Back to Journals » Journal of Inflammation Research » Volume 17
Protective Effect of Rosavin Against Intestinal Epithelial Injury in Colitis Mice and Intestinal Organoids
Authors Luo H , Guo M, Li M, Zhao Y, Shen J, Du F, Chen Y, Deng S, Sun Y, Gu L, Li W, Li X, Chen M, Xiao Z , Wang S, Wu X
Received 18 April 2024
Accepted for publication 22 August 2024
Published 4 September 2024 Volume 2024:17 Pages 6023—6038
DOI https://doi.org/10.2147/JIR.S474368
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Haoming Luo,1,2,* Miao Guo,3,4,* Mingxing Li,1 Yueshui Zhao,1 Jing Shen,1 Fukuan Du,1 Yu Chen,1 Shuai Deng,1 Yuhong Sun,1 Li Gu,1 Wanping Li,1 Xiaobing Li,1 Meijuan Chen,1 Zhangang Xiao,1,5,6 Shengpeng Wang,3,4 Xu Wu1,7
1Cell Therapy & Cell Drugs of Luzhou Key Laboratory, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646100, People’s Republic of China; 2Department of Pharmacy, Three Gorges University Hospital of Traditional Chinese Medicine & Yichang Hospital of Traditional Chinese Medicine, Yichang, Hubei, 443003, People’s Republic of China; 3State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, People’s Republic of China; 4Macao Centre for Research and Development in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, People’s Republic of China; 5Department of Pharmacy, Gulin County Hospital of Traditional Chinese Medicine, Luzhou, Sichuan, 646500, People’s Republic of China; 6School of Pharmacy, Sichuan College of Traditional Chinese Medicine, Mianyang, Sichuan, 621000, People’s Republic of China; 7Department of Paediatric Care, Luzhou People’s Hospital, Luzhou, Sichuan, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shengpeng Wang, State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, People’s Republic of China, Email [email protected] Xu Wu, Cell Therapy & Cell Drugs of Luzhou Key Laboratory, Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646100, People’s Republic of China, Email [email protected]
Introduction: Rhodiola species have been utilized as functional foods in Asia and Europe for promoting health. Research has demonstrated that Rhodiola has the potential to alleviate inflammatory bowel disease (IBD) in animal models. However, the specific active components and the underlying mechanism for ameliorating intestinal damage remain unclear. This study aims to explore the relieving effect of Rosavin (Rov), a known active constituent of Rhodiola, in IBD and the regulatory mechanisms.
Methods: The therapeutic effect of Rov was evaluated using a murine model of acute colitis induced by dextran sulfate sodium salt (DSS). Inflammatory cytokines and neutrophil activation markers were measured by corresponding kits. Immunohistochemistry, immunofluorescence, TUNEL, and EdU assays were applied to investigate the tight conjunction proteins expression, epithelial marker expression, number of apoptotic cells, and epithelial proliferation, respectively. The protection effect of Rov on gut epithelial injury was assessed using TNF-α-induced intestinal organoids. Additinally, RNA sequencing was applied to observe the genetic alteration profile in these intestinal organoids.
Results: Oral administration of Rov significantly attenuated weight loss and restored colon length in mice. Notably, Rov treatment led to decreased levels of pro-inflammatory cytokines and neutrophil activation markers while increasing anti-inflammatory factors. Importantly, Rov restored intestinal despair by increasing the number of Lgr5+ stem cells, Lyz1+ Paneth cells and Muc2+ goblet cells in intestines of colitis mice, displaying reduced epithelial apoptosis and recovered barrier function. In TNF-α-induced intestinal organoids, Rov facilitated epithelial cell differentiation and protected against TNF-α-induced damage. RNA sequencing revealed upregulation in the gene expression associated with epithelial cells (including Lgr5+, Lyz1+ and Muc2+ cells) proliferation and defensin secretion, unveiling the protective mechanisms of Rov on the intestinal epithelial barrier.
Discussion: Rov holds potential as a natural prophylactic agent against IBD, with its protective action on the intestinal epithelium being crucial for its therapeutic efficacy.
Keywords: rosavin, colitis, inflammatory bowel disease, organoid, intestinal stem cell
Graphical Abstract:

Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is an immune-related disorder primarily affecting the gastrointestinal tract.1 Since the latter half of the 20th century, there has been a remarkable increase in the incidence of IBD in North America and Europe. Additionally, populations previously considered “low-risk”, such as those in Japan and India, have also been affected.2 Apart from genetic factors, research has shown that interactions involving disrupted intestinal barrier function, gut microbiota, and environmental factors contribute to the onset and progression of colitis.3 Among these, the intestinal epithelium acts as a natural barrier. However, disruption of the gut epithelial barrier and impairment of crypts are typical features of IBD, which may lead to sustained intestinal inflammation and barrier dysfunction.4,5 These findings are particularly attractive for investigating the mechanisms of intestinal epithelial damage and repair in the prevention and pathogenesis of colitis.
