Back to Journals » International Journal of General Medicine » Volume 18
Prognostic Impact of Anemia in Patients with Significant Mitral Regurgitation: A Multicenter Cohort Study
Authors Lv J , Lu Q, Li Z, Ye Y, Zhang B, Wang W, Zhao Q, Zhang H, Zhao Z, Wang B, Liu Q, Guo S, Yu Z, Duan Z, Zhao Y, Gao R, Xu H, Ge J, Wu Y
Received 3 December 2024
Accepted for publication 23 April 2025
Published 28 April 2025 Volume 2025:18 Pages 2303—2318
DOI https://doi.org/10.2147/IJGM.S509171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Junxing Lv,1– 4,* Qianhong Lu,1,2,* Zhe Li,1,2 Yunqing Ye,1,2 Bin Zhang,1,2 Weiwei Wang,1,2 Qinghao Zhao,1,2 Haitong Zhang,1,2 Zhenyan Zhao,1,2 Bincheng Wang,1,2 Qingrong Liu,1,2 Shuai Guo,1,2 Zikai Yu,1,2 Zhenya Duan,1,2 Yanyan Zhao,1,5 Runlin Gao,1,2 Haiyan Xu,1,2,* Junbo Ge,3,4,* Yongjian Wu1,2,* for the CHINA-VHD collaborators
1Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100730, People’s Republic of China; 2Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100037, People’s Republic of China; 3Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, Shanghai, 200032, People’s Republic of China; 4Cardiovascular Technology and Device Innovation Unit, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100730, People’s Republic of China; 5Medical Research & Biometrics Center, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100037, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongjian Wu, Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, 167 Beilishi Road, Xicheng District, Beijing, 100037, People’s Republic of China, Tel +8610-13701387189, Email [email protected] Junbo Ge, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai, 200032, People’s Republic of China, Email [email protected]
Background and Aim: Anemia may affect cardiac function and outcomes in cardiovascular diseases. However, there is scarce evidence on the impact of anemia in patients with mitral valve dysfunction. This study sought to investigate the prevalence of anemia in patients with significant mitral regurgitation (MR), as well as its association with outcomes.
Methods: A total of 4339 patients with moderate or greater MR in the China Valvular Heart Disease study were included in this analysis. Anemia was determined according to the World Health Organization definition. The primary outcome of this study was two-year all-cause mortality, and the secondary outcome was the composite of death and hospitalization for heart failure.
Results: Anemia was present in 33.1% (1435/4339) of the study population. During a median follow-up of 732 (704– 748) days, 426 (9.8%) patients died and 686 (15.8%) experienced the composite endpoint. Both anemia and hemoglobin were independently associated with two-year outcomes (all P < 0.001). Similar results were observed in patients with conservatively managed MR, left ventricular ejection fraction ≤ 60%, or in subsets according to New York Heart Association functional class (I/II–IV), the diagnosis of heart failure, severity of valvular lesion, etiology of MR, and the presence of malnutrition. The combination of anemia with left atrial dilatation or impaired left ventricular systolic function identified high-risk patients with significantly poor survival, and the inclusion of anemia to EuroSCORE II model enhanced risk prediction in MR.
Conclusion: Anemia was common in patients with MR, and it was a significant predictor of poor prognosis. The high prevalence and negative impact of anemia make it as an important risk factor for prognostic evaluation and clinical decision-making.
Keywords: mitral regurgitation, anemia, hemoglobin, prognosis
Introduction
Anemia is a common blood disorder in patients with cardiovascular diseases,1–4 and it is not an innocent bystander to cardiac abnormalities or elevated mortality risk.5,6 Although the mechanism of anemia contributing to poor cardiac function and clinical outcomes remains to be further elucidated, it has been established as a prognostic factor in patients with heart failure, coronary artery disease, and congenital heart disease.4,6,7 There is also emerging evidence implying a large burden of anemia in valvular heart disease (VHD). In patients undergoing transcatheter aortic valve intervention, anemic individuals accounted for up to 64.4% of patients across different studies,8–13 and experienced significantly impaired exercise performance and survival.9,11,12 Besides aortic stenosis, anemia may also be frequently present in other types of VHD, such as mitral regurgitation (MR), causing a negative impact on cardiac function, functional status, as well as clinical outcomes.
There are limited data on the prevalence and prognostic impact of anemia in MR. Several studies reported inconsistent distributions of anemia in patients undergoing transcatheter mitral valve intervention (TMVI),14–18 as well as the associations of anemia or hemoglobin with functional MR severity or all-cause mortality.19,20 Nevertheless, TMVI candidates generally represent a group of high-risk, surgically inoperable patients with various cardiac and non-cardiac comorbidities. Little is known about the presence of anemia and its association with outcomes in MR population regardless of therapeutic strategy. Besides, the combined predictive effects of anemia with well-established risk factors, as well as its incremental value over existing risk assessment tools are not investigated. In current guidelines for the management of VHD, the importance of hematologic parameters for risk assessment is overlooked in patients with MR.21,22
Therefore, the present study sought to investigate the prevalence and prognostic impact of anemia in a large, representative series of patients with significant MR.
