Back to Journals » Pragmatic and Observational Research » Volume 15
Preserved Ratio Impaired Spirometry in US Primary Care Patients Diagnosed with Chronic Obstructive Pulmonary Disease
Authors Evans A, Tarabichi Y, Pace WD , Make B , Bushell N, Carter V, Chang KL, Fox C, Han MK, Kaplan A , Kocks JWH , Le Lievre C , Roussos A, Skolnik N, Soriano JB, Yawn BP, Price D
Received 17 May 2024
Accepted for publication 20 November 2024
Published 13 December 2024 Volume 2024:15 Pages 221—232
DOI https://doi.org/10.2147/POR.S478721
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ruth Murray
Alexander Evans,1 Yasir Tarabichi,2 Wilson D Pace,3,4 Barry Make,5 Nicholas Bushell,6 Victoria Carter,7 Ku-Lang Chang,8 Chester Fox,9 MeiLan K Han,10 Alan Kaplan,11,12 Janwillem WH Kocks,13– 15 Chantal Le Lievre,6 Alexander Roussos,6 Neil Skolnik,10,16 Joan B Soriano,17 Barbara P Yawn,10 David Price1,6,7,18
1Observational and Pragmatic Research Institute, Singapore, Singapore; 2Center for Clinical Informatics Research and Education, MetroHealth, Cleveland, OH, USA; 3DARTNet Institute, Aurora, CO, USA; 4Department of Family Medicine, Anschutz Medical Campus University of Colorado, Aurora, CO, USA; 5Department of Medicine, National Jewish Hospital, Denver, CO, USA; 6Optimum Patient Care, Brisbane, Queensland, Australia; 7Optimum Patient Care, Oakington, Cambridge, UK; 8Lucas Research, a Centricity Research Company, Morehead City, NC, USA; 9University at Buffalo, Buffalo, NY, USA; 10University of Minnesota, Minneapolis, MN, USA; 11Family Physician Airways Group of Canada, Stouffville, Ontario, Canada; 12University of Toronto, Toronto, Canada; 13General Practitioners Research Institute, Groningen, the Netherlands; 14Groningen Research Institute Asthma and COPD (GRIAC), University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; 15Department of Pulmonology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; 16Abington Jefferson Health, Jenkintown, PA, USA; 17School of Medicine, Universitat de les Illes Balears, Palma, Spain; 18Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, UK
Correspondence: David Price, Observational and Pragmatic Research Institute, 22 Sin Ming Lane, #06-76, Midview City, Singapore, 573969, Singapore, Tel +65 3105 1489, Email [email protected]
Background: Preserved ratio impaired spirometry (PRISm) represents a population with spirometry results that do not meet standardized COPD obstruction criteria, yet present with high respiratory symptom burden and might benefit from respiratory management and treatment. We aimed to determine prevalence of PRISm in US primary care patients diagnosed with COPD, describe their demographic, clinical, and CT scan characteristics.
Methods: An observational registry study utilizing the US APEX COPD registry, composed of patients diagnosed with COPD aged 35+ years. Demographic and clinical data were collected from EHRs and complemented by questionnaires. Multivariable logistic regression was performed to assess whether PRISm predicts lung function decline.
Results: Prevalence of PRISm within a primary care population clinically diagnosed with COPD was 23.6% (678/2866, 95% CI 22.0– 25.1). Those with PRISm were more likely female (55.9% vs 46.9%), younger (66.3± 11.1 vs 69.2± 10.3 years), with a greater mean BMI (33.5± 9.2 vs 27.8± 7.2 kg/m2), more often African American or Hispanic (37.2% vs 26.3%), and with fewer current smokers (33.1% vs 36.8%) when compared to those meeting COPD spirometry criteria (all p< 0.05). Compared to COPD GOLD 0 patients, individuals with PRISm had greater BMI (33.5± 9.2 vs 30.6± 7.8), and were more likely current smokers (33.1% vs 23.4%), both p< 0.05. Patients with PRISm had similar respiratory symptoms (chronic bronchitis, CAT, and mMRC) to overall COPD patients, but more frequently than GOLD 0 COPD patients (p< 0.01). Emphysema was more commonly reported in CT scans from patients with PRISm 70.3% (260/369, 95% CI 65.8– 75.3) than those with GOLD 0 COPD 64.1% (218/340, 95% CI 58.8– 69.2) (p< 0.05). PRISm status was not predictive of lung function decline.
