neubiorev.2022.104925
51. Bilder DA, Noel JK, Baker ER, et al. Systematic review and meta-analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev Neuropsychol. 2016;41:245–260. doi:10.1080/87565641.2016.1243109
52. Sharman R, Sullivan K, Young R, McGill J. Executive function in adolescents with PKU and their siblings: associations with biochemistry. Mol Genet Metab Rep. 2015;4:87–88. doi:10.1016/j.ymgmr.2015.08.001
53. Cleary M, Trefz F, Muntau AC, et al. Fluctuations in phenylalanine concentrations in phenylketonuria: a review of possible relationships with outcomes. Mol Genet Metab. 2013;110:418–423. doi:10.1016/j.ymgme.2013.09.001
54. Anastasoaie V, Kurzius L, Forbes P, Waisbren S. Stability of blood phenylalanine levels and IQ in children with phenylketonuria. Mol Genet Metab. 2008;95:17–20. doi:10.1016/j.ymgme.2008.06.014
55. Hood A, Grange DK, Christ SE, Steiner R, White DA. Variability in phenylalanine control predicts IQ and executive abilities in children with phenylketonuria. Mol Genet Metab. 2014;11:445–451. doi:10.1016/j.ymgme.2014.01.012
56. de Almeida Duarte CM, Piazzon FB, Rocco IS, de Mello CB. Influence of blood phenylalanine level variations on the development of executive functions and social cognition in children with phenylketonuria. J Pediatr. 2023;99:507–513. doi:10.1016/j.jped.2023.04.003
57. Feldmann R, Schallert M, Nguyen T, Och U, Rutsch F, Weglage J. Children and adolescents with phenylketonuria display fluctuations in their blood phenylalanine levels. Acta Paediatr. 2019;108:541–543.
58. Romani C, Manti F, Nardecchia F, et al. Adult cognitive outcomes in phenylketonuria: explaining causes of variability beyond average Phe levels. Orphanet J Rare Dis. 2019;14:273.
59. Scala I, Riccio MP, Marino M, Bravaccio C, Parenti G, Strisciuglio P. Large neutral amino acids (LNAAs) supplementation improves neuropsychological performances in adult patients with phenylketonuria. Nutrients. 2020;12:1092. doi:10.3390/nu12041092
60. White DA, Antenor-Dorsey JA, Grange DK, et al. White matter integrity and executive abilities following treatment with tetrahydrobiopterin (BH4) in individuals with phenylketonuria. Mol Genet Metab. 2013;110:213–217. doi:10.1016/j.ymgme.2013.07.010
61. Christ SE, Moffitt AJ, Peck D, White DA. The effects of tetrahydrobiopterin (BH4) treatment on brain function in individuals with phenylketonuria. Neuroimage Clin. 2013;3:539–547. doi:10.1016/j.nicl.2013.08.012
62. Reach G. A novel conceptual framework for understanding the mechanism of adherence to long term therapies. Patient Prefer Adherence. 2008;2:7–19.
63. Wroe AL. Intentional and unintentional nonadherence: a study of decision making. J Behav Med. 2002;25:355–372. doi:10.1023/A:1015866415552
64. Kalenscher T, Pennartz CM. Is a bird in the hand worth two in the future? The neuroeconomics of intertemporal decision-making. Prog Neurobiol. 2008;84:284–315. doi:10.1016/j.pneurobio.2007.11.004
65. Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi:10.1901/jeab.1991.55-233
66. Ainslie G. Willpower with and without effort. Behav Brain Sci. 2020;44:e30. doi:10.1017/S0140525X20000357
67. Reach G. How is patient adherence possible? A novel mechanistic model of adherence based on humanities. Patient Prefer Adherence. 2023;17:1705–1720. doi:10.2147/PPA.S419277
68. Wainwright K, Romanowich P, Crabtree MA. Associations between impulsivity and self-care adherence in individuals diagnosed with Type 2 or prediabetes. PLoS One. 2022;17:e0263961. doi:10.1371/journal.pone.0263961
69. Datye KA, Moore DJ, Russell WE, Jaser SS. A review of adolescent adherence in type 1 diabetes and the untapped potential of diabetes providers to improve outcomes. Curr Diab Rep. 2015;15:51. doi:10.1007/s11892-015-0621-6
70. Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953–957. doi:10.1164/rccm.200511-1706PP
71. Ferrand RA, Briggs D, Ferguson J, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Trop Med Int Health. 2016;21:325–333. doi:10.1111/tmi.12656
72. Beghini M, Pichler M, Tinnefeld FC, et al. Poor adherence during adolescence is a risk factor for becoming lost-to-follow-up in patients with phenylketonuria. Mol Genet Metab Rep. 2024;39:101087. doi:10.1016/j.ymgmr.2024.101087
73. Willoughby T, Good M, Adachi PJ, Hamza C, Tavernier R. Examining the link between adolescent brain development and risk taking from a social-developmental perspective. Brain Cogn. 2013;83:315–323. doi:10.1016/j.bandc.2013.09.008
74. Reach G. Obedience and motivation as mechanisms for adherence to medication: a study in obese type 2 diabetic patients. Patient Prefer Adherence. 2011;5:523–531. doi:10.2147/PPA.S24518
75. Fogarty JS. Reactance theory and patient noncompliance. Soc Sci Med. 1997;45:1277–1288. doi:10.1016/S0277-9536(97)00055-5
76. Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi:10.1016/j.dr.2007.08.003
77. Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28:78–106. doi:10.1016/j.dr.2007.08.002
78. Shulman EP, Smith AR, Silva K, et al. The dual systems model: review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. doi:10.1016/j.dcn.2015.12.010
79. Bickel WK, Snider SE, Quisenberry AJ, Stein JS, Hanlon CA. Competing neurobehavioral decision systems theory of cocaine addiction: from mechanisms to therapeutic opportunities. Prog Brain Res. 2016;223:269–293.
80. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
81. Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi:10.1523/JNEUROSCI.1062-06.2006
82. Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54:1344–1354. doi:10.1016/j.neuroimage.2010.08.067
83. Gokmen Ozel H, Ahring K, Bélanger-Quintana A, et al. Overweight and obesity in PKU: the results from 8 centres in Europe and Turkey. Mol Genet Metab Rep. 2014;1:483–486. doi:10.1016/j.ymgmr.2014.11.003
84. Rodrigues C, Pinto A, Faria A, et al. Is the phenylalanine-restricted diet a risk factor for overweight or obesity in patients with phenylketonuria (PKU)? A systematic review and meta-analysis. Nutrients. 2021;13:3443. doi:10.3390/nu13103443
85. Tankeu AT, Pavlidou DC, Superti-Furga A, Gariani K, Tran C. Overweight and obesity in adult patients with phenylketonuria: a systematic review. Orphanet J Rare Dis. 2023;18:37. doi:10.1186/s13023-023-02636-2
86. Luengo-Pérez LM, Fernández-Bueso M, Ambrojo A, et al. Body composition evaluation and clinical markers of cardiometabolic risk in patients with phenylketonuria. Nutrients. 2023;15:5133. doi:10.3390/nu15245133
87. Barlow P, Reeves A, McKee M, Galea G, Stuckler D. Unhealthy diets, obesity and time discounting: a systematic literature review and network analysis. Obes Rev. 2016;17:810–819. doi:10.1111/obr.12431
88. Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev. 2018;84:225–244. doi:10.1016/j.neubiorev.2017.11.020
89. Bickel WK, Moody LN, Koffarnus M, Thomas JG, Wing R. Self-control as measured by delay discounting is greater among successful weight losers than controls. J Behav Med. 2018;41:891–896. doi:10.1007/s10865-018-9936-5
90. Dassen FCM, Houben K, Allom V, Jansen A. Self-regulation and obesity: the role of executive function and delay discounting in the prediction of weight loss. J Behav Med. 2018;41:806–818.
91. Du Z, Li J, Huang J, et al. Executive functions in predicting weight loss and obesity indicators: a meta-analysis. Front Psychol. 2021;11:604113.
92. Appelhans BM, Tangney CC, French SA, Crane MM, Wang Y. Delay discounting and household food purchasing decisions: the SHoPPER study. Health Psychol. 2019;38:334–342.
93. Bénard M, Bellisle F, Kesse-Guyot E, et al. Impulsivity is associated with food intake, snacking, and eating disorders in a general population. Am J Clin Nutr. 2019;109:117–126. doi:10.1093/ajcn/nqy255
94. Zhang W, Li G, Manza P, et al. Functional abnormality of the executive control network in individuals with obesity during delay discounting. Cereb Cortex. 2022;32:2013–2021. doi:10.1093/cercor/bhab333
95. Weller RE, Cook EW 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi:10.1016/j.appet.2008.04.010
96. Hosseini-Kamkar N, Morton JB. Sex differences in self-regulation: an evolutionary perspective. Front Neurosci. 2014;8:233.
