Back to Journals » Journal of Inflammation Research » Volume 17
Pretreatment with Indole-3-Propionic Acid Attenuates Lipopolysaccharide-Induced Cardiac Dysfunction and Inflammation Through the AhR/NF-κB/NLRP3 Pathway
Authors Zhang Y , Li S, Fan X, Wu Y
Received 4 April 2024
Accepted for publication 7 August 2024
Published 13 August 2024 Volume 2024:17 Pages 5293—5309
DOI https://doi.org/10.2147/JIR.S466777
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yiqiong Zhang,1,2,* Shanshan Li,1,2,* Xiaojuan Fan,1 Yue Wu1,2
1Department of Cardiovascular Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 2Key Laboratory of Environment and Genes Related to Diseases, Ministry of Education, Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaojuan Fan; Yue Wu, Email [email protected]; [email protected]
Background: Patients with sepsis frequently develop septic cardiomyopathy, which is known to be closely related to excessive inflammatory responses. Indole-3-propionic acid (IPA) is a tryptophan metabolite with anti-inflammatory properties that have been demonstrated in various studies. In this study, we investigated the underlying mechanisms and therapeutic role of IPA in septic cardiomyopathy.
Methods: To investigate the role of IPA in septic cardiomyopathy, we constructed a lipopolysaccharide (LPS)-induced rat model of septic cardiomyopathy, and treated rats with IPA. Inflammatory factors and the NF-κB/NLRP3 pathway were evaluated in myocardial tissues and cells after IPA treatment using RT-qPCR, ELISA, Western blotting, and immunohistochemistry. To further elucidate the role of the aryl hydrocarbon receptor (AhR), we detected changes in inflammatory mediators and the NF-κB/NLRP3 pathway in in vivo and in vitro models of septic cardiomyopathy, which were treated with the AhR antagonist CH-223191 and/or AhR agonist FICZ.
Results: IPA supplementation improved cardiac dysfunction in rats with septic cardiomyopathy. IPA reduced inflammatory cytokine release and inhibited NF-κB/NLRP3 signaling pathway in myocardial tissue and in H9c2 cells. CH-223191 impaired the anti-inflammatory effect of IPA in LPS-treated cells, whereas FICZ exerted the same effect as IPA. IPA also exhibited anti-inflammatory activity by binding to the AhR. Our results indicated that IPA attenuated septic cardiomyopathy in rats via AhR/NF-κB/NLRP3 signaling.
Conclusion: Our study revealed that IPA improved left heart dysfunction and myocardial inflammation caused by sepsis via AhR/NF-κB/NLRP3 signaling, suggesting that IPA is a potential therapy for septic cardiomyopathy.
Keywords: Indole-3-propionic acid, septic cardiomyopathy, cardiac function, inflammation, aryl hydrocarbon receptor
Introduction
Systemic inflammatory response syndrome (SIRS), defined as sepsis, is a condition induced by infection or infectious factors. Unless controlled promptly, septic shock can progress to become life-threatening.1 Sepsis remains a major threat to global health, despite declining morbidity and mortality. In 2017, there were 48.9 million cases of sepsis and 11 million deaths linked to sepsis worldwide, accounting for 19.7% of all deaths.2,3 Septic cardiomyopathy is a common complication of sepsis or septic shock, and typically manifests as left ventricular dilatation, reduced ejection fraction, and decreased contractility.4 The pathogenesis of sepsis-induced cardiac dysfunction includes the dysregulation of inflammatory mediators, mitochondrial dysfunction, oxidative stress, and endothelial dysfunction.5 Inhibiting one or more of these processes would be of great therapeutic significance for septic cardiomyopathy.
In the last few years, increasing attention has been paid to the function of intestinal flora metabolites. These metabolites are biologically active and have various functions. Indole-3-propionic acid (IPA), an intestinal flora metabolite of dietary tryptophan, has been shown to have various effects in different studies.6 Fang et al found that IPA attenuated sepsis by improving intestinal flora disorders in septic mice.7 Huang et al revealed that IPA promoted macrophage phagocytosis and mitigated liver and lung damage by activating the aryl hydrocarbon receptor (AhR).8 IPA protected against diastolic dysfunction and reduced myocardial inflammation in heart failure with preserved ejection fraction (HFpEF).9 IPA pretreatment has been shown to effectively inhibit the upregulation of Toll-like receptor 4 (TLR4), MyD88, and nuclear factor-kappaB (NF-κB) and subsequent maturation of IL-1β and NLRP3 inflammasome in lipopolysaccharide (LPS)-treated C2C12 cells.10 IPA administration has also been shown to attenuate chondrocyte inflammation and extracellular matrix degradation via the AhR/NF-κB axis in in vivo and in vitro models of osteoarthritis.11 Additionally, IPA could repress proinflammatory cytokine levels and improve liver injury in non-alcoholic steatohepatitis (NASH) rats.12 Therefore, IPA has anti-inflammatory effects in several organs, but its role in septic cardiomyopathy and cardiomyocytes is unclear.