Materials and Methods
Study Population
The study population was from the database of the China Valvular Heart Disease (China-VHD; NCT03484806) registry, which was a nationwide, multicenter, prospective, observational cohort study for patients (≥18 years) with significant (≥moderate) VHD. From April to June 2018, patients with at least moderate VHD, as defined by echocardiography, were consecutively recruited from inpatient wards and outpatient clinics at 46 medical centers in China. Details of the study design and quality control measures have been described and published elsewhere.23 The study was approved by the Institutional Review Board at Fuwai Hospital, National Center for Cardiovascular Diseases of China (Approval No. 2017–968) and conformed to the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from each patient before registration. Among 13917 patients enrolled in the China-VHD study, 6673 (47.9%) had moderate or greater MR. Patients with significant aortic stenosis (n = 283), aortic regurgitation (n = 953), mitral stenosis (n = 406), or pulmonary valve disease (n = 42) were excluded. We also excluded patients with previous valvular intervention (n = 115), infective endocarditis (n = 87), missing value on hemoglobin (n = 444), and those without any follow-up data (n = 4). As a result, a total of 4339 patients were included in this analysis (Figure 1).
 |
Figure 1 Flowchart of this study. Abbreviation: China-VHD, China Valvular Heart Disease; MR, mitral regurgitation; AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis. |
Echocardiography
Comprehensive transthoracic two-dimensional and Doppler echocardiography with standard ultrasound systems (Supplementary Material 1) was performed on each patient in the China-VHD study. Cardiac chamber was quantified according to recommendations from the American Society of Echocardiography and the European Association of Cardiovascular Imaging,24 and left ventricular ejection fraction (LVEF) was obtained by the biplane modified Simpson method. The echocardiographic definition of significant MR has been described and published previously,25 and was also detailed in Supplementary Material 1.
Definitions
Venous blood samples were collected and tested during the same period of the echocardiography. Anemia was determined according to the World Health Organization (WHO) definition,26 which was a hemoglobin level <120g/L in women and <130g/L in men. Baseline risk, nutritional status, and cardio-renal-hepatic function were assessed by EuroSCORE II and Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score (Supplementary Material 1), geriatric nutritional risk index (GNRI), and cardio-renal-hepatic (CRH) score, respectively, which were calculated according to previous publications.25,27–32
Follow-up and Endpoints
Follow-up was conducted by clinical visits, medical records, or telephone calls at six months, 12 months, 18 months, and two years. All-cause mortality was the primary outcome of the present analysis. The secondary outcome was the composite of all-cause death and hospitalization for heart failure (HHF). If no event occurred within two years, patients were censored at the date of the last contact or valvular intervention if performed during follow-up.
Statistical Analysis
Baseline characteristics of patients with and without anemia were summarized and compared. Categorical variables were presented by numbers and proportions and compared by Chi-square test. Continuous variables were reported as mean ± standard deviation (SD) or the median value with interquartile range (IQR) and compared using the Mann–Whitney U-test.
The associations of both WHO-defined anemia and hemoglobin with outcomes were investigated in the present study. To examine the shape of the relationship between hemoglobin and outcomes, restricted cubic splines with three knots at 25th, 50th, and 75th percentiles were adopted. Kaplan-Meier curves were constructed according to the existence of anemia, and the survival differences between groups were examined by the Log rank test. We also used Cox proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Following variables were adjusted in multivariable analyses: age, sex, body mass index (BMI), smoking status, hypertension, hyperlipidemia, diabetes, coronary artery disease, cardiomyopathy, atrial fibrillation or flutter, chronic lung disease, chronic kidney disease, New York Heart Association (NYHA) functional class (I–II/III–IV), creatinine, albumin, left atrial end-diastolic dimension (LA), left ventricular end-diastolic dimension (LVEDD), LVEF, pulmonary hypertension, significant tricuspid regurgitation (TR), severity of MR, and mitral valve intervention. The proportional hazards assumptions were examined by the Schoenfeld residual plots and log–log survival plots.
The combined prognostic effects of the presence of both anemia and other parameters, including echocardiographic indices with guideline-recommended thresholds (LA ≥ 55mm; LVEF ≤ 60%), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and GNRI were assessed by examining the associations between the existence of risk factors (two, one, or zero) and outcomes. To further assess the incremental value of anemia over existing risk prediction tools and well-established prognostic factors, we added anemia to EuroSCORE II, the MAGGIC score, as well as the adjusted model, which included variables mentioned previously, and evaluated the improvement of predictive performance. The differences between models were compared by C index, category-less net reclassification improvement index (NRI), integrated discrimination improvement index (IDI), likelihood ratio test for nested models, and Bayesian Information Criterion (BIC).