Interpretation: One in four primary care patients with clinically diagnosed COPD in a large US registry fulfil the spirometric definition of PRISm rather than COPD, but suffers from emphysema in CT and significant respiratory symptoms.
Keywords: APEX, COPD, PRISm, US primary care, COPD exacerbations, electronic health records, patient reported outcomes
Introduction
Chronic obstructive pulmonary disease (COPD) represents a global healthcare challenge characterized by airflow limitation, systemic inflammation, and a significant impact on patients, quality of life.1–4 As part of standard guidance, COPD diagnosis requires an abnormal post-bronchodilator spirometry result with a pre-defined ratio of forced expiratory volume in the first second (FEV1) over forced vital capacity (FVC) of 0.7.5–7 It has been suggested that, beyond diagnosis of COPD and other conditions, spirometry testing helps to identify overall lung health.8 However, the clinical spectrum of what is diagnosed in primary care as COPD is heterogenous, and some patients present with spirometry results that challenge conventional diagnostic patterns, or receive a diagnosis without any confirmatory spirometry.9,10 In addition, COPD is often not diagnosed until a patient is hospitalised as a result of worsening of their lung condition encompassing a COPD exacerbation. A UK study identified that up to 85% of opportunities for early diagnosis were missed in Primary Care11 and may include symptomatic cases where spirometry does not meet the standard for diagnosis, yet may meet criteria for preserved ratio impaired spirometry (PRISm).
PRISm, defined as an FEV1/FVC ratio ≥0.7 combined with a reduced FEV1 predicted value of <80%, has emerged as a spirometric pattern observed in a subset of patients with clinical features suggestive of future incident COPD.12,13 PRISm prevalence has been shown to be around 6–20% worldwide, although geographical variation does occur.14–20 Unlike the more typical pattern of airflow limitation characterized by a reduced FEV1/FVC ratio, either by a fixed ratio <0.7 or <lower limit of normal (LLN), patients with PRISm present with higher or “preserved” FEV1/FVC ratio despite demonstrable airflow impairment, raising diagnostic dilemmas and challenging traditional definitions of COPD.10 PRISm is often associated with younger age, greater BMI, and a greater frequency of comorbidities, presenting as an alternative clinical entity prior to COPD.13 The earlier age of appearance could present opportunities for earlier intervention, diagnosis and management.13,21 Patients with PRISm have also been shown to have greater rates of morbidity and mortality than individuals with normal postbronchodilator spirometry, particularly related to cardiovascular events and risk.19,22–24 PRISm is not currently recognised within the definition of COPD developed by national and international guidelines, although it has been raised for potential inclusion in future expanded definitions.5 This muddying of objective rules for diagnosis underscores the value of clinician decision-making in the diagnostic process. While spirometry is a cornerstone in the evaluation of COPD, additional diagnostic tools, such as lung imaging, diffusion capacity measurements, and clinical assessments, may be required to confirm the diagnosis and rule out alternative respiratory conditions.25
In this study, we aim to determine the frequency and determinants of PRISm within a population of patients with diagnosed COPD served by US primary care providers, and how this subgroup compares to those whose spirometry results meet current COPD diagnostic criteria.
Methods
Design
This is a historical, observational registry study using patient data from the APEX in COPD registry. The APEX in COPD registry contains longitudinal Primary Care EHR data and a subset of patients with linked patient reported outcomes (PRO), spirometric, and CT scan data.26–28 The study was constructed, performed, and reported in accordance with all relevant regulatory/ethical requirements, namely: the European Network Centres for Pharmacoepidemiology and Pharmacovigilance (study reference number: EUPAS29401), study registration with the European Union Electronic Register of Post-Authorization studies was also undertaken; Approval for this study was granted by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee (ADEPT0520). Central ethics (Institutional Review Board, IRB) approval was obtained from the American Academy of Family Physicians for most sites (AAFP; IRB reference number: 19–349). Additional ethics approval was obtained from one site that required its own board to approve.