In recent years, the safe and highly active properties of functional foods and its derivatives have led to a heightened focus among many research teams on the development of dietary remedies to develop individualized treatments for diseases including IBD.6–8 Rhodiola species include several valuable plants that are widely used in Europe and east countries for preventing diseases of cardiovascular diseases, neurodegenerative diseases, diabetes, sepsis, and cancer.9 Previous studies by our research team found that Rhodiola crenulata extract had anti-inflammatory effects, regulated intestinal flora, and alleviated acute colitis in mice.10 However, the mechanism of action of its active ingredients lacks in-depth investigation. Rosavin (Rov), a key physiologically active compound of Rhodiola species, has been shown to effectively treat intestinal radiation injury.11 It was found that Rov significantly improved the cell viability of the irradiated IEC-6 cells.11 Furthermore, its combination with 12 probiotics, prebiotics and zinc could effectively alleviate acute colitis.12 These findings indicate that Rov could play a positive role in the treatment of IBD. Therefore, exploring the protective effects of Rov on IBD and the underlying mechanisms is essential for novel therapeutics.
The establishment of in vitro 3D miniature intestinal organoid cultures achieves long-term cultivation of intestinal epithelium in vitro while maintaining the original differentiation ability of intestinal epithelial cells.13,14 This technology has gradually been applied in stem cell research, disease models, and regenerative medicine. In long-term cultures, individual crypts in the intestine undergo fission to produce multiple crypts, while also generating villus-like epithelial structural domains containing all differentiated cell types.13 Multiple studies have shown that in intestinal epithelial organoid culture systems, intestinal stem cells (ISCs) reproduce the self-renewal and differentiation abilities observed in mature intestines.13,15,16 Therefore, intestinal organoids provide an excellent perspective for more directly exploring the mechanisms of action of natural products in IBD.
In this study, the dextran sulfate sodium salt (DSS)-induced acute colitis model and an in vitro culture of mouse intestinal organoid model were established. The aim is to reveal the potential therapeutic effects of Rov on colitis and its role in intestinal barrier repair. The results of current study indicate that Rov is a potential natural prophylactic for IBD, and its protective and restorative effects on the intestinal epithelium regeneration are key factors in its therapeutic response.
Materials and Methods
Materials and Chemicals
Rov (purity>99%) was brought from Chengdu Must Biotechnology (Chengdu, China). 5’-Aminosalicylic acid (5’ASA) was acquired from Shanghai Aladdin Biochemical Technology (Shanghai, China). DSS (36–50 kDa) was obtained from International Laboratory USA (San Francisco, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for mouse IL-1β, IL-4, IL-17, IFN-γ, TNF-α, TGF-β1 and myeloperoxidase (MPO) were sourced from R&D Systems, Inc. (Minneapolis, MN, USA). IntestiCult™ organoid growth medium (Mouse) was obtained from StemCell technologies (Vancouver, Canada). Matrigel (#356234) was obtained from Corning (NY, USA). Zonula occludens (ZO)-1, occludin and Muc2 antibodies were provided by Servicebio (Wuhan, China). LGR5/GPR49 rabbit pAb was obtained from ABclonal Technology Co.,Ltd. (Wuhan, China). Protease K and colorimetric TUNEL apoptosis assay kits were obtained from Beyotime Biotechnology (Nanjing, China). Click-iT™ EdU kit (#C10337) was provided by Thermo Fisher Scientific Inc (Waltham, MA, USA).
Animals
Male C57BL/6J mice (6–8 weeks, 20–22 g) were sourced from Spelford Biotechnology (Beijing, China). All experimental procedures were performed in compliance with the guidelines for the care and use of experimental animals set forth by the National Institutes of Health and were approved by the Ethics Committee of Southwest Medical University (Approval No: 20220817–009). The mice were housed in the Animal Experimental Center of Southwest Medical University under specific pathogen-free (SPF) conditions, with a 12-hour light-dark cycle and free access to water. Prior to the commencement of the experiments, the mice were acclimatized to the environment for one week with adaptive feeding.