Subgroup analyses were performed based on the therapeutic strategy of MR (conservative management/valvular intervention), the diagnosis of heart failure, symptomatic status (NYHA I/II–IV), NT-proBNP (NT-proBNP ≥ 2034 pg/mL/NT-proBNP < 2034 pg/mL), LA (≥55 mm/<55 mm), LVEF (>60%/≤60%), the existence of significant TR (yes/no), severity of MR (moderate/severe MR), etiology (primary/secondary MR), and the presence or absence of GNRI-defined malnutrition (GNRI ≤ 98).30 Missing data (≤2.9% for each variable) and imputation methods were shown in Supplementary Table 1. Statistical significance was defined as a two-tailed P < 0.05. All analyses were performed using R software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
Of 4339 patients in the present study, the mean age was 62.84 ± 12.88 years and 2552 (58.8%) were male. Patients with anemia accounted for 33.1% (1435/4339) in the overall population, with mild anemia (110g/L ≤ the hemoglobin level < 120g/L [female]/130g/L [male]) in 894 patients, moderate anemia (80g/L ≤ the hemoglobin level < 110g/L) in 471 patients, and severe anemia (the hemoglobin level <80g/L) in 70 patients. Baseline characteristics according to the existence of anemia were shown in Table 1. Compared with those without anemia, anemic patients were older, and had a higher prevalence of hypertension, diabetes, coronary artery disease, chronic lung disease, and kidney disease (all P < 0.001). Patients with anemia were less likely to use warfarin, while they were more frequently taking antiplatelet therapy at baseline (all P < 0.001). Compared with patients without anemia, individuals with anemia had higher C-reactive protein, poorer nutritional status, higher surgical risk, as well as worse cardio-renal-hepatic function (all P < 0.001). Notably, there was only a modest correlation of hemoglobin with albumin (Spearman r = 0.31, P < 0.001) or nutritional index (Spearman r = 0.31, P < 0.001), despite reaching statistical significance.
 |
Table 1 Baseline Characteristics |
Association of Anemia and Hemoglobin with Outcomes
Of 4339 patients in the present study, 426 (9.8%) died during a median follow-up of 732 (704–748) days. The cumulative one-year and two-year survival was 92.3% and 88.9%. In restricted cubic spline analysis, there was a monotonic decrease in the risk of events with the increasing hemoglobin level (Figure 2). Both anemia and the hemoglobin level were independently associated with two-year mortality (Table 2; anemia, adjusted HR [95% CI], 1.686 [1.370–2.075], P < 0.001; hemoglobin, adjusted HR [95% CI], 0.987 [0.982–0.992], P < 0.001). When assessing the relationships of anemia and the hemoglobin level with the composite endpoint of all-cause death and HHF, they remained independent prognostic factors in patients with significant MR (Figure 3; Table 3; anemia, adjusted HR [95% CI], 1.509 [1.283–1.775], P < 0.001; hemoglobin, adjusted HR [95% CI], 0.990 [0.986–0.994], P < 0.001). Additional adjustment for NT-proBNP, EuroSCORE II, use of warfarin, aspirin, P2Y12 inhibitor, β blocker, angiotensin converting enzyme inhibitor/angiotensin receptor blocker, and diuretics yielded similar results (n = 2769; all-cause mortality: anemia, adjusted HR [95% CI], 1.465 [1.144–1.875], P = 0.002; hemoglobin, adjusted HR [95% CI], 0.993 [0.987–0.999], P = 0.028; composite endpoint: anemia, adjusted HR [95% CI], 1.385 [1.137–1.687], P = 0.001; hemoglobin, adjusted HR [95% CI], 0.993 [0.988–0.998], P = 0.005). Compared with those without anemia, patients with mild, moderate, or severe anemia had significantly higher risks of two-year adverse events (all-cause mortality: mild anemia vs no anemia, adjusted HR [95% CI], 1.554 [1.227–1.969], P < 0.001; moderate anemia vs no anemia, adjusted HR [95% CI], 1.780 [1.344–2.356], P < 0.001; severe anemia vs no anemia, adjusted HR [95% CI], 3.313 [2.046–5.364], P < 0.001; composite endpoint: mild anemia vs no anemia, adjusted HR [95% CI], 1.360 [1.127–1.641], P = 0.001; moderate anemia vs no anemia, adjusted HR [95% CI], 1.734 [1.390–2.165], P < 0.001; severe anemia vs no anemia, adjusted HR [95% CI], 2.417 [1.552–3.766], P < 0.001).
