Back to Journals » Journal of Pain Research » Volume 18
Pocket Fills for Intrathecal Pump Delivery Systems: A Narrative Review
Authors Banks DW , Jevotovsky DS , Oehlermarx W , Broachwala M, Gulati A, Chakravarthy K
Received 3 February 2025
Accepted for publication 7 July 2025
Published 10 July 2025 Volume 2025:18 Pages 3519—3526
DOI https://doi.org/10.2147/JPR.S520502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Dylan W Banks,1 David S Jevotovsky,1 Whitman Oehlermarx,1 Mustafa Broachwala,2 Amitabh Gulati,3 Krishnan Chakravarthy2
1Department of Physical Medicine and Rehabilitation, New York University, New York, NY, USA; 2Department of Anesthesiology and Pain Medicine, University of California San Diego, San Diego, CA, USA; 3Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Correspondence: Krishnan Chakravarthy, Department of Anesthesiology and Pain Medicine, University of California San Diego, 3350 La Jolla Village Drive, San Diego, CA, 92161, USA, Email [email protected]
Abstract: Intrathecal drug delivery systems (IDDS) are an increasingly common treatment option in the management of refractory chronic pain. IDDS allow for highly customizable administration of medication directly into the intrathecal space, optimizing therapeutic benefit while minimizing systemic side effects. Understanding potential complications of IDDS is key for patient safety. This narrative review examines pocket fills, a potential complication that occurs when inadvertently missing the port on the intrathecal pump reservoir during a pump refill, resulting in the injection of the medication into the surrounding subcutaneous tissue. It is suspected that pocket fill events are vastly underreported and understudied despite posing serious risk for patient safety. Given the limited existing research discussing pocket fills, this narrative review will provide an overview of pocket fills including the anatomy of the intrathecal pump placement, risk factors for pocket fills, preventative techniques, as well as post pocket fill recommendations. Key preventative techniques highlighted include the application of firm pressure throughout the procedure, imaging guidance, as well as post procedure monitoring and device interrogation. As there is a lack of clinical guidelines for pocket fill prevention, we advise tailoring these strategies to available resources and individual patient needs. As IDDS utilization continues to evolve in clinical practice, future quality improvement initiatives could focus on the development of standardized refill protocols, simulation-based training courses and competency assessments, while future research initiatives could focus on comparative analyses of pump refill complication rates under imaging guidance in comparison to template guidance, as well as early detection monitoring technology.
Keywords: pocket fill, intrathecal pump, chronic pain, safety
Introduction
Intrathecal drug delivery systems (IDDS), also known as intrathecal pumps, are increasingly common in the management of refractory chronic pain. Utilization of IDDS has steadily increased since their development in the 1980’s.1 While initially IDDS were only considered as a salvage therapy, encouraging evidence supporting its use in both malignant and nonmalignant pain conditions has broadened its application.2,3 Despite recent advancements, some note that the utilization of intrathecal management is outpacing the current research and thus knowledge gaps exist.1 One area that this is particularly true, and the focus of this review, is pocket fills – a potential complication that occurs during a pump refill when medication is injected into the surrounding subcutaneous tissue instead of the pump itself.
IDDS include an implanted subcutaneous pump reservoir attached to an intrathecal catheter, allowing for the direct delivery of medication to the central nervous system.1 By utilizing this targeted delivery method, pain management can be optimized while minimizing systemic side effects associated with the medication.4 Common indications include cancer related pain, refractory pain associated with axial neck or back pain, failed back surgery syndrome, radicular pain, complex regional pain syndrome, and spasticity.1,5 Commonly administered intrathecal medications include morphine, hydromorphone, fentanyl, bupivacaine, ziconotide, clonidine, and baclofen.5,6
Though intrathecal pumps are an effective treatment modality, it is crucial to understand potential complications to maximize safe utilization. The decision to implement an intrathecal pump is complex, and thus proper patient counseling on these risks is essential.1 There are risks associated with the surgical implantation of the device, the process of filling the pump reservoir with the desired medication, as well as risks associated with the medications themselves.7 Risks associated with the surgical implantation of the device include bleeding, spinal cord injury, cerebrospinal fluid leakage, and infection.8 A key complication to be aware of during the intrathecal pump refill process are pocket fills.