The AhR is a ligand-activated transcription factor. It has been reported that IPA could alleviate metabolic disorders13 and attenuate inflammation through its receptor AhR.8,11 The TLR4, NF-κB and NOD-like receptor 3 (NLRP3) pathways have gained considerable attention in septic cardiomyopathy over the past few years. Various studies have shown that suppression of the TLR4/NF‐κB/NLRP3 pathway can improve left ventricular dysfunction and alleviate myocardial inflammation in mice with septic cardiomyopathy.14,15 Additionally, Sun et al revealed that IPA upregulated AhR production, inhibited the NF‐κB/NLRP3 signaling pathway, and reduced the release of inflammatory cytokines in APP/PS1 mice.16 Fang et al also demonstrated that IPA exerted neuroprotective effects in mice with sepsis-related encephalopathy by inhibiting the NLRP3 inflammasome.17 Therefore, we assume that IPA supplementation inhibits the TLR4/NF‐κB/NLRP3 signaling pathway and attenuates myocardial injury in septic cardiomyopathy rats.
We assessed the role of IPA in an animal model of septic cardiomyopathy. Our findings indicate that IPA enhances cardiac function via the AhR/NF-κB/NLRP3 axis. This study is the first to investigate the therapeutic benefits of IPA for septic cardiomyopathy, providing new insights into the treatment of this condition.
Methods
Materials
LPS and IPA were obtained from Sigma (St Louis, MO, USA). AhR agonist FICZ, AhR antagonist CH-223191, and NF-κB antagonist BAY 11–7082 were purchased from Medchem Express (Shanghai, China). Primary antibodies against IL-1β, IL-6, TNF-α, MyD88, caspase-1, NLRP3, and CD68 were purchased from Abcam (Cambridge, UK). Primary antibodies against NF-κB p65 and Phospho-NF-κB p65 were acquired from Cell Signaling Technology (Danvers, MA, USA). Primary antibodies against GSDMD, GAPDH, and Vinculin were purchased from Proteintech Group (Rosemont, USA). The secondary antibodies against rabbits and mice were acquired from Cell Signaling Technology (Danvers, MA, USA). ELISA kits for rat cardiac troponin T (c-TnT) and brain natriuretic peptide (BNP) levels were obtained from Elabscience Biotechnology (Wuhan, China). And the IPA ELISA kit was obtained from Shyqbio (Shanghai, China). H9c2 cells and raw264.7 cells were purchased from Procell Life Science&Technology Co, Ltd. (Wuhan, China). All other chemicals in this study were analytical grade.
Animals
The Animal Care and Use Committees of Xi’an Jiaotong University (Xi’an, Shaanxi, China) approved all animal procedures for animal welfare. The experiments followed the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011). The rats were maintained at 26°C under a 12:12 h light/12:12 h dark cycle for two weeks prior to the experiment, with free access to food and water.
Male Sprague–Dawley rats (8 weeks) were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). The rats were randomly divided into the following four groups: (1) Control group (Control), in which rats were intraperitoneally administered saline; (2) LPS group (LPS), in which rats were intraperitoneally administered LPS (10 mg/kg);18–20 (3) IPA group (IPA), in which rats were treated with IPA (20 mg/kg/day)8,11,12 by gavage for 7 consecutive days before saline injection; (4) LPS+IPA group (LPS+IPA), in which rats were treated with IPA (20 mg/kg/day) by gavage for 7 consecutive days before LPS injection; and (5) LPS+IPA+CH-223191 group (LPS+IPA+CH), in which rats were intraperitoneally administered CH-223191 (5 mg/kg/day)8 for 7 consecutive days before IPA gavage.
Echocardiography
After anesthesia with isoflurane, echocardiograms were performed on rats using a MyLab ultrasound (Esaote SpA, Genoa, Italy). Cardiac function was evaluated 24 h after saline or LPS administration, and the following parameters were collected: ejection fraction (EF), fractional shortening (FS), left ventricle (LV) end-systolic diameter (LVEDd), and LV end-diastolic diameter (LVEDs).
Elisa
At the end of the experiment, the rats were sacrificed, and serum was collected. The serum levels of cardiac Troponin T (cTn-T), B-type natriuretic peptide (BNP) proteins and IPA were determined according to the manufacturer’s instructions.