Patients
Patients from participating Primary Care sites were considered eligible if they were aged 35 years or older at time of COPD diagnosis, with the presence of a diagnostic code compatible with COPD (ICD9CM or ICD10CM), or a COPD compatible review code at any time in their EHR. This included bronchitis, emphysema, α1-antitrypsin deficiency, and mixed COPD/asthma. Patients were to be considered active if they had an appointment within the last two years, and with current COPD with either having (i) been diagnosed/re-coded with COPD in the last year or (ii) had a prescription for a COPD inhaler in the last two years and a COPD diagnosis (including diagnoses with an end date) or (iii) active patient reported COPD symptoms. According to our study protocol, explicitly investigating patients without spirometric confirmation of a COPD diagnosis, no positive result in spirometry (ie, post-BD FEV1/FVC ratio <0.7) was necessary for inclusion. When assigning groupings for lung-function decline and GOLD status, most patients had recorded values for post-bronchodilator spirometry (n=2415, 84.3%). In instances where post-bronchodilator findings were not available, pre-bronchodilator results (n=451, 15.7%) were used. When investigating longitudinal lung function decline, pre- and post-bronchodilator values were compared against results produced in the same manner. Predicted values were calculated in line with American Thoracic Society reference equations.29,30
Patients were excluded for any of the following criteria: (i) they were participating in a COPD drug therapy clinical trial at the time of enrollment (ii) had a life expectancy <12 months from the date of enrollment (iii) had an active cancer diagnosis in the last 3 years (excluding non-melanoma skin cancer) or (iv) were receiving palliative care.
Sites
For this study, sites within five healthcare organizations located in five US states, namely Texas, Ohio, Colorado, New York, and North Carolina, provided access to a limited EHR dataset through a data use agreement of all patients with COPD who did not specifically opt out of the project.
Freetext Analysis
Spirometry results were collected from EHR and through manual transcription from digital scans of reports. For CT scan analysis, text was scanned searching for a positive interpretation for emphysema and/or airway wall thickening. Synonyms within the search criteria for emphysema included: overinflation, bulla, low attenuation, and air sac destruction. Airway wall thickening was defined as the presence of a variety of interpretations, including: airway wall thickening, asthma, bronchitis, bronchiectasis, and presence of mucus. Responses were excluded when found to be in proximity to negative connections pre- or post-appearance, eg, “not”, “incorrectly diagnosed”, “exclude”, “nil”, “no”. There was a 5-word distance investigated pre/post-keyword and negative connections.
Statistical Analysis
We endorsed STROBE guidance for observational research, and the STROBE checklist is appended.31 STATA version 14 (College Station, TX, US) and R version 3.6 (Vienna, Austria) were used to conduct all statistical analyses and data handling. For the demographic, clinical, and patient reported elements of the study, descriptive statistics were calculated. All available data (non-missing) was summarized. Categorical variables were expressed as counts (%), while numerical variables were expressed as means (standard deviation).
For the exploratory analysis, multivariable logistic regression was performed. GOLD 0 was defined by the presence of symptoms of COPD, an FEV1/FVC ratio ≥0.7, and an FEV1 >80% predicted, and was used as the reference category to examine lung function decline.5 A threshold of lung function decline was defined as an annual decrease in FEV1 of >140 mL. Age at first spirometric reading, gender, smoking status, and baseline FEV1 were used as covariates. As the number of patients in severe and very severe GOLD airflow limitation were small, both were grouped; GOLD spirometric groups 1 and 2 were also combined. Statistical significance was set at p<0.05.
Results
Patient Population
A total of 2866 patients were identified to be eligible for analysis from the APEX COPD Registry for the purpose of the study (Figure 1). Additional patient-reported data was available for 1354 patients. PRISm was found to be present in 23.6% (678/2866) of patients with available spirometry. Among those meeting COPD obstruction criteria, 638 (22.3%) were categorised as GOLD 0, 211 (7.4%) were GOLD 1, 833 (29.1%) GOLD 2, 420 (14.7%) GOLD 3, and 86 (3.0%) GOLD 4 (Figure 2). In Table 1, those participants with PRISm were compared with those with obstructive COPD (post-BD ratio <0.7), and then with GOLD 0.