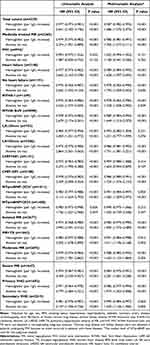 |
Table 2 Association of Anemia and Hemoglobin with Mortality |
 |
Table 3 Association of Anemia and Hemoglobin with the Composite Outcome |
 |
Figure 3 Kaplan-Meier curves according to the presence of anemia. (A) Kaplan-Meier curves for survival. (B) Kaplan-Meier curves for event-free survival. |
Association of Anemia and Hemoglobin with Outcomes in Subgroups of Patients
Of 3365 patients with medically managed MR, 404 (12.0%) died during a median follow-up of 732 (613–749) days, and the cumulative one-year and two-year survival was 90.4% and 85.8%, respectively. In multivariable analyses, both anemia and the hemoglobin level were independently associated with mortality in patients under medial management (Table 2; anemia, adjusted HR [95% CI], 1.705 [1.377–2.111], P < 0.001; hemoglobin, adjusted HR [95% CI], 0.986 [0.981–0.991], P < 0.001), and they were also significant predictors of the composite outcome (Table 3; anemia, adjusted HR [95% CI], 1.500 [1.269–1.773], P < 0.001; hemoglobin, adjusted HR [95% CI], 0.989 [0.985–0.993], P < 0.001). The prognostic values of anemia and hemoglobin remained significant regardless of the diagnosis of heart failure, symptomatic status, TR, severity of MR, etiology of valvular disease, and the presence of malnutrition, and were also robust in patients with LVEF ≤60% or NT-proBNP ≥ 2034 pg/mL (Table 2 and Table 3; Supplementary Table 2). However, neither anemia nor the hemoglobin level was associated with outcomes in 974 patients undergoing mitral valve intervention or in those with LVEF > 60% after multivariable adjustment (Table 2 and Table 3). The two-year outcomes for the four groups stratified by the presence of anemia and severe MR were shown in Supplementary Figure 1.
Combined Prognostic Effects of Anemia and Other Parameters for Outcomes
Patients with both anemia and LA ≥ 55mm had the highest risk of all-cause mortality (Figure 4; Supplementary Table 3; anemia and LA ≥ 55mm vs neither, adjusted HR [95% CI], 2.669 [1.731–4.117], P < 0.001; anemia only vs neither, adjusted HR [95% CI], 1.791 [1.417–2.264], P < 0.001; LA ≥ 55mm only vs neither, adjusted HR [95% CI], 2.087 [1.394–3.125], P<0.001), and the composite endpoint (anemia and LA ≥ 55mm vs neither, adjusted HR [95% CI], 1.933 [1.366–2.736], P < 0.001; anemia only vs neither, adjusted HR [95% CI], 1.518 [1.267–1.818], P < 0.001; LA ≥ 55mm only vs neither, adjusted HR [95% CI], 1.354 [0.983–1.867], P = 0.064), compared with those with one or neither of risk factors. Similarly, concomitant anemia and LVEF ≤ 60% was independently associated with poorer outcomes (Figure 4; Supplementary Table 3). The combined prognostic effects of anemia and NT-proBNP or GNRI were also pronounced (Figure 5 and Supplementary Figure 2; Supplementary Table 3).
Incremental Prognostic Value of Anemia
The inclusion of anemia status to the EuroSCORE II system significantly enhanced its prediction of mortality in patients with MR (Supplementary Table 4; NRI [95% CI]: 0.235 [0.180–0.285], P < 0.001; IDI [95% CI]: 0.026 [0.015–0.040], P < 0.001; likelihood ratio test P < 0.001). An improvement of risk assessment was also observed after adding anemia status to the MAGGIC score (Supplementary Table 4; NRI [95% CI]: 0.236 [0.186–0.291], P < 0.001; IDI [95% CI]: 0.011 [0.004–0.021], P < 0.001; likelihood ratio test P < 0.001). Moreover, when introducing anemia status to the adjusted model of the present study, similar results were obtained (Supplementary Table 4).
Discussion
In this large, multicenter, observational cohort study of 4339 patients with significant MR, we investigated the prevalence of anemia, as well as its impact on two-year outcomes. The present study showed that anemia was present in 33.1% of patients with MR, and both anemia presence and the hemoglobin level were independently associated with all-cause mortality and the composite endpoint of death or HHF. The combination of anemia with left atrial enlargement, impaired left ventricular systolic function, a high NT-proBNP level, or malnutrition was associated with significantly poorer prognosis. In patients with significant MR, anemia status provided complementary prognostic value beyond EuroSCORE II and the MAGGIC risk score, and had the potential to guide clinical risk stratification, as well as being a therapeutic target for improving outcomes.
Both VHD and anemia are closely related to the aging process,33,34 and it is warranted to infer that the co-existence of these two disorders will continue to increase in general population. However, the prevalence of anemia in VHD is generally under-investigated so far. Although several studies conducted in TMVI candidates reported a prevalence of 19.6%–58.7% for anemia,14–18,35 scarce evidence indicated the distribution of anemic individuals in MR patients regardless of therapeutic strategy, and data were particularly limited in Asian population. Our study found that anemic patients accounted for approximately one third of population with moderate or greater MR, which was similar with the data of the study conducted in the BIOSTAT-CHF (A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure) cohort (36.2%).36 This could be explained by the high proportion (72.6%) of patients with the diagnosis of heart failure in our study population. To our best knowledge, the current study for the first time revealed a high prevalence of anemia in a large, Chinese cohort of both medically managed and corrected MR, which called for more attention to this blood disorder in clinical management and research of mitral valve disease.