Immunohistochemistry
After dewaxing, dehydration, and antigen repair, heart tissue sections were treated with 3% H2O2 for 10 min and 10% goat serum for 30 min at 37°C to remove endogenous peroxidase. The slides were incubated overnight at 4°C in a CD68 primary antibody solution. Subsequently, the sections were incubated with a secondary antibody against rabbit IgG at 37°C for 1 h and with DAB for 5 min at room temperature. Finally, the sections were observed under a microscope and photographed.
Tunel
Paraffin sections of heart tissue were deparaffinized in xylene for 10 min followed by fresh xylene for 10 min. Sections were deparaffinized sequentially with anhydrous ethanol for 5 min, 90% ethanol for 2 min, 70% ethanol for 2 min, distilled water for 2 min, and then DNase-free protease potassium (20 μg/mL) was added to the sections. After incubation at 37°C for 30 min, the potassium protease was washed 3 times with phosphate-buffered saline. TUNEL test solution was then added and incubated without light at 37°C for 1 h. The sections were then washed 3 times with phosphate-buffered saline, and finally sealed with fluorescence quenching sealing solution and observed under the fluorescence microscope. The apoptosis level of cardiomyocyte was assessed by calculating the ratio of TUNEL-positive cells to the total cells.
Cell Culture and Treatment
H9c2 cells were cultured in high-glucose Dulbecco’s modified eagle medium (DMEM, Gibco) with 1% penicillin/streptomycin and fetal bovine serum (FBS; 10%). When the cell density reached 70%–80%, H9c2 cells were primed with different drug treatments. The groups were as follows: (1) control group; (2) LPS group, in which the cells were exposed to LPS (10 μg/mL) for 24 h to induce cardiomyocyte injury;21–23 (3) IPA group, in which the cells were exposed to IPA (500 μM) for 24 h;12,24 (4) LPS+IPA group, in which the cells were treated with LPS and IPA for 24 h; (5) LPS+IPA+CH-223191 group, in which the cells were treated with CH-2232191 (10 μM) 2 h before LPS and IPA stimulation;9,25 (6) LPS+FICZ group, in which the cells were treated with LPS and FICZ (400 nM) for 24h;26 (7) LPS+BAY 11–7082 group, in which the cells were treated with BAY 11–7082 (10 μM) 2 h before LPS stimulation;27,28 (8) LPS+BAY 11–7082+IPA group, in which the cells were treated with BAY 11–7082 (10 μM) 2 h before LPS and IPA stimulation. After these treatments, cells from different groups were collected for protein or RNA extraction and analysis.
Raw264.7 cells were cultured in in high-glucose Dulbecco’s modified eagle medium (DMEM, Gibco) with 1% penicillin/streptomycin and fetal bovine serum (FBS; 10%). Cells were pretreated with IPA (500 μM)12 for 1 h, followed by 500 ng/mL lipopolysaccharide treatment for an additional 6h. After these treatments, cells from different groups were collected for protein or RNA extraction and analysis.
Western Blot
Proteins and cell lysates from the ventricle tissue were extracted using RIPA. Next, 40 μg of proteins were separated on SDS/PAGE gels and transferred to 0.22-μm PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk in TBS containing 0.1% Tween-20 (TBST) for 1 h and then incubated with primary antibodies overnight at 4°C. After being washed with TBST for 30 min, the membranes were incubated with the appropriate secondary antibodies for 1 h at room temperature. After a 30-min wash, the membranes were visualized using a chemiluminescence apparatus. The band intensities were measured and quantified using ImageJ.
Real-Time PCR
RNA was isolated from the cells using an RNA Isolation Kit (Takara, 9767) following the manufacturer’s protocol. Subsequently, cDNA was synthesized using a PrimeScript RT kit (Takara, RR047A). The target genes were amplified and quantified using Fast Start Essential DNA Green Master (Roche, 06924204001). The obtained data were analyzed using the 2−ΔΔCq method, with GAPDH serving as the internal standard control. The primers used in our study were as follows: GGCACAGTCAAGGCTGAGAATG (forward) and ATGGTGGTGAAGACGC-CAGTA (reverse) for rat GAPDH; CCGTTTCTACCTGGAGTTTGT (forward) and GTTTGCCGAGTAGACCTCATAG (reverse) for rat IL-6; CGTGTTCATCCGTTCTCTACC (forward) and GCAATCCAGGCCACTACTT (reverse) for rat TNF-α; GGTGAAGGTCGGTGTGAACG (forward) and CTCGCTCCTGGAAGATGGTG (reverse) for raw cells GAPDH; GGTGTGTGACGTTCCCATTA (forward) and ATTGAGGTGGAGAGCTTTCAG (reverse) for raw cells IL-1β; TAGTCCTTCCTACCCCAATTTCC (forward) and TTGGTCCTTAGCCACTCCTTC (reverse) for raw cells IL-6; CGATGGGTTGTACCTTGTCTAC (forward) and GCAGAGAGGAGGTTGACTTTC (reverse) for raw cells TNF-α.