 |
Table 1 Demographic Characteristics of PRISm, GOLD 0 and Obstructive COPD Patients in the US APEX COPD Registry* |
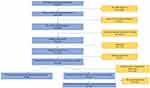 |
Figure 1 Flowchart of stages in patient inclusion and exclusion. |
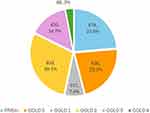 |
Figure 2 Prevalence of PRISm, obstructive COPD (GOLD 1 to 4) and of GOLD 0 groups in the APEX COPD Registry. |
Baseline Characteristics
Gender differed between populations, with the PRISm group showing more women (55.9%) compared to the COPD groups overall (46.9%, p<0.001), but fewer women than GOLD 0 population (60.5%, p=0.097) (Table 1). The age distribution was younger within the PRISm 66.3±11.1 years and GOLD 0 groups 66.3±11.5 years than in GOLD 1–4 COPD groups (69.2±10.4 p<0.001). The PRISm population had a higher mean BMI of 33.5±9.2, compared to the Obstructive COPD (GOLD 1 to 4) and GOLD 0 groups with a mean BMI of 27.8±7.2 and 30.6±7.8 respectively (both p<0.001). In examining ethnicity, while all groups had a majority of Caucasian individuals, the PRISm group had a similar percentage of Caucasians (62.8%) compared to the GOLD 0 (63.9%) (p=0.611), and significantly lower to the Obstructive COPD group (73.7%, p<0.001). Finally, patients in the Obstructive COPD group were more likely to be current or ex-smokers (93.0%), with PRISm having a much larger proportion of never smokers (17.0%) (all P<0.001).
Clinical Characteristics
All spirometry comparisons between PRISm and the other groups were highly significant, as grouping was according to spirometry thresholds. Overall, patients with COPD were more likely to have experienced an exacerbation in the previous year (49.8% vs 43.6 in PRISm and 39.5% in GOLD 0, both p<0.001) (Table 2). On symptomology, Obstructive COPD patients were more likely to have the presence of Chronic Bronchitis registered in the EHRs (63.1) than PRISm (56.3%) or GOLD 0 (44.4%) patients (all p<0.001), but interestingly PRISm had similar COPD Assessment Test scores ≥10 to the Obstructive COPD group but a higher proportion of modified Medical Research Council (mMRC) dyspnea scores ≥2 (p<0.001).
 |
Table 2 Clinical Characteristics of PRISm, GOLD 0 and Obstructive COPD Patients in the US APEX COPD Registry* |
CT Scans
CT scan data was available for 54.4% of PRISm patients, 53.3% of GOLD 0 patients and 51.4% of Obstructive COPD. Analysis of free text notes in CT scans revealed 70.5% of PRISm patients with available CT scans had a positive interpretation of emphysema in their notes (Table 3). This percentage was lower in the GOLD 0 population, but still substantial, where 64.1% also presented emphysema in some form or severity. Airway wall thickening was present in 34.4% of PRISm patients greater than the 25.9% of GOLD 0 patients, with more frequent bronchiectasis in PRISm group (32.0% vs 22.9%) (p<0.001). The GOLD 0 subgroup had a higher percentage of patients with a CT, but the PRISm subgroup had more CTs per patient (data not shown). Of note, the prevalence of both bronchiectasis and airway thickening was higher in PRISm than GOLD0, and quite similar to those with Obstructive COPD (Table 3), a finding that highlights the clinical relevance of PRISm.
 |
Table 3 Interpretation of CT Scans for Evidence of Lung Structural Change |
Lung Function Decline
The risk of decreasing lung function in PRISm and GOLD severity groups was analysed using multivariate logistic regression. PRISm (p=0.792) and other stages were not proven to be significant predictors of >140mL decrease in FEV1 over time (Table 4).
 |
Table 4 Logistic Regression Model for FEV1 Progression in PRISm and Obstructive COPD Populations |
Discussion
Summary of Results
To improve the diagnosis and management of COPD within Primary Care settings, it is essential to gain insights into the patient population investigated. Our study describes the burden of PRISm within a well-defined US cohort of diagnosed COPD patients who had spirometry assessment, with enhanced supplementary data of PRO, longitudinal spirometry, and CT scan imaging with text interpretation. PRISm represents a substantial subgroup (23.6%) of nearly one in four patients who were diagnosed with COPD despite not meeting current COPD diagnostic criteria. (GOLD 2023). While not meeting obstruction criteria, these patients present an opportunity for early recognition of respiratory symptom burden and management potentially at a younger age at presentation) compared to the obstructive COPD populations.