Pocket fills are a complication that can occur during pump refills when the needle inadvertently misses the reservoir port, leading to the medication being injected into the surrounding subcutaneous tissue rather than the pump itself (Figure 1). While the incidence is reported to be 1 in 10,000 procedures, it is estimated that the true incidence may be much higher in part due to unreported complications or those complications unbeknownst to the provider.9 Research regarding pocket fills is a current knowledge gap in IDDS management, partially driven by poorly defined epidemiologic data as suggested above, but also due to limited discussion in clinical guidelines. Additionally, it is important to recognize that patients who are using IDDS often have complicated neurologic conditions and thus the symptoms of withdrawal or overdose of their pump medication may be attributed to other causes especially if the complication is relatively minor. This warrants attention, as pocket fills may result in both immediate and delayed risks that are potentially lethal.6 The specific risks are often related to the medication being administered being either overdosed or underdosed.
 |
Figure 1 Intrathecal drug delivery system reservoir with surrounding pocket fill. |
Immediate risks stem from the subcutaneous injection of large boluses of highly concentrated medications. As the subcutaneous tissues are rich in capillaries, the medication is absorbed peripherally via diffusion before entering systemic circulation.10 With opioids, pocket fill events can lead to respiratory depression and altered mentation requiring naloxone and advanced care.9 Though less common, cases of overdose from baclofen pocket fill events have also been reported resulting in respiratory depression and altered mentation.11 Additional symptoms of overdose from either opioids or baclofen include hypotonia, hypotension, nausea, vomiting, or seizures.12 While also less common, overdose from ziconotide may result in symptoms including psychosis, ataxia and a reduced level of cognition, though the existing reports indicate these side effects are often shorter lived and less severe than those seen in opioids.13 For clonidine, overdose can lead to an early hypertensive crisis followed by hypotension or myocardial infarction.9 Additional symptoms from clonidine overdose include confusion, profuse sweating, dysarthria, or respiratory depression.12 Adverse events from pocket fills utilizing local anesthetics such as bupivacaine are generally infrequent and thus suspected to be better tolerated.14
Delayed risks include drug withdrawal from underdosing if the pump reservoir is left empty in a patient who has developed a tolerance. This is most often seen with opioids or baclofen and may require oral replacement until intrathecal delivery is resumed.9 Withdrawal of opioids presents with piloerection, myalgias, diarrhea, or autonomic hyperactivity.12 Withdrawal of baclofen often presents with an exacerbation of spasticity in addition to fever, sweating, pruritus and in severe cases cognitive changes.12 Withdrawal of clonidine may lead to a sympathomimetic crisis and significant hypertension.12 Abrupt withdrawal of ziconotide is generally well tolerated without significant adverse events.12
Though pocket fills are a known complication of intrathecal pumps and carry significant risk, very limited literature exists discussing their detection or prevention, and is thus an important area of ongoing focus given the increasing utilization of this modality. This narrative review aims to summarize the existing evidence on pocket fills as well as provide a beneficial guide to prevention techniques.