Statistical Analysis
All results are presented as the mean ± SD and were analyzed using GraphPad Prism 9.0. Multiple comparisons among different groups were analyzed by one-way ANOVA followed by Bonferroni’s test (multiple groups). Statistical significance was considered when P < 0.05.
Results
IPA Improved Cardiac Dysfunction in Septic Cardiomyopathy
After IPA gavage for 7 consecutive days, the concentration of IPA in the serum of rats was significantly elevated (Figure S1). We investigated the effects of IPA on cardiac function and myocardial damage in a rat model of septic cardiomyopathy. Our findings revealed that IPA reduced LPS-induced left ventricular dysfunction and improved EF and FS (Figure 1A–D). In addition, we examined the serum BNP and Troponin T levels in each group. The results showed that IPA administration reversed elevated BNP and Troponin T levels after LPS stimulation, which in turn reduced myocardial damage (Figure 1E and F). Therefore, we conclude that IPA treatment prevents LPS-induced cardiac damage.
IPA Attenuated Cardiac Inflammation and Inhibited NF-κB/NLRP3 Signaling in Septic Cardiomyopathy
We detected changes in inflammatory factors in the rat myocardium. The expression levels of IL-1β, IL-6, and TNF-α were significantly elevated in the LPS group, but not in the control group. However, after IPA administration, the levels of IL-1β, IL-6, and TNF-α were significantly reduced (Figure 2A–D). We also examined macrophage infiltration in the myocardial tissues of each group. The data demonstrated that macrophage infiltration was attenuated in the myocardial tissues of rats with septic cardiomyopathy supplemented with IPA (Figure 2E and F). And we found that IPA attenuated LPS-induced macrophage inflammatory activation (Figure S2).
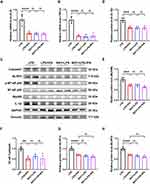
We evaluated the effects of IPA on myocardial inflammatory pathways in rats with septic cardiomyopathy. The results indicated that the protein levels of MyD88 and NF-κB, which are downstream of TLR4, were increased by LPS treatment (Figure 3A–C). In addition, the expression of caspase-1 and NLRP3 significantly increased in septic cardiomyopathy, suggesting activation of the NLRP3 inflammasome (Figure 3D and E). However, IPA supplementation inhibited the activation of the above proteins. Furthermore, TUNEL staining showed that LPS injection significantly induced cardiomyocyte apoptosis, which was attenuated by IPA treatment (Figure 3F and G). Thus, IPA attenuated cardiac inflammation and suppressed the activation of the NF-κB/NLRP3 signaling pathway in LPS-treated rats.
Protective Effects of IPA are Dependent on AhR
Previous studies have demonstrated that IPA exerts its anti-inflammatory activity via the AhR.8,11 Hence, we hypothesized that IPA exerts cardioprotective effects via the AhR. To verify our hypothesis, we treated septic rats with CH-223191. We found that treatment with CH-223191 significantly suppressed the protective effect of IPA on the hearts of septic rats. Rats in the IPA+CH223191 group had worse cardiac function (Figure 4B and C) and more severe myocardial injury (Figure 4D and E) than those in the LPS+IPA group. Additionally, the expression of proinflammatory factors was upregulated in CH-223191- and IPA-treated septic rats (Figure 4F–I). We then examined the expression of proteins associated with the NF-κB/NLRP3 pathway, and found elevated protein expression of MyD88, caspase-1, and NLRP3, and increased phosphorylation of NF-κB (Figure 5). These results show that CH223191 weakens the inhibitory effect of IPA on the NF-κB/NLRP3 pathway. In summary, IPA played an anti-inflammatory role by activating the AhR.
IPA Reduced Inflammation and Inhibited NF-κB/NLRP3 Signaling in a Septic Cardiomyopathy Cell Model
We next examined the anti-inflammatory effects of IPA in in vitro models. The results showed that the mRNA expression levels of IL-6 and TNF-α were significantly elevated in H9c2 cells after LPS stimulation (Figure 6A and B). However, their elevated expression levels were reversed after IPA treatment. We also evaluated the effect of IPA on the NF-κB/NLRP3 pathway in LPS-treated cells. Similarly, the LPS-induced upregulation of proteins such as MyD88, NF-κB and NLRP3 was reversed by IPA treatment. (Figure 6C–H). Our data demonstrated that IPA played a protective role against LPS-treated H9c2 cells by inhibiting the inflammatory pathway.