In the present study, we found that anemia was an independent predictor of outcomes in patients with significant MR. Compared with those without anemia, anemic patients had a nearly 70% higher risk of two-year mortality. Meanwhile, analyzed as a continuous variable, the increasing hemoglobin level was significantly and monotonically associated with a lower risk of adverse events. These results were generally in line with some previous findings in patients undergoing TMVI.14,15,17,37 However, our study extended to previous work by examining the relationship of anemia with outcomes in a large, prospectively enrolled, representative cohort of MR population, which for the first time enabled comprehensive analyses in various subgroups of patients. The prognostic value of anemia was significant in patients with conservatively managed MR and was consistent regardless of symptomatic status (NYHA I/II–IV), heart failure diagnosis, severity of MR, and etiology of valvular disease. Nevertheless, anemia was only related to poor outcomes in patients with LVEF ≤ 60% or NT-proBNP ≥ 2034pg/mL, rather than in those with normal left-sided systolic function, which was most likely explained by the notion that maintaining sufficient cardiac function was crucial to compensate for tissue hypoxia caused by anemia and mitigate its negative impact on outcomes. Based on this finding, we speculate that the cardio-anemia interaction is a clinically relevant pathophysiological process in the course of MR and acts as a major driver of prognosis.
Regarding the association of anemia with outcomes, an important issue is that whether its prognostic value is independent of malnutrition, which is also frequently present in patients with MR and significantly impairs survival.30 The present study filled this knowledge gap by investigating the prognostic significance of anemia in MR with consideration of the potential impact from nutritional status. We found that the hemoglobin level was only modestly correlated with albumin and GNRI, which was a nutritional risk score recently validated in secondary MR,30 and confirmed the robust prognostic value of anemia in patients with or without malnutrition, respectively. All these results implied that anemia itself, as a hematologic parameter, should be considered as a prognostic factor in patients with significant MR, instead of a pure surrogate marker of poor nutrition or frailty.
Another novel finding of this study was that MR patients with both anemia and left atrial dilatation or impaired left ventricular function had significantly poorer outcomes, in comparison with those with one or neither of indices. In the management of MR, current guidelines underscored the importance of left cardiac dimensions and function to inform clinical decision-making.21,22 However, recent studies, including those adopting machine learning techniques for feature selection, found that blood parameters could effectively complement or even perform beyond well-known echocardiographic indices in risk stratification of MR.25,38,39 Besides assessing other prognostic indicators, the additional detection of anemia may assist in identifying individuals at a higher risk for intensive therapy and close monitoring. Notably, our analysis also demonstrated that the anemia status provided incremental value over the MAGGIC risk score as well as the surgical risk assessment model, which further supported its unique prognostic role in routine clinical practice.
The high event risk associated with anemia raises the question of whether anemia itself could serve as a therapeutic target to improve prognosis in patients with MR. In clinical practice, active screening for the underlying causes of anemia in MR patients should be prioritized as the initial step to guide subsequent management of anemia. The updated European Society of Cardiology guidelines for heart failure recommended intravenous iron supplementation in symptomatic patients with heart failure and reduced ejection fraction or mildly reduced ejection fraction, and iron deficiency, to alleviate heart failure symptoms and improve quality of life.40 Intravenous iron therapy should also be considered to reduce the risk of heart failure hospitalization.40 Notably, the benefits associated with intravenous iron therapy in patients with chronic heart failure and iron deficiency were found to be independent of anemia presence,41 and a prior randomized, double-blind trial showed that treatment with darbepoetin alfa failed to improve clinical outcomes and was related to a higher risk of thromboembolic adverse events in patients with systolic heart failure and anemia.42 The sodium-glucose co-transporter 2 (SGLT2) inhibitor is one of the guideline-recommended ‘Fantastic Four’ medications for the treatment of heart failure.40,43,44 A post-hoc analysis of the placebo-controlled Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial found that dapagliflozin corrected anemia more often compared with placebo and improved outcomes regardless of baseline anemia status in patients with heart failure and reduced ejection fraction.45 The mechanisms of the beneficial effects of SGLT2 inhibitors on anemia include improving iron utilization efficiency and increasing erythropoietin levels through the restoration of fibroblast-like function in the renal tubular interstitium.46–49 On the other hand, the Ertugliflozin for Functional Mitral Regurgitation (EFFORT) trial demonstrated that ertugliflozin significantly improved left ventricular global longitudinal strain and left atrial remodeling, and reduced functional MR in patients with heart failure and functional MR.50 In light of all these benefits of the SGLT2 inhibitor, it may be the most promising medication for MR patients with anemia. Dedicated large randomized controlled trials should be conducted to assess the impact of SGLT2 inhibitors on anemia as well as prognosis in patients with MR.