Discussion
Intrathecal Pump Placement
IDDS have two main components: an intrathecal catheter and a pump reservoir (Figure 2). The catheter is inserted into the intrathecal space, allowing for direct delivery of the medication to the central nervous system. The catheter is attached to a battery powered medication reservoir that is surgically implanted into the abdominal subcutaneous tissue. Within each reservoir is a reservoir access point. Most reservoirs have either 20 to 40 mL capacities.15 The pumps are highly programmable and allow for specific rate and timing adjustments to be made for medication administration.15 All programming adjustments are made by an external hand held device that allows for easy physician and patient management.15
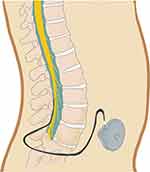 |
Figure 2 Intrathecal drug delivery system demonstrating intrathecal catheter and pump reservoir. |
The two main types of intrathecal pumps include Medtronic’s SynchroMed II and Flowonix Prometra II. The choice of which to pursue is often driven by features including battery life, magnetic resonance imaging compatibility, desired programming features, and clinical availability. Although a detailed comparative analysis is outside of the scope of this review, several key features are worth noting as they pertain to pocket fill risk. It is important to note that Flowonix Prometra II does feature a raised refill port, which may facilitate palpation guided refills in comparison to Medtronic’s SynchroMed II more recessed port design. Both devices feature a silicon rubber port septum. Medtronic’s refill port is designed to withstand up to 500 punctures, while Flowonix’s port is designed to withstand up to 1,000 punctures. Both devices require a 22-gauge noncoring needle for reservoir refills which is necessary to ensure the silicon port is not damaged. Lastly, both devices feature comparable subcutaneous suture systems, utilized to reduce pump migration or rotation which would increase the risk of pocket fill events.16,17 No direct comparisons of pocket fill event rates between devices are reported in the literature.
Intrathecal Pump Refilling Procedure
Refilling an intrathecal pump is the most common procedure performed on the pump after the installation process.9 Recent advancements have allowed for pumps to operate for 1 to 3 months without requiring a refill.15 To refill an intrathecal pump, providers often utilize the provided pump template to facilitate identification of the pump orientation and identify the reservoir filling port. After sterilizing the procedural field and identifying the pump orientation, a needle is inserted into the access point, and the previous medication is aspirated before new medication is administered into the reservoir.9
A 2015 observational study assessing the accuracy of template-guided injections compared to fluoroscopic-guided controls. This study raised concern over the accuracy of template-guided injections. The authors found that template-guided injection sites were an average 8.2 mm away from the center of the reservoir filling site, highlighting the poor accuracy and error margin.18
Contributing Risk Factors
While refilling an intrathecal pump is a common outpatient procedure, there are several factors that may influence the ease at which this procedure is performed and increase the risk of an inadvertent pocket fill. These risk factors are important to consider prior to all refills to mitigate the associated risks.
Pump location and orientation: There is inherent variation in the pump locations and overall orientation based upon both surgical technique and surgeon preference.7 Additional anatomic differences may influence IDDS orientation, adding to the inherent variation between pumps.7 Given these distinctions, some pumps may exhibit hypermobility resulting in pump rotation or inversion, increasing the risk of pocket fills.9
Anatomic differences: Patient-specific anatomy also plays a large role in the ease at which this procedure is performed. Patients with excess subcutaneous tissue (ie obesity), excess scar tissue surrounding their reservoir filling port, deep surgical implantation, or peri-pump seromas can all add to the procedural challenges of refilling an intrathecal pump.9,19 For patients identified as having challenging anatomy, special attention should be paid to risk reduction techniques. Moreover, these patients may benefit from consideration of specialized protocols or pre-fill imaging given their increased risk.
Multiple refill attempts: Given the above technical challenges, multiple attempts at needle insertion for pump refills not only increase procedural pain for the patient but can also increase the subsequent risk of infection in addition to septum damage and potential future leakage.19 Repeated punctures of the silicon reservoir port septum may, in theory, lead to slow leaks which could result in an undetected and delayed pocket fill event. Close surveillance of patients with frequent pump refills may be advantageous.
Provider-related risks: Lastly, provider dependent risks exist. As there is a lack of standardized training guiding pump refills, provider inexperience may lead to higher rates of inadvertent pocket fills.
Incidence of Pocket Fills
The incidence of pocket fills has historically been challenging to accurately determine and is an understudied area in the realm of intrathecal pumps. It is expected that the true incidence is much higher than that which is reported, in part due to unreported complications or those complications unbeknownst to the provider.9 There is an increasing demand for improved reporting of pocket fill events for more accurate depiction of its incidence.