AhR Mediated the Beneficial Effects of IPA
We further investigated the role of AhR in in vitro experiments. We treated H9c2 cells with the AhR antagonist CH-223191. Consistent with previous results, IPA reversed the expression of IL-6 and TNF-α, and suppressed the NF-κB/NLRP3 signaling pathway. After treatment with CH-223191, the mRNA expression of inflammatory factors was elevated (Figure 7A and B), and NF-κB/NLRP3 pathway-related proteins were activated (Figure 7C–H). These results are consistent with those of in vivo experiments and indicated that the cardioprotective effect of IPA depended on AhR activation.
AhR Activation Inhibited Inflammation and NF-κB/NLRP3 Signaling
To further explore the effect of AhR, H9c2 cardiomyocytes were treated with the AhR agonist, FICZ. The RT-PCR results showed that FICZ reduced the expression of IL-6 and TNF-α (Figure 8A and B). We also examined the effect of FICZ on the NF-κB/NLRP3 pathway in LPS-treated cardiomyocytes. The upregulation of MyD88, NF-κB, and NLRP3 expression was reversed by FICZ treatment (Figure 8C–H). These results demonstrated that AhR activation exerted an anti-inflammatory effect, which was consistent with the results of IPA-treated cells.
The Effect of IPA Was Approved by AhR / NF-κB / NLRP3 Pathway
We further investigated the involvement of NF-κB / NLRP3 in the effects of IPA on inflammation in in vitro models. We treated H9c2 cells treated with NF-κB antagonist, BAY 11–7082. The mRNA expression levels of TNF-α and IL-6 were successfully alleviated with IPA treatment in LPS-treated cells. However, this therapeutic effect of IPA was weakened in cells treated by BAY 11–7082 (Figure 9A and B). Consistently, Western blotting revealed that IPA significantly inhibited the expression of NF-κB/NLRP3 pathway related proteins, whereas BAY 11–7082 attenuated the protective effect of IPA (Figure 9C–H). The findings indicated that NF-κB inhibition blunted the effect of IPA in alleviating LPS-caused inflammation.
Discussion
IPA inhibited cardiomyocyte inflammation and attenuated cardiac dysfunction in septic cardiomyopathy through the AhR/NF-kB/NLRP3 mechanism. Our in vivo findings showed that IPA protected cardiac function by attenuating cardiomyocyte inflammation. Furthermore, in vitro studies have demonstrated that IPA activated AhR and inhibited the NF-κB/NLRP3 pathway in cardiomyocytes. Our findings indicated that IPA had protective effects against septic cardiomyopathy.
Sepsis is defined as a dysregulated host response to infection that results in life-threatening multiple organ dysfunction. Prolonged inflammation may lead to persistent organ damage and long-term mortality.29 Sepsis often causes congestive heart failure with reduced ejection fraction, which worsens patient prognosis and increases mortality.30,31 Several studies have attempted to elucidate the pathophysiological mechanisms of septic cardiomyopathy. The release of inflammatory factors, infiltration of macrophages, and activation of inflammatory pathways have been observed in LPS-treated animal models.14,32 These findings suggest that inflammation in cardiomyocytes contributes to the progression of septic cardiomyopathy. Our study showed that intraperitoneal injection of LPS (10 mg/kg) into rats for 24 h not only induced septic myocardial dysfunction and myocardial injury, but also promoted the expression of inflammatory factors in cardiac tissue, including IL-1β, TNF-α, and IL-6.
The NF-κB/NLRP3 signaling pathway is critical for the pathophysiology of septic cardiomyopathy. Upon LPS stimulation, TLR4 was specifically activated, followed by activation of the downstream NF-κB/NLRP3 signaling pathway to produce inflammatory cytokines.33,34 IL-1β, TNF-α, and IL-6 are major inflammatory factors contributing to myocardial dysfunction in sepsis. NF-κB is activated and upregulates NLRP3 expression following LPS recognition by TLR4. The NLRP3 inflammasome participated in the development of septic cardiomyopathy, and inhibition of the NLRP3 signaling axis reduced LPS-induced cardiac injury.35 In the present study, we examined changes in NF-κB/NLRP3 pathway-related proteins in patients with septic cardiomyopathy. We found that the NF-κB/NLRP3 pathway was activated in LPS-treated in vivo models, and we obtained the same results by treating H9c2 cells with LPS. Our results confirm the rapid and severe cardiac inflammation caused by sepsis.