97. Rovelli V, Longo N. Phenylketonuria and the brain. Mol Genet Metab. 2023;139:107583.
98. Boettiger CA, Mitchell JM, Tavares VC, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi:10.1523/JNEUROSCI.2551-07.2007
99. Kelm MK, Boettiger CA. Effects of acute dopamine precusor depletion on immediate reward selection bias and working memory depend on catechol-O-methyltransferase genotype. J Cogn Neurosci. 2013;25:2061–2071. doi:10.1162/jocn_a_00464
100. Wagner B, Clos M, Sommer T, Peters J. Dopaminergic modulation of human intertemporal choice: a diffusion model analysis using the D2-receptor antagonist haloperidol. J Neurosci. 2020;40:7936–7948. doi:10.1523/JNEUROSCI.0592-20.2020
101. Boot E, Hollak CEM, Huijbregts SCJ, et al. Cerebral dopamine deficiency, plasma monoamine alterations and neurocognitive deficits in adults with phenylketonuria. Psychol Med. 2017;47:2854–2865. doi:10.1017/S0033291717001398
102. Joutsa J, Voon V, Johansson J, Niemelä S, Bergman J, Kaasinen V. Dopaminergic function and intertemporal choice. Transl Psychiatry. 2015;5:e491. doi:10.1038/tp.2014.133
103. Hase A, Jung SE, aan het Rot M. Behavioral and cognitive effects of tyrosine intake in healthy human adults. Pharmacol Biochem Behav. 2015;133:1–6. doi:10.1016/j.pbb.2015.03.008
104. Aquili L. The role of tryptophan and tyrosine in executive function and reward processing. Int J Tryptophan Res. 2020;13:1178646920964825. doi:10.1177/1178646920964825
105. Ruocco AC, Rodrigo AH, Carcone D, McMain S, Jacobs G, Kennedy JL. Tryptophan hydroxylase 1 gene polymorphisms alter prefrontal cortex activation during response inhibition. Neuropsychology. 2016;30:18–27. doi:10.1037/neu0000237
106. Neufang S, Akhrif A, Herrmann CG, et al. Serotonergic modulation of ‘waiting impulsivity’ is mediated by the impulsivity phenotype in humans. Transl Psychiatry. 2016;6:e940. doi:10.1038/tp.2016.210
107. Wieber F, Thürmer JL, Gollwitzer PM. Promoting the translation of intentions into action by implementation intentions: behavioral effects and physiological correlates. Front Hum Neurosci. 2015;9:395. doi:10.3389/fnhum.2015.00395
108. Zogg JB, Woods SP, Sauceda JA, Wiebe JS, Simoni JM. The role of prospective memory in medication adherence: a review of an emerging literature. J Behav Med. 2012;35:47–62. doi:10.1007/s10865-011-9341-9
109. Dolansky MA, Hawkins MA, Schaefer JT, et al. Association between poorer cognitive function and reduced objectively monitored medication adherence in patients with heart failure. Circ Heart Fail. 2016;9:e002475. doi:10.1161/CIRCHEARTFAILURE.116.002475
110. Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I, HIV Neurobehavioral Research Center (HNRC) Group. Prospective memory in HIV-1 infection. J Clin Exp Neuropsychol. 2006;28:536–548. doi:10.1080/13803390590949494
111. Contardo C, Black AC, Beauvais J, Dieckhaus K, Rosen MI. Relationship of prospective memory to neuropsychological function and antiretroviral adherence. Arch Clin Neuropsychol. 2009;24:547–554. doi:10.1093/arclin/acp046
112. Avci G, Sheppard DP, Tierney SM, Kordovski VM, Sullivan KL, Woods SP. A systematic review of prospective memory in HIV disease: from the laboratory to daily life. Clin Neuropsychol. 2018;32:858–890. doi:10.1080/13854046.2017.1373860
113. Luna PM, López-Paz JF, García M, et al. Cognitive functioning in adults with phenylketonuria in a cohort of Spanish patients. Behav Neurol. 2023;2023:9681740. doi:10.1155/2023/9681740
114. Channon S, Goodman G, Zlotowitz S, Mockler C, Lee PJ. Effects of dietary management of phenylketonuria on long-term cognitive outcome. Arch Dis Child. 2007;92:213–218. doi:10.1136/adc.2006.104786
115. Bartus A, Palasti F, Juhasz E, et al. The influence of blood phenylalanine levels on neurocognitive function in adult PKU patients. Metab Brain Dis. 2018;33:1609–1615. doi:10.1007/s11011-018-0267-6
116. Pilotto A, Zipser CM, Leks E, et al. Phenylalanine effects on brain function in adult phenylketonuria. Neurology. 2021;96:e399–e411. doi:10.1212/WNL.0000000000011088
117. Palermo L, Geberhiwot T, MacDonald A, Limback E, Hall SK, Romani C. Cognitive outcomes in early-treated adults with phenylketonuria (PKU): a comprehensive picture across domains. Neuropsychology. 2017;31:255–267. doi:10.1037/neu0000337
118. Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM. The impact of cognitive function on medication management: three studies. Health Psychol. 2010;29:50–55. doi:10.1037/a0016940
119. Haynes RB. Introduction. In: Haynes RB, Taylor DW, Sackett DL, editors. Compliance in Health Care. Baltimore: Johns Hopkins University Press; 1979:1–7.
120. Reach G, Chenuc G, Maigret P, Elias-Billon I, Martinez L, Flipo RM. Implication of character traits in adherence to treatment in people with gout: a reason for considering nonadherence as a syndrome. Patient Prefer Adherence. 2019;13:1913–1926. doi:10.2147/PPA.S227329
121. Reach G, Benarbia L, Benhamou PY, et al. An unsafe/safe typology in people with type 2 diabetes: bridging patients’ expectations, personality traits, medication adherence, and clinical outcomes. Patient Prefer Adherence. 2022;16:1333–1350. doi:10.2147/PPA.S365398
122. Martinez M, Harding CO, Schwank G, Thöny B. State-of-the-art 2023 on gene therapy for phenylketonuria. J Inherit Metab Dis. 2024;47:80–92. doi:10.1002/jimd.12651
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