Limitations
Several limitations should be noted in the current analysis. First, this was an observational cohort study. Unmeasured confounders might exist and affect the present findings. However, in comparison with previous work, this study represented a crucial step demonstrating the prognostic impact of anemia in MR, with a large sample size, rigorous adjustment, and comprehensive analysis. Second, the specific interventions for anemia were not collected in the China-VHD study, and therefore we could not evaluate their potential values for improving outcomes in patients with MR. Such problem may only be solved in future randomized controlled trials. Third, in recent years, a series of studies have confirmed the therapeutic effects of SGLT2 inhibitors on anemia in patients with heart failure.45,48 However, the China-VHD study (as well as the current analysis) did not include data on the use of SGLT2 inhibitors, because they were not widely adopted for the treatment of heart failure or VHD during the design phase of the China-VHD study. Further studies are needed to elucidate the beneficial effects of SGLT2 inhibitors on anemia or iron deficiency in patients with MR. Fourth, the use of the mineralocorticoid receptor antagonist, which was also one of the guideline-recommended ‘Fantastic Four’ drugs for treating heart failure,40,43,44 was not documented in the China-VHD database, so we could not perform analyses with consideration of this medication. Fifth, the prevalence of anemia was reported in a clinical registry cohort, instead of from a random sampling survey, and thus might be subject to bias. However, the China-VHD cohort was consecutively enrolled in 46 high-level academic hospitals across mainland China,23 and could be representative of VHD patients in routine clinical practice. Sixth, the history of heart failure-related hospitalizations and some echocardiographic indices, such as e’ and E/e’, were not collected in the China-VHD study. Finally, the China-VHD database did not include data on the etiology of anemia, so the detailed causes of anemia were not discriminated in the current analysis and the prognostic significance of anemia was not analyzed according to its etiology. Previous studies focusing on patients with heart failure showed that over 70% of anemia in heart failure was attributed to iron deficiency,51 which was associated with malnutrition, renal disease, inflammation, fluid overload, malabsorption, and antiplatelet medications.36 Defective iron utilization and bone marrow suppression are also non-negligible causes of anemia.51–53 Although the mechanisms of the development of anemia in MR could not be clarified by the current analysis, our study suggested that anemia in patients with significant MR was related to malnutrition, inflammation, and cardio-renal-hepatic co-dysfunction, and demonstrated the independent prognostic impact of anemia in patients with or without malnutrition respectively.
Conclusions
Anemia was prevalent in patients with significant MR and was an independent predictor of two-year outcomes regardless of symptomatic status, the diagnosis of heart failure, severity of MR, and etiology of the valvular lesion. The combination of anemia with left atrial dilatation and impaired left ventricular systolic function identified high-risk individuals with significantly poor prognosis, and this blood disorder provided complementary value beyond the traditional risk prediction model as well as established prognostic indices. The detection of anemia may enable better clinical risk stratification in patients with MR, and the potential value of anemia correction should be evaluated in future research.
Data Sharing Statement
The data in the current study are available from the corresponding authors on reasonable request.
Ethical Approval and Consent Statement
The study was approved by the Institutional Review Board at Fuwai Hospital, National Center for Cardiovascular Diseases of China (Approval No. 2017-968) and conformed to the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from each patient before registration.
Acknowledgments
The authors are grateful for all collaborators of the China-VHD study for data collection, data entry, and monitoring.
Funding
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences [grant number 2017-12M-3-002]; the Fundamental Research Funds for the Central Universities [grant number 3332022018]; and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences [grant number 2021-I2M-C&T-B-029].
Disclosure
The authors declare no conflicts of interest.
References
1. Beladan CC, Botezatu SB. Anemia and management of heart failure patients. Heart Fail Clin. 2021;17(2):195–206. doi:10.1016/j.hfc.2020.12.002
2. Curtain JP, Adamson C, Docherty KF, et al. Prevalent and incident anemia in PARADIGM-HF and the effect of sacubitril/valsartan. JACC Heart Fail. 2023;11(7):749–759. doi:10.1016/j.jchf.2022.12.012
3. De Larochellière H, Puri R, Eikelboom JW, Rodés-Cabau J. Blood disorders in patients undergoing transcatheter aortic valve replacement: a review. JACC Cardiovasc Interv. 2019;12(1):1–11. doi:10.1016/j.jcin.2018.09.041
4. Dimopoulos K, Diller GP, Giannakoulas G, et al. Anemia in adults with congenital heart disease relates to adverse outcome. J Am Coll Cardiol. 2009;54(22):2093–2100. doi:10.1016/j.jacc.2009.06.050
5. Silverberg DS, Wexler D, Blum M, et al. The interaction between heart failure, renal failure and anemia - the cardio-renal anemia syndrome. Blood Purif. 2004;22(3):277–284. doi:10.1159/000078698
6. Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110(2):149–154. doi:10.1161/01.CIR.0000134279.79571.73
7. da Silveira AD, Ribeiro RA, Rossini AP, et al. Association of anemia with clinical outcomes in stable coronary artery disease. Coron Artery Dis. 2008;19(1):21–26. doi:10.1097/MCA.0b013e3282f27c0a
8. Seiffert M, Conradi L, Gutwein A, et al. Baseline anemia and its impact on midterm outcome after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2017:89:E44–E52. doi:10.1002/ccd.26563.