In a 2011 study with data ranging from 1996 to 2010, 351 cases of pocket fill were reported to Medtronic.9 Eight of these pocket fills resulted in lethal complications, 270 of which resulted in serious complications, and 58 which resulted in minor complications. From this data, Gofeld et al estimated the overall prevalence is 1 in 10,000 procedures.9 In a 2018 study assessing data since 2010 with 77,584 pump refills in 6,179 implanted pumps, there were 9 reported pocket filling events and an additional 17 reports of symptoms consistent with pocket fills ultimately unable to be confirmed as such.7
As utilization of IDDS continues to climb, ongoing efforts to accurately assess pocket fill incidence will enhance our understanding of its impact.
Preventing Pocket Fills
Few techniques have been reported in the literature to improve the safety profile of intrathecal pump refills and reduce pocket fill events. The absence of clear procedural guidelines contributes to inter-provider variability. The existing studies focus on multiple domains of risk reduction, including prevention of pocket fills, early detection of pocket filling events, as well as post procedure protocol when a pocket fill is suspected. To our knowledge, the existing literature regarding pocket fill preventative techniques is limited, largely restricted to case reports in addition to one cadaveric study and one prospective observational study.6,9,20
Pressure: A foundational principle in pocket fill prevention includes holding constant pressure on the needle throughout the entirety of the procedure with the goal of reducing potential needle migration were the patient to move.7 It is also important to mention that the syringe should be held in close approximation to the patient to ensure laxity of the syringe-needle catheter to ensure pressure can be maintained on the needle. This is utilized in conjunction with the provided template which aids in determining pump location and orientation.
Ultrasound: Ultrasound is increasingly utilized for both procedural planning and procedural guidance. One cadaveric study assessing the use of ultrasound for intrathecal pump refills found that ultrasound allowed for easy detection of pump inversion, a potential complication that can lead to pocket fills.9 Ultrasound guidance allows for easier identification of the reservoir filling port which, if absent, provides immediate feedback that the pump may be inverted or flipped (Figure 3). Use of ultrasound subsequently reduces the risk of refill attempts when the filling port is inaccessible. The Doppler function has been reported as a means of detecting extra-pump spread during the injection (Figure 4).9 Some providers use Doppler in conjunction with a two syringe model, in which a small volume of sterile saline is injected to confirm appropriate needle placement prior to aspiration of the saline and injection of the desired medication. Despite these benefits, utilization of ultrasound is not commonplace, perhaps due to the additional step required for procedural set-up and the cumbersome nature of the device.9
 |
Figure 3 Ultrasound image of intrathecal pump reservoir fill port with stars marking the area for potential fluid accumulation in pocket fills. |
 |
Figure 4 Ultrasound image of intrathecal pump with Doppler function demonstrating flow within the needle and reservoir. |
Fluoroscopy: The use of procedural fluoroscopy is another technique described in the literature, specifically utilized for challenging cases such as with obesity (Figure 5).18 Though this technique may aid in visualization of the reservoir access port, fluoroscopic access is generally not readily available and thus not a commonly utilized technique.9 Additional limitations to this technique include the added cost of fluoroscopic guidance in addition to radiation exposure.18
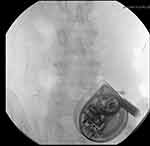 |
Figure 5 Fluoroscopic image of intrathecal pump with star marking reservoir filling port. |