Gut microbiota and its metabolites are associated with human health and disease.36–38 Tryptophan is an essential amino acid critical for maintaining physiological homeostasis. Dietary tryptophan is mainly converted to IPA via Clostridium sporogenes in the intestine.39 IPA regulated intestinal barrier function and decreased intestinal inflammation by binding to the pregnane X receptor (PXR).40,41 In septic mice, IPA reduced sepsis-induced mortality by modulating intestinal flora and reducing the serum levels of inflammatory cytokines.7 In human and murine models of sepsis, IPA has been shown to improve survival and host control of infection.8 IPA supplementation attenuated diastolic dysfunction, oxidative stress, inflammation, and intestinal epithelial barrier damage in the preserved ejection fraction (HFpEF) mouse model.9 Our study showed that IPA treatment attenuated cardiac dysfunction and myocardial inflammatory injury in rats with septic cardiomyopathy. The expression of the pro-inflammatory factors IL-1β, IL-6, and TNF-α was significantly lower in the LPS + IPA group than in the LPS group. In addition, IPA inhibited the expression of NF-κB/NLRP3 pathway-associated proteins, including NF-κB, MyD88, caspase-1, and NLRP3. Similarly, IPA inhibited NF-κB/NLRP3 pathway activation and reduced mRNA expression of inflammatory factors in LPS-treated H9c2 cells. In addition, IPA inhibited the expression of pyroptosis-related protein GSDMD in LPS-induced cells (Figure S3). Our results revealed that IPA improved cardiac function and myocardial inflammatory injury in an in vivo and in vitro model of septic cardiomyopathy.
The AhR is a ligand-activated transcription factor that integrates environmental, dietary, microbial, and metabolic cues to regulate complex transcriptional programs.42 The AhR is expressed in multiple tissues and is involved in many aspects of health and disease. An increasing number of studies have shown that endogenous metabolites exert anti-inflammatory effects in various tissues by activating the AhR. Zhuang et al found that 3-IAld (Indole-3-aldehyde) attenuated inflammation in IL-1β-induced chondrocytes through the AhR/NF-κB signaling pathway.43 Qiao et al revealed that quinolinic acid suppressed NLRP3 inflammasome activation and inflammatory cytokine secretion by activating AhR in a model of psoriasis.44 Moreover, dietary supplementation with tryptophan or AhR ligands protected mice against E. coli-induced mastitis by activating the AhR.45 These studies demonstrated that AhR activation inhibits the NF-κB/NLRP3 pathway, which in turn exerts anti-inflammatory effects. To verify that AhR mediates the anti-inflammatory effects of IPA, we treated septic rats and LPS-intervened H9c2 cells with the AhR antagonist CH-223191. Our results showed that supplementation with CH-223191 attenuated the cardioprotective effects of IPA in septic rats. Compared to the LPS+IPA group, the LPS+IPA+CH-223191 group exhibited the aggravation of myocardial injury and activation of the NF-κB/NLRP3 inflammatory pathway. We obtained the same results in H9c2 cells treated with CH-223191. We also used FICZ (AhR agonist) to treat H9c2 cells. We found that AhR activation inhibited the NF-κB/NLRP3 pathway and reduced the release of inflammatory factors. Finally, we treated LPS-induced cells with the NF-κB antagonist BAY 11–7082. We found that BAY 11–7082 could impair the anti-inflammatory function of IPA. The results revealed that the effect of IPA was approved by AhR/NF-κB/NLRP3 pathway. Our results demonstrated that inhibition of the AhR diminished the anti-inflammatory effects of IPA, whereas activation of the AhR produced the same anti-inflammatory effects, revealing that the endogenous IPA suppressed the NF-κB/NLRP3 signaling pathway via AhR activation.
Our study has some limitations. A major shortcoming is that we did not validate the function of AhR in knockout mice, which should be investigated in future studies. In addition, our animal and cellular models were obtained from rats, whereas primary cardiomyocyte samples from human patients would make this work even more meaningful.
Conclusion
Collectively, our results demonstrated that IPA attenuated cardiomyocyte inflammation and suppressed NF-κB/NLRP3 signaling partially through the AhR, which effectively improved cardiac function and alleviated myocardial inflammation in LPS-induced septic cardiomyopathy rats. This treatment offers a potentially therapeutic strategy for septic cardiomyopathy.
Acknowledgments
This paper is available as a preprint on [Research Square] at: [https://www.researchsquare.com/article/rs-3993405/v1].