Under international standards for COPD concordant spirometry, those in our study who are allocated to the PRISm subgroup would be considered a COPD misdiagnosis. The differences in smoking status, lung function, and symptomatology are crucial factors in the recognition of early identification of chronic lung disease in the absence of confirmatory spirometry. This is demonstrated in the current study by the differences in symptoms between the groups. These are notable and provide valuable insights into the varying clinical profiles. Individuals with obstructive COPD, particularly in the Obstructive COPD (Ratio <0.7) and those with moderate COPD (Ratio <0.7 and %pred FEV1 <80%) groups, exhibit a higher prevalence of chronic bronchitis, indicating an advanced disease along the COPD severity progression. However, a greater percentage of individuals in the PRISm groups report high symptom burden, as evidenced by a CAT (COPD Assessment Test) score of ≥10, which represents an effect on activities of daily-life, similar to those with established Obstructive COPD and significantly greater mMRC, and therefore a recommendation for further management. The finding that a large proportion of PRISm patients have an mMRC of ≥2, reflects a high degree of dyspnea and functional impairment. This is compounded in the investigation of CT scans, where ~70% of PRISm and GOLD 0 patients with CTs have shown to have a positive interpretation of emphysema. However, this may be skewed higher due to confirmation bias, where arrangement of tests is clinician-driven, and is a result of recognition when the wider symptomology and supplementary tests suggest the presence of COPD without a confirmatory spirometry. These findings suggest that individuals with obstructive COPD may have reduced lung function compared to those with PRISm. However, they may not experience more severe and impactful respiratory symptoms, possibly due to receiving better management as a result of a confirmed diagnosis of COPD due to impaired airflow limitation. This finding highlights the clinical significance of COPD-related symptoms at the point of clinician-patient interaction. Interestingly, PRISm individuals had more dyspnoea with mMRC≥2 yet with less chronic bronchitis than in obstructive COPD, while the rate of CAT≥10 did not differ (Table 2); this apparent clinical inconsistency might be due to imbalance in age, gender and other respiratory determinants, or because of small sample sizes.
For individuals with PRISm, emphasis might be placed on preventive measures. This could include smoking cessation interventions, vaccinations, and lifestyle modifications to reduce the risk of developing COPD or exacerbations which could lead to further loss of lung function in the future. In contrast, for those with obstructive COPD, clinical decisions would likely revolve around optimizing disease management. Earlier recognition and diagnosis of COPD can allow for increased, personalization of individualized care plans considering comorbidities, age, and overall health status. Regular monitoring of lung function and symptom assessment would be integral to gauge treatment efficacy and adjust therapeutic strategies as needed.
Strengths of this study include its basis in primary care, longitudinal assessment and the single, protocolised assessment of all measurements and relevant PROs, exploring a well-studied COPD population. However, some limitations are worth considering. Overall, there was a lack of consistent, annual spirometry in the registry; however, this is representative of the need for regular scheduled tests and digitization of lung function reports into clinical systems. Similar issues occur with CT scans, where greater insight for management of disease could be provided to clinicians should results be clearly presented in the EHR. Perhaps, the very often used threshold of a lung decline of 140 mL in FEV1 per year could be challenged as arbitrary for progression within COPD severities; within our multivariate analyses those with moderate COPD were the closest to reach significance, as likely those with severe or very severe airflow limitation have no room to reach such a threshold, and later they are censored or right truncated to perform a valid spirometry. Should this be further investigated, it would be prescient to conduct a sensitivity analysis to explore a wider range of cut-offs to define annual lung function decline. Finally, real-world registry data is inherently lower in validity compared to clinical trial data, which would be more complete as part of the trial design and variable selection. Inherently, patients with higher severity of disease are more likely to have PFTs or imaging performed and recorded due to being further ahead in the progression of their disease. Imaging bias is likely mitigated by the fact that the majority of the CT scans were performed for lung cancer screening and not as part of a respiratory evaluation. To conduct an automatic quantification of emphysema or of airway wall thickening by AI or else was beyond the scope of our research. And we must insist as a strength of our study the free-text review of EHRs and radiologist reports. However, future research might include the search for radiologic abnormalities in PRISm using quantitative or parametric response mapping CTs.30 All of these limitations could be considered representative of real-world evidence in US primary care, collected over numerous sites and regions. The sheer size of the APEX dataset of >17,000 patients lends to its strength of being generalizable to the entire US population.