Device interrogation: After administering the medication, it is advised to compare the volume of injected medication to that which is recorded by the reservoir fill sensor. A 2017 study assessing 221 intrathecal pump refills across 9 patients sought to assess these volumetric differences.6 They found an average difference of 0.4 mL for 20 mL reservoirs and a difference of 1.3 mL for 40 mL reservoirs. Notably, of the 221 pump refills reported, six resulted in overdose symptoms which were suspected consequences of pocket fill events. Within this sub-grouping, the authors found a volumetric difference of 1.15 mL to 4.5 mL for 20 mL reservoirs and 2.08 mL to 4.88 mL in 40 mL reservoirs. The study therefore concluded that volume discrepancies greater than 1 mL in 20 mL reservoirs and 2 mL in 40 mL reservoirs should be considered at risk for pocket fills events. Interestingly, the authors suggested that the expected allowable variance is likely secondary to micro-air bubbles in the syringes.6 The value of assessing volumetric differences for pocket fill detection is highlighted in this additional case report discussing a patient with multiple rounds of volumetric discrepancies of clonidine, prompting surgical excision of her IDDS which was found to have scarring of the reservoir’s silicon septum with multiple gouges that resulted in small volume leakage.20
Post procedure protocol: Post intrathecal pump refill, it is advised to have the patient remain in the office for 45 minutes post procedure to allow for early detection of adverse effects and facilitate an immediate response if a pocket fill event is suspected.7
Post Pocket Fill Recommendations
In cases of suspected pocket fill events, even if asymptomatic, it is advised to closely monitor the patient for a minimum of 2 hours to facilitate a quick medical response if indicated.6 The exact duration of monitoring time may also be influenced by the specific medication and dose injected. In the event of a pocket fill event, it is advised to aspirate the pocket fill if possible and to thoroughly rinse the area with sterile saline.6
Ultrasound can also be beneficial post procedure for visualization of suspected pocket fills. As described in one case report, the utilization of ultrasound allowed for detection of the fluid surrounding the pump and subsequent aspiration of it. Early aspiration resulted in significantly less systemic absorption of the injected medication and potentially reducing lethal side effects from the pocket fill event.21
Conclusions
As IDDS become more prevalent in managing refractory chronic pain, understanding the associated complications, such as pocket fills, is crucial for ensuring patient safety. Key gaps in knowledge that exist include underreporting within epidemiologic data – possibly due to low complication rate reporting or lack of provider awareness – in addition to limited discussion of pocket fills within clinical guidelines. This narrative review highlights the risks associated with pocket fills, including immediate risks in the form of acute overdose from the subcutaneous absorption of medication, and delayed risks in the form of pump underdosing. In either case, the risks are directly associated with the medication being administered. Key preventative techniques discussed include improved awareness, standardized procedural protocols including post refill device interrogation, as well as the adoption of advanced imaging techniques, such as ultrasound or fluoroscopy. Additionally, for patients identified as being high risk based on challenging anatomy (eg scar tissue formation, pump rotation, body habitus), pre-fill imaging and specialized protocols may serve an important role in risk reduction. Ongoing education and rigorous monitoring practices are essential to mitigate risks and optimize patient outcomes. In line with this, the development of simulation-based training courses or competency assessments may be a direction for future quality improvement initiatives. Additionally, clinical guidelines promoting standardized refill protocols could serve to reduce the variance across practices and promote a more systematic approach. In keeping with this, an additional direction for future quality improvement initiatives could be a deeper assessment of pocket fill events to better determine common missteps and expand upon data-driven prevention strategies. Lastly, as the field evolves, prioritizing research in this area will be critical to advancing best practices. Future research directions may include developing tracking systems to more accurately report incidence rates, comparative analyses of complication rates between image guided and template guided pump refills, as well as exploring potential smart detection technologies.
Abbreviations
IDDS, Intrathecal drug delivery systems.
Disclosure
Dr. Amitabh Gulati is a consultant for Medtronic, AIS Healthcare, SPR Therapeutics, Nalu Medical, Neurovasis, Hinge Health, Tersera Medical, Menda Health, Symmetric, Edenos and Veritas Pharma. Dr. Krishnan Chakravarthy is a consultant for Medtronic, NXSTIM Inc., Mainstay and Vertos. The authors report no other conflicts of interest in this work.