This paper is available as a preprint on [Sciety] at: [https://sciety.org/articles/activity/10.21203/rs.3.rs-3993405/v1].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Key R&D Program of China (2019YFA0802300 to Yue Wu).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/S0140-6736(18)30696-2
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
3. Kempker JA, Martin GS. A global accounting of sepsis. Lancet. 2020;395(10219):168–170. doi:10.1016/S0140-6736(19)33065-X
4. Flynn A, Mani BC, Mather PJ. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart Fail Rev. 2010;15(6):605–611. doi:10.1007/s10741-010-9176-4
5. Liu YC, Yu MM, Shou ST, Chai YF. Sepsis-induced Cardiomyopathy: mechanisms and Treatments. Front Immunol. 2017;8. doi:10.3389/fimmu.2017.01021
6. Su XM, Gao YH, Yang RC. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells-Basel. 2022;11(15):2296.
7. Fang H, Fang MX, Wang YR, et al. Indole-3-Propionic Acid as a Potential Therapeutic Agent for Sepsis-Induced Gut Microbiota Disturbance. Microbiol Spectr. 2022;10(3). doi:10.1128/spectrum.00125-22.
8. Huang ZB, Hu Z, Lu CX, et al. Gut microbiota-derived indole 3-propionic acid partially activates aryl hydrocarbon receptor to promote macrophage phagocytosis and attenuate septic injury. Front Cell Infect Mi. 2022;12:1015386.
9. Wang YC, Koay YC, Pan C, et al. Indole-3-Propionic Acid protects against heart failure with preserved ejection fraction. Circ Res. 2024;134(4):371–389. doi:10.1161/CIRCRESAHA.123.322381
10. Du L, Qi RL, Wang J, Liu ZH, Wu ZL. Indole-3-Propionic Acid, a functional metabolite of clostridium sporogenes, promotes muscle tissue development and reduces muscle cell inflammation. Int J Mol Sci. 2021;22(22):12435. doi:10.3390/ijms222212435
11. Zhuang HM, Ren XS, Jiang FZ, Zhou PH. Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol Med. 2023;29(1). doi:10.1186/s10020-023-00614-9
12. Zhao ZH, Xin FZ, Xue YQ, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med. 2019;51(9):1–14. doi:10.1038/s12276-019-0304-5
13. Niu B, Pan T, Xiao Y, et al. The therapeutic potential of dietary intervention: based on the mechanism of a tryptophan derivative-indole propionic acid on metabolic disorders. Crit Rev Food Sci. 2023.
14. Luo M, Yan DS, Sun QS, et al. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J Cell Biochem. 2020;121(4):2994–3004. doi:10.1002/jcb.29556
15. Liu Q, Zhu J, Kong B, Shuai W, Huang H. Tirzepatide attenuates lipopolysaccharide-induced left ventricular remodeling and dysfunction by inhibiting the TLR4/NF-kB/NLRP3 pathway. Int Immunopharmacol. 2023;120:110311. doi:10.1016/j.intimp.2023.110311
16. Sun J, Zhang YH, Kong Y, et al. Microbiota-derived metabolite Indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain, Behavior, and Immunity. 2022;106:76–88. doi:10.1016/j.bbi.2022.08.003
17. Fang H, Wang YR, Deng J, et al. Sepsis-Induced Gut Dysbiosis Mediates the Susceptibility to Sepsis-Associated Encephalopathy in Mice. Msystems. 2022;7(3). doi:10.1128/msystems.01399-21.
18. Zhu XF, Sun M, Guo HM, et al. Verbascoside protects from LPS-induced septic cardiomyopathy via alleviating cardiac inflammation, oxidative stress and regulating mitochondrial dynamics. Ecotox Environ Safe. 2022;233:113327. doi:10.1016/j.ecoenv.2022.113327
19. Chen H, Liu Q, Liu X, Jin J. Berberine attenuates septic cardiomyopathy by inhibiting TLR4/NF-kappaB signalling in rats. Pharm Biol. 2021;59(1):121–128. doi:10.1080/13880209.2021.1877736
20. Xiong X, Lu L, Wang Z, et al. Irisin attenuates sepsis-induced cardiac dysfunction by attenuating inflammation-induced pyroptosis through a mitochondrial ubiquitin ligase-dependent mechanism. Biomed Pharmacother. 2022;152(113199):113199. doi:10.1016/j.biopha.2022.113199
21. Liu H, Hu Q, Ren K, Wu P, Wang Y, Lv C. ALDH2 mitigates LPS-induced cardiac dysfunction, inflammation, and apoptosis through the cGAS/STING pathway. Mol Med. 2023;29(1):171. doi:10.1186/s10020-023-00769-5
22. Chen L, Liu P, Feng X, Ma C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med. 2017;21(12):3178–3189. doi:10.1111/jcmm.12871