When spirometry results are inconclusive, clinicians can take a wider and more varied approach to a diagnosis of COPD. This includes considering the patient’s demographic and clinical situation, assessing spirometry metrics beyond just FEV1/FVC, arranging imaging and interpretation, evaluating symptoms using standardized tools, and inquiring about exacerbation history.
These insights also enable clinicians to provide more informed management decisions, such as lifestyle modifications, pharmacological options, pulmonary rehabilitation, and perhaps earlier vaccination with RSV, pneumococcus and else for younger than 65-yr individuals, to meet the specific needs of each patient. Giving strength to clinician decision-making must be reinforced with further tests, which become crucial for clarifying COPD diagnosis and management in cases where spirometry alone may not provide a clear diagnosis.
Conclusion
This study provides evidence that one in four patients currently diagnosed with COPD in Primary Care, actually fulfil the spirometric criteria of PRISm rather than COPD, with a symptom burden and rates of imaging of emphysema similar to those with established spirometric confirmed COPD.
Abbreviations
APEX, Advancing the Patient EXperience; BMI, Body mass index; CAT, COPD Assessment Test; CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; CT, Computed Tomography; EHR, electronic health record; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, The Global Initiative for Chronic Obstructive Lung Disease; LLN, limit of normal; mMRC, modified Medical Research Council; PRISm, Preserved Ratio Impaired Spirometry; PRO, Patient-Reported Outcomes.
Acknowledgments
We would also like to acknowledge Shilpa Suresh (MSc) and Thuy Tien Vuong (BSc) of the Observational and Pragmatic Research Institute (OPRI), Singapore, for editorial and formatting assistance which supported the development of this publication.
Funding
APEX COPD is established and maintained by Optimum Patient Care (OPC) Global Limited; and research was conducted by the Observational & Pragmatic Research Institute Pte Ltd (OPRI). The establishment of the APEX registry was initially co-funded by OPC Global and Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). OPC Global retains intellectual property rights to the APEX registry.
Disclosure
Yasir Tarabichi, Chester Fox and Joan B Soriano report no conflicts of interest in this work. Wilson Pace has received funding via subcontracts with his organization from CDC, PCORI, NIH, Boehringer Ingelheim, ONC, Tabula Rasa Healthcare, and Astra-Zeneca; his organization received consulting fees for his work from Boehringer Ingelheim; he is/was on an Advisory board and Executive Committee member (unpaid) for COPD Foundation 360 Network; he owns stock through a trust in Johnson and Johnson, Eli Lily, Novo-Nordisk, Pfizer, Novartis, Moderna, and Amgen; he received grant and writing support for an unrelated project from Boehringer Ingelheim and grant from Circasso for collecting FeNO data; and was an unpaid member of the Colorado Medicaid Provider Rate Review Committee. In addition, he provided free ICS and distribution support for a large PCORI around asthma care - clinical site management for entire project for Teva. Barry Make reports funding from the NHLBI for the COPDGene study; grants and medical advisory boards from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Regeneron, Sanofi and Sunovian; CME personal fees from WebMD, National Jewish Health, American College of Chest Physicians, American Lung Association, Projects in Knowledge, Hybrid Communications, SPIRE Learning, Ultimate Medical Academy, Catamount Medical, Eastern Pulmonary Society, Catamount Medical Communications Medscape, Eastern VA Medical Center, Academy Continued Healthcare Learning, and Mt. Sinai Medical Center; royalties from Up-To-Date, Wolters Kluwer Health; medical advisory boards from Novartis, Phillips, Third Pole, Science 24/7, and