References
1. Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(2):96–132. doi:10.1111/ner.12538
2. Hayek SM, Deer TR, Pope JE, Panchal SJ, Patel VB. Intrathecal therapy for cancer and non-cancer pain. Pain Physician. 2011;14(3):219–248. doi:10.36076/ppj.2011/14/219
3. Pope J, Poree L, McRoberts WP, Falowski S, Deer T. Consent decree: physician and institution ramifications? Neuromodulation. 2015;18(8):653–656. doi:10.1111/ner.12374
4. Deer TR, Smith HS, Burton AW, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician. 2011;14(3):E283–312. doi:10.36076/ppj.2011/14/E283
5. Cho SR. Intrathecal baclofen therapy: pros and cons. Ann Rehabil Med. 2023;47(1):1–3. doi:10.5535/arm.23003
6. Maino P, Rsgm P, Koetsier E. Intrathecal pump refills, pocket fills, and symptoms of drug overdose: a prospective, observational study comparing the injected drug volume vs. the drug volume effectively measured inside the pump. Neuromodulation. 2017;20(7):733–739. doi:10.1111/ner.12597
7. Abd-Elsayed A. Pocket fill during intrathecal pump refill: is it a frequent occurrence? Neuromodulation. 2019;22(6):761. doi:10.1111/ner.13029
8. Gerges T, Mavropoulos A, Levin D, Dao T, Acquadro M. A rare intrathecal pump complication caused by prolonged seroma leading to a potential pump pocket fill: a near miss. Cureus. 2023;15(11):e48651. doi:10.7759/cureus.48651
9. Gofeld M, McQueen CK. Ultrasound-guided intrathecal pump access and prevention of the pocket fill. Pain Med. 2011;12(4):607–611. doi:10.1111/j.1526-4637.2011.01090.x
10. Kim H, Park H, Lee SJ. Effective method for drug injection into subcutaneous tissue. Sci Rep. 2017;7(1):9613. doi:10.1038/s41598-017-10110-w
11. Tinnirello A, Santi C. Intrathecal catheter puncture followed by a pocket fill after a pump refill: a case report and review of the literature. Pain Med Case Rep. 2022;6(3):115–118.
12. Delhaas EM, Fjpm H. Complications associated with intrathecal drug delivery systems. BJA Educ. 2020;20(2):51–57. doi:10.1016/j.bjae.2019.11.002
13. Webster LR. The relationship between the mechanisms of action and safety profiles of intrathecal morphine and ziconotide: a review of the literature: intrathecal mechanisms of action and safety. Pain Med. 2015;16(7):1265–1277. doi:10.1111/pme.12666
14. Deer TR, Serafini M, Buchser E, Ferrante FM, Hassenbusch SJ. Intrathecal bupivacaine for chronic pain: a review of current knowledge. Neuromodulation. 2002;5(4):196–207. doi:10.1046/j.1525-1403.2002.02030.x
15. Rauck R, Deer T, Rosen S, et al. Accuracy and efficacy of intrathecal administration of morphine sulfate for treatment of intractable pain using the Prometra(®) Programmable Pump. Neuromodulation. 2010;13(2):102–108. doi:10.1111/j.1525-1403.2009.00257.x
16. Medtronic SynchroMed II Programmable pump implant manual. Available from: https://www.medtronic.com/content/dam/medtronic-wide/public/united-states/products/neurological/synchromed-ii-product-manual.pdf.
17. Flowonix Prometra® Pump. Available from: https://flowonix.com/healthcare-provider/products/prometra-pump.
18. Maino P, Koetsier E, Rsgm P. The accuracy of template-guided refill technique of intrathecal pumps controlled by fluoroscopy: an observational study. Neuromodulation. 2015;18(5):428–432. doi:10.1111/ner.12212
19. Pinho S, Ferreira A, Calado D, Hatia M, Faria F. Ultrasound-guided intrathecal baclofen pump refilling method for management of spasticity in a complex clinical case. Cureus. 2022;14(11):e31537. doi:10.7759/cureus.31537
20. Perruchoud C, Bovy M, Rutschmann B, Durrer A, Buchser E. Silicone septum leakage at the origin of a drug overdose in a patient implanted with an intrathecal pump. Neuromodulation. 2013;16(5). doi:10.1111/j.1525-1403.2012.00523.x
21. Peccora CD, Ross EL, Hanna GM. Aberrant intrathecal pump refill: ultrasound-guided aspiration of a substantial quantity of subcutaneous hydromorphone. Reg Anesth Pain Med. 2013;38(6):544–546. doi:10.1097/AAP.0000000000000008
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