23. Wu M, Huang Z, Huang W, et al. microRNA-124-3p attenuates myocardial injury in sepsis via modulating SP1/HDAC4/HIF-1alpha axis. Cell Death Discov. 2022;8(1):40. doi:10.1038/s41420-021-00763-y
24. Xue H, Chen X, Yu C, et al. Gut Microbially Produced Indole-3-Propionic Acid Inhibits Atherosclerosis by Promoting Reverse Cholesterol Transport and Its Deficiency Is Causally Related to Atherosclerotic Cardiovascular Disease. Circ Res. 2022;131(5):404–420. doi:10.1161/CIRCRESAHA.122.321253
25. Volkova M, Palmeri M, Russell KS, Russell RR. Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovasc Res 2011;90(2):305–314. doi:10.1093/cvr/cvr007
26. Li YY, Wang XJ, Su YL, et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol Sin. 2022;43(6):1495–1507. doi:10.1038/s41401-021-00781-7
27. Cui S, Chen X, Li J, et al. Endothelial CXCR2 deficiency attenuates renal inflammation and glycocalyx shedding through NF-kappaB signaling in diabetic kidney disease. Cell Commun Signal. 2024;22(1):191. doi:10.1186/s12964-024-01565-2
28. Chen Z, Zhang M, Zhao Y, et al. Hydrogen Sulfide Contributes to Uterine Quiescence Through Inhibition of NLRP3 Inflammasome Activation by Suppressing the TLR4/NF-kappaB Signalling Pathway. J Inflamm Res. 2021;14:2753–2768. doi:10.2147/JIR.S308558
29. Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Investig. 2016;126(1):23–31. doi:10.1172/JCI82224
30. Arfaras-Melainis A, Polyzogopoulou E, Triposkiadis F, et al. Heart failure and sepsis: practical recommendations for the optimal management. Heart Fail Rev. 2020;25(2):183–194. doi:10.1007/s10741-019-09816-y
31. Wardi G, Joel I, Villar J, et al. Equipoise in Appropriate Initial Volume Resuscitation for Patients in Septic Shock With Heart Failure: results of a Multicenter Clinician Survey. J Intensive Care Med. 2020;35(11):1338–1345. doi:10.1177/0885066619871247
32. Guo T, Jiang ZB, Tong ZY, Zhou Y, Chai XP, Xiao XZ. Shikonin Ameliorates LPS-Induced Cardiac Dysfunction by SIRT1-Dependent Inhibition of NLRP3 Inflammasome. Front Physiol. 2020;11. doi:10.3389/fphys.2020.570441
33. Yan JH, Zhang JW, Wang YA, et al. Rapidly Inhibiting the Inflammatory Cytokine Storms and Restoring Cellular Homeostasis to Alleviate Sepsis by Blocking Pyroptosis and Mitochondrial Apoptosis Pathways. Adv Sci. 2023;10(14). doi:10.1002/advs.202207448
34. Guo YJ, Zhang HQ, Lv Z, et al. Up-regulated CD38 by daphnetin alleviates lipopolysaccharide-induced lung injury via inhibiting MAPK/NF-κB/NLRP3 pathway. Cell Commun Signal. 2023;21(1). doi:10.1186/s12964-023-01041-3
35. Li N, Zhou H, Wu HM, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. doi:10.1016/j.redox.2019.101215
36. Zhang T, Cheng JK, Hu YM. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev. 2022;81:101739. doi:10.1016/j.arr.2022.101739
37. Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70(6):1174–1182. doi:10.1136/gutjnl-2020-323071
38. Tang WHW, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120(7):1183–1196. doi:10.1161/CIRCRESAHA.117.309715
39. Dodd D, Spitzer MH, Van Treuren W, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–+. doi:10.1038/nature24661
40. Venkatesh M, Mukherjee S, Wang HW, et al. Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity. 2014;41(2):296–310. doi:10.1016/j.immuni.2014.06.014
41. Li JJ, Zhang L, Wu T, Li YF, Zhou XJ, Ruan Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J Agr Food Chem. 2021;69(5):1487–1495. doi:10.1021/acs.jafc.0c05205
42. Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19(3):184–197. doi:10.1038/s41577-019-0125-8
43. Zhuang HM, Li B, Xie T, et al. Indole-3-aldehyde alleviates chondrocytes inflammation through the AhR-NF-ΚB signalling pathway. Int Immunopharmacol. 2022;113:109314. doi:10.1016/j.intimp.2022.109314
44. Qiao P, Zhang C, Yu J, et al. Quinolinic Acid, a Tryptophan Metabolite of the Skin Microbiota, Negatively Regulates NLRP3 Inflammasome through AhR in Psoriasis. J Invest Dermatol. 2022;142(8):2184–2193. doi:10.1016/j.jid.2022.01.010
45. Zhao CJ, Hu XY, Bao LJ, Wu KY, Feng LJ, Qiu M, Hao HY, Fu YH, Zhang NS: Aryl hydrocarbon receptor activation by tryptophan metabolism alleviates-induced mastitis in mice. PLoS Pathog 2021, 17(7).
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.