Verona; grants from Pearl, US Department of Defense; personal fees from Optimum Patient Care Global Limited, Quintiles, Web MD, outside the submitted work. He also presented at GOLD conference. MeiLan K. Han reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, Amgen, UpToDate, Altesa Biopharma, Medscape, NACE, MDBriefcase, Integrity and Medwiz. She has received either in kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation and the American Lung Association. She has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. She has received stock options from Meissa Vaccines and Altesa Biopharma. Alan Kaplan reports speakers bureau or Advisory Board for AstraZeneca, ALK, Belus, Boehringer Ingelheim, Covis, Eisai, GSK, Idorsia, Merck Frosst, Moderna, Novo Nordisk, Pfizer, Sanofi, Teva, Trudel, Valeo. Ku-lang Chang declares grant funding from Abbvie, Afimmune, Akero Therapeutics, Inc. Anji Pharma, Arrowhead Pharmaceuticals, Astrazeneca Pharmaceuticals, Axcella Health Inc, Boehringer Ingelheim Pharmaceuticals, Eli Lilly and Company, Enanta Pharmaceuticals, Inc, Esperion, Galectin Therapeutics, Gilead Sciences, Inc, Glympse Bio, Hanmi Pharmaceutical Co, LTD, Intercept Pharmaceuticals, Inventiva Pharma, Kowa Pharmaceuticals America, Inc, Lexicon Pharmaceuticals, Madrigal Pharmaceuticals, Inc, Merck & Co., Inc, Metacrine, Inc, Moderna, New Amsterdam Pharma, Novartis Pharmaceuticals, Novartis Pharmaceuticals Corp, Novo Nordisk, Romark Laboratories, Sagimet Biosciences, Sanofi Pasteur, Inc, Valneva, Viking Therapeutics, Zydus Discovery DMCC. Janwillem W. H. Kocks Janwillem Kocks reports grants, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, GSK; grants and personal fees from Chiesi, Teva; non-financial support from Mundi Pharma; personal fees from MSD, COVIS Pharma, ALK-Abello; grants from Valneva outside the submitted work; and Janwillem Kocks holds <5% shares of Lothar Medtec GmbH and 72.5% of shares in the General Practitioners Research Institute. Neil Skolnik is on advisory boards for AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Novartis, Bayer, Genentech, Abbott, Novo Nordisk, Heartland, Astellas, Proteomics International, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline; Payment for lectures/speaking engagements from AstraZeneca and Boehringer Ingelheim; Research Support from Sanofi, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. Barbara Yawn received fees for consulting and serving on COPD-related advisory boards for GlaxoSmithKline, AstraZeneca, Novartis, Boehringer Ingelheim, and Teva; received travel support from Boehringer Ingelheim and AstraZeneca for international presentations and received COPD-related investigator-initiated research funds from GlaxoSmithKline, Sanofi, Boehringer Ingelheim, AstraZeneca, Mylan and Novartis. Alexander Evans is an employee of Observational and Pragmatic Research Institute. Victoria Carter is an employee of Optimum Patient Care Ltd. Chantal Le Lievre, Alexander Roussos, and Nicholas Bushell are employees of Optimum Patient Care, Australia. David Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Inside Practice, GlaxoSmithKline, Medscape, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Medscape, Teva Pharmaceuticals.; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. Adeloye D, Song P, Zhu Y, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi:10.1016/S2213-2600(21)00511-7
2. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147
3. Hurst JR, Skolnik N, Hansen GJ, et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Internal Med. 2020;73:1–6. doi:10.1016/j.ejim.2019.12.014
4. Selzler A-M, Habash R, Robson L, et al. Self-efficacy and health-related quality of life in chronic obstructive pulmonary disease: a meta-analysis. Patient Educ Couns. 2020;103(4):682–692. doi:10.1016/j.pec.2019.12.003
5. 2024 GOLD Report. Global initiative for chronic obstructive lung disease – GOLD. Available from: https://goldcopd.org/2024-gold-report/.
6. Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Primary Care Respir J. 2009;18(3):216–223. doi:10.4104/pcrj.2009.00055
7. Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest. 2006;129(6):1509–1515. doi:10.1378/chest.129.6.1509
8. Bakke PS, Rönmark E, Eagan T, et al. Recommendations for epidemiological studies on COPD. Eur Respir J. 2011;38(6):1261–1277. doi:10.1183/09031936.00193809
9. Schermer TR, Robberts B, Crockett AJ, et al. Should the diagnosis of COPD be based on a single spirometry test? NPJ Primary Care Res Med. 2016;26(1):1–8. doi:10.1038/npjpcrm.2016.59
10. Katherine E, Lowe M, Anzueto A. COPDGene® 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chron Obstructive Pulm Dis. 2019;6(5):384–399. doi:10.15326/jcopdf.6.5.2019.0149
11. Jones RCM, Price D, Ryan D, et al. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med. 2014;2(4):267–276. doi:10.1016/S2213-2600(14)70008-6
12. Adibi A, Sadatsafavi M. Looking at the COPD spectrum through “PRISm”. Eur Respir J. 2020;55(1):1902217. doi:10.1183/13993003.02217-2019
13. Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15(1):89. doi:10.1186/s12931-014-0089-y
14. Kim J, Lee C-H, Lee HY, Kim H. Association between comorbidities and preserved ratio impaired spirometry: using the Korean national health and nutrition examination survey IV–VI. Respiration. 2022;101(1):25–33. doi:10.1159/000517599
15. He D, Sun Y, Gao M, et al. Different risks of mortality and longitudinal transition trajectories in new potential subtypes of the preserved ratio impaired spirometry: evidence from the English longitudinal study of aging. Front Med. 2021;8:755855. doi:10.3389/fmed.2021.755855
16. Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the first national health and nutrition examination survey follow up study. Thorax. 2003;58(5):388–393. doi:10.1136/thorax.58.5.388
17. Guerra S, Sherrill DL, Venker C, et al. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(6):499–504. doi:10.1136/thx.2009.126052
18. Kaise T, Sakihara E, Tamaki K, et al. Prevalence and characteristics of individuals with preserved ratio impaired spirometry (PRISm) and/or impaired lung function in Japan: the OCEAN study. Int J Chronic Obstr. 2021;Volume 16:2665–2675. doi:10.2147/COPD.S322041
19. Higbee DH, Granell R, Smith GD, Dodd JW. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK biobank cohort analysis. Lancet Respir Med. 2022;10(2):149–157. doi:10.1016/S2213-2600(21)00369-6
20. Mannino DM, McBurnie MA, Tan W, et al. Restricted spirometry in the burden of lung disease study. Int J Tuberc Lung Dis. 2012;16(10):1405–1411. doi:10.5588/ijtld.12.0054
21. Park HJ, Byun MK, Rhee CK, et al. Significant predictors of medically diagnosed chronic obstructive pulmonary disease in patients with preserved ratio impaired spirometry: a 3-year cohort study. Respir Res. 2018;19(1):1–11. doi:10.1186/s12931-018-0896-7
22. Krishnan S, Tan WC, Farias R, et al. Impaired Spirometry and COPD Increase the Risk of Cardiovascular Disease: a Canadian Cohort Study. Chest. 2023;164(3):637–649. doi:10.1016/j.chest.2023.02.045
23. Zheng J, Zhou R, Zhang Y, et al. Preserved ratio impaired spirometry in relationship to cardiovascular outcomes: a large prospective cohort study. Chest. 2023;163(3):610–623. doi:10.1016/j.chest.2022.11.003
24. Wijnant S, De Roos E, Kavousi M, et al. Preserved ratio impaired spirometry (PRISm) and mortality: the Rotterdam study. Eur Respir J. 2019;54(suppl 63):PA4421.
25. Wei X, Ding Q, Yu N, et al. Imaging features of chronic bronchitis with preserved ratio and impaired spirometry (PRISm). Lung. 2018;196(6):649–658. doi:10.1007/s00408-018-0162-2
26. Fox C, Pace W, Brandt E, et al. Variation in demographic and clinical characteristics of patients with COPD receiving care in US primary care: data from the advancing the patient experience (APEX) in COPD registry. POR. 2022;13:17–31. doi:10.2147/POR.S342736
27. Edwards CL, Kaplan AG, Yawn BP, et al. Development of the advancing the patient experience in COPD registry: a modified delphi study. Chronic Obstr Pulm Dis. 2021;8(1):135–151. doi:10.15326/jcopdf.2020.0154
28. Yawn B, Kaplan A, Pace WD, et al. Advancing the patient experience (APEX) in COPD registry: study design and strengths. J Am Board Fam Med. 2021;34(1):22–31. doi:10.3122/jabfm.2021.01.200351
29. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi:10.1164/ajrccm.159.1.9712108
30. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Res J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312
31. Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Relationship Between BMI and Lung Function in Populations with Different Characteristics: A Cross-Sectional Study Based on the Enjoying Breathing Program in China
Tang X, Lei J, Li W, Peng Y, Wang C, Huang K, Yang T
International Journal of Chronic Obstructive Pulmonary Disease 2022, 17:2677-2692
Published Date: 18 October 2022
Clinical Concepts for Triple Therapy Use in Patients with COPD: A Delphi Consensus
Miravitlles M, Acharya S, Aggarwal B, Fernandes FL, Dreyse J, Jardim JR, Juthong S, Levy G, Sivori M
International Journal of Chronic Obstructive Pulmonary Disease 2023, 18:1853-1866
Published Date: 28 August 2023