9. DeLarochellière H, Urena M, Amat-Santos IJ, et al. Effect on outcomes and exercise performance of anemia in patients with aortic stenosis who underwent transcatheter aortic valve replacement. Am J Cardiol. 2015;115(4):472–479. doi:10.1016/j.amjcard.2014.11.033
10. Shuvy M, Mewa J, Wolff R, et al. Preprocedure anemia management decreases transfusion rates in patients undergoing transcatheter aortic valve implantation. Can J Cardiol. 2016;32(6):732–738. doi:10.1016/j.cjca.2015.08.018
11. Van Mieghem NM, Nuis RJ, Tzikas A, et al. Prevalence and prognostic implications of baseline anaemia in patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2011;7(2):184–191. doi:10.4244/EIJV7I2A32
12. Nuis RJ, Sinning JM, Rodés-Cabau J, et al. Prevalence, factors associated with, and prognostic effects of preoperative anemia on short- and long-term mortality in patients undergoing transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2013;6(6):625–634. doi:10.1161/CIRCINTERVENTIONS.113.000409
13. Rheude T, Pellegrini C, Michel J, et al. Prognostic impact of anemia and iron-deficiency anemia in a contemporary cohort of patients undergoing transcatheter aortic valve implantation. Int J Cardiol. 2017;244:93–99. doi:10.1016/j.ijcard.2017.06.024
14. Raposeiras-Roubin S, Adamo M, Freixa X, et al. A score to assess mortality after percutaneous mitral valve repair. J Am Coll Cardiol. 2022;79(6):562–573. doi:10.1016/j.jacc.2021.11.041
15. Kaneko H, Neuss M, Okamoto M, Weissenborn J, Butter C. Impact of preprocedural anemia on outcomes of patients with mitral regurgitation who underwent MitraClip implantation. Am J Cardiol. 2018;122(5):859–865. doi:10.1016/j.amjcard.2018.05.028
16. Hellhammer K, Balzer J, Zeus T, et al. Percutaneous mitral valve repair using the MitraClip system in patients with anemia. Int J Cardiol. 2015;184:399–404. doi:10.1016/j.ijcard.2015.02.081
17. Iliadis C, Metze C, Körber MI, Baldus S, Pfister R. Association of iron deficiency, anaemia, and functional outcomes in patients undergoing edge-to-edge mitral valve repair. ESC Heart Fail. 2020;7(5):2379–2387. doi:10.1002/ehf2.12778
18. Bhardwaj B, Karuparthi PR, Desai R, Fong HK, Aggarwal K. Anemia among patients undergoing transcatheter mitral valve repair: from the national inpatient sample in the United States. Cureus. 2020;
19. Tigen K, Karaahmet T, Kirma C, et al. The association of functional mitral regurgitation and anemia in patients with non-ischemic dilated cardiomyopathy. Cardiol J. 2010;17(3):274–280.
20. Simpson TF, Kumar K, Samhan A, et al. Clinical predictors of mortality in patients with moderate to severe mitral regurgitation. Am J Med. 2022;135(3):380–385.e3. doi:10.1016/j.amjmed.2021.09.004
21. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi:10.1093/eurheartj/ehab395
22. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227. doi:10.1161/CIR.0000000000000923
23. Lv J, Ye Y, Li Z, et al. Prognostic value of modified model for end-stage liver disease scores in patients with significant tricuspid regurgitation. Eur Heart J Qual Care Clin Outcomes. 2023;9(3):227–239. doi:10.1093/ehjqcco/qcac027
24. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi:10.1016/j.echo.2014.10.003
25. Lv J, Zhang B, Ye Y, et al. Assessment of cardio-renal-hepatic function in patients with valvular heart disease: a multi-biomarker approach-the cardio-renal-hepatic score. BMC Med. 2023;21(1):257. doi:10.1186/s12916-023-02971-y
26. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37.
27. Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–44,discussion744–45. doi:10.1093/ejcts/ezs043
28. Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
29. Cereda E, Pedrolli C. The geriatric nutritional risk index. Curr Opin Clin Nutr Metab Care. 2009;12(1):1–7. doi:10.1097/MCO.0b013e3283186f59
30. Scotti A, Coisne A, Granada JF, et al. Impact of malnutrition in patients with heart failure and secondary mitral regurgitation: the COAPT trial. J Am Coll Cardiol. 2023;82(2):128–138. doi:10.1016/j.jacc.2023.04.047
31. Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404–1413. doi:10.1093/eurheartj/ehs337
32. Lv J, Xu H, Ye Y, et al. Meta-analysis global group in chronic heart failure score for the prediction of mortality in valvular heart disease. ESC Heart Fail. 2024;11(1):349–365. doi:10.1002/ehf2.14586
33. Messika-Zeitoun D, Baumgartner H, Burwash IG, et al. Unmet needs in valvular heart disease. Eur Heart J. 2023;44(21):1862–1873. doi:10.1093/eurheartj/ehad121
34. Michalak SS, Rupa-Matysek J, Gil L. Comorbidities, repeated hospitalizations, and age ≥ 80 years as indicators of anemia development in the older population. Ann Hematol. 2018;97(8):1337–1347. doi:10.1007/s00277-018-3321-x
35. Ahuja KR, Nazir S, Ariss RW, et al. Derivation and validation of risk prediction model for 30-day readmissions following transcatheter mitral valve repair. Curr Probl Cardiol. 2023;48(3):101033. doi:10.1016/j.cpcardiol.2021.101033
36. van der Wal HH, Grote Beverborg N, Dickstein K, et al. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur Heart J. 2019;40(44):3616–3625. doi:10.1093/eurheartj/ehz680
37. Puls M, Lubos E, Boekstegers P, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J. 2016;37(8):703–712. doi:10.1093/eurheartj/ehv627
38. Zhang B, Xu H, Zhang H, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide in elderly patients with valvular heart disease. J Am Coll Cardiol. 2020;75(14):1659–1672. doi:10.1016/j.jacc.2020.02.031
39. Zweck E, Spieker M, Horn P, et al. Machine learning identifies clinical parameters to predict mortality in patients undergoing transcatheter mitral valve repair. JACC Cardiovasc Interv. 2021;14(18):2027–2036. doi:10.1016/j.jcin.2021.06.039
40. McDonagh TA, Metra M, Adamo M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627–3639. doi:10.1093/eurheartj/ehad195
41. Filippatos G, Farmakis D, Colet JC, et al. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. Eur J Heart Fail. 2013;15(11):1267–1276. doi:10.1093/eurjhf/hft099
42. Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368(13):1210–1219. doi:10.1056/NEJMoa1214865
43. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
44. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021;42(6):681–683. doi:10.1093/eurheartj/ehaa1012
45. Docherty KF, Curtain JP, Anand IS, et al. Effect of dapagliflozin on anaemia in DAPA-HF. Eur J Heart Fail. 2021;23(4):617–628. doi:10.1002/ejhf.2132
46. Sano M. A role of sodium-glucose co-transporter 2 in cardiorenal anemia iron deficiency syndrome. Int J mol Sci. 2023;24(6):5983. doi:10.3390/ijms24065983
47. Asada N, Takase M, Nakamura J, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121(10):3981–3990. doi:10.1172/JCI57301
48. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. doi:10.1111/dom.12127
49. Docherty KF, Welsh P, Verma S, et al. Iron deficiency in heart failure and effect of dapagliflozin: findings from DAPA-HF. Circulation. 2022;146(13):980–994. doi:10.1161/CIRCULATIONAHA.122.060511
50. Kang DH, Park SJ, Shin SH, et al. Ertugliflozin for functional mitral regurgitation associated with heart failure: EFFORT trial. Circulation. 2024;149(24):1865–1874. doi:10.1161/CIRCULATIONAHA.124.069144
51. Nanas JN, Matsouka C, Karageorgopoulos D, et al. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48(12):2485–2489. doi:10.1016/j.jacc.2006.08.034
52. Opasich C, Cazzola M, Scelsi L, et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J. 2005;26(21):2232–2237. doi:10.1093/eurheartj/ehi388
53. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138(1):80–98. doi:10.1161/CIRCULATIONAHA.118.030099
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Decrease in Hb and Hypoproteinemia: Possible Predictors of Complications in Neonates with Late-Onset Sepsis in a Developing Country
Cai N, Liao W, Chen Z, Tao M, Chen S
International Journal of General Medicine 2022, 15:6583-6589
Published Date: 13 August 2022
Dapagliflozin Improves Erythropoiesis and Iron Metabolism in Type 2 Diabetic Patients with Renal Anemia
Osonoi T, Shirabe S, Saito M, Hosoya M, Watahiki N, Douguchi S, Ofuchi K, Katoh M
Diabetes, Metabolic Syndrome and Obesity 2023, 16:1799-1808
Published Date: 20 June 2023
Anemia in Heart Failure: A Perspective from 20-Year Bibliometric Analysis
Yang Q, Dong T, Lyu D, Xue D, Zhuang R, Ma L, Zhang L
International Journal of General Medicine 2024, 17:1845-1860
Published Date: 2 May 2024
Infective Endocarditis Complicated by Severe Mitral Regurgitation and Markedly Elevated Troponin Levels as A Prognostic Marker: A Case Report
Dahir OF, Hassan MO, Adan AS, Abdi AE, Abdi IA
Research Reports in Clinical Cardiology 2025, 16:9-14
Published Date: 26 March 2025
Predictive Value of Dynamic Changes in Hemoglobin Levels During Early Pregnancy for the Development of Anemia During Pregnancy
Liu L, Li X
International Journal of Women's Health 2025, 17:1829-1835
Published Date: 17 June 2025