DSS-Induced Colitis Mice Model
After acclimatization, the mice were divided into 5 groups (n = 10 per group), including Ctrl, DSS, DSS+5’ASA, DSS+L-Rov, and DSS+H-Rov groups. Except for Ctrl group, the remaining four groups of mice were induced with acute colitis by freely drinking water containing 3% DSS from day 0 to day 6. Subsequently, their drinking water was switched to sterile water. From day 0 to day 10, mice in the DSS, DSS+5’ASA, DSS+L-Rov, and DSS+H-Rov groups received daily oral administrations of water, 5’ASA (200 mg/kg), and Rov (12.5 and 25 mg/kg), respectively. The mice in the Ctrl group were given normal drinking water and orally administered water daily. The selection of dosage was based on a preliminary experiment as well as referring to previous reports where the commonly used oral dosage ranged from 10 to 200 mg/kg.11,17,18
Throughout the experimental period, the mice had free access to drinking water and food. Body weight was monitored daily, and the disease activity index (DAI) was evaluated by summing weight loss (scored from 0 to 4), stool consistency (scored from 0 to 4), and rectal bleeding (scored from 0 to 4). On day 11, the mice were sacrificed after anesthesia. The entire length of the colon and rectum (including cecum to rectum) was measured, and ileum and colon tissue samples were collected. Segments (1.5 cm) of the distal ileum and colon were placed in 4% paraformaldehyde (PFA) solution for fixation, followed by paraffin embedding and sectioning. Similarly, segments of the ileum and colon were washed, placing in RNAlater at −20 °C or directly storing in −80°C. Blood samples were centrifuged at 3000 rpm/min and 12000 rpm/min at 4 °C for 5 min and 10 min, respectively, to acquire serum, which were then stored at −80 °C. Notably, 3–5 specimens from each group were randomly selected for subsequent analysis.
Culture of Intestinal Organoids
Male C57BL/6J mice were euthanized to collect the proximal segment of the small intestine, followed by cutting into pieces, and washing with cold PBS. The tissue fragments were incubated in 5 mM ethylene diamine tetraacetic acid (EDTA) with PBS for 15 min on ice. After removal of EDTA medium, vigorous suspension and centrifugation were conducted to enrich crypts. Pelleted crypts were resuspended and seeded (1000–2000 crypts per well) in Matrigel on a prewarmed 24-well plate, followed by an incubation period of 15 min at 37 °C. Subsequently, pre-prepared organoid medium (IntestiCult™ organoid growth medium) was added once the substrate gel had solidified. After 3 hours, the crypts would aggregate into a spherical structure, with budding occurring within 2–4 days. The culture medium was refreshed three times a week. After 7–10 days, the crypts reached maturity.
TNF-α-Mediated Epithelial Injury in Intestinal Organoids
To detect the reparative effect of Rov, intestinal organoids were treated with TNF-α to induce intestinal damage.19 Intestinal organoids were categorized into four groups, including Ctrl, Rov, TNF-α, and TNF-α+Rov groups. All groups were given complete medium on day 0. TNF-α, and TNF-α+Rov groups were exposed to 500 ng·mL−1 TNF-α for 24 h on day 3. Rov and TNF-α+Rov groups received 1.0 μM Rov starting from day 0 of the organoid culture. In a preliminary test, it was found that at the concentration range of 0.5–5 μM, the number of building organoids were increased, while the high concentration of Rov at 10 μM slightly decreased the organoid development, indicating that Rov was not cytotoxic to organoids at a concentration up to 5 μM. Consequently, the Rov concentration of 1 μM was selected for subsequent study.
Histological Analysis
Proximal colon tissues were fixed by formalin and embedded in paraffin. Following dewaxing, the paraffin sections were stained with hematoxylin and eosin (H&E), dehydrated and sealed. They were then examined under a microscope, and images were captured and analyzed using the Nikon Eclipse E100 microscope (Nikon Instruments Inc., Tokyo, Japan). The scoring standard of H&E stained tissue was conducted according to previous reports.20 The heights of five complete villi and the depths of five crypts in the small intestine were measured using the Nikon Eclipse Ci-L microscope (Nikon Instruments Inc., Tokyo, Japan). Image analysis was performed using Image J software (Version 1.48v, NIH, USA), followed by calculation of averages.
Biochemical Determination
The concentrations of serum IL-1β, IL-4, IL-17, IFN-γ, TNF-α, TGF-β1, and MPO were detected by ELISA kits according to the manufacturer’s instruction.
Immunohistochemistry (IHC) Analysis
To evaluate the expression of tight junction proteins (such as ZO-1 and occludin) in the intestines, IHC analysis was conducted following our previously established protocol.7 The staining was visualized using diaminobenzidine (DAB) and counterstained with hematoxylin (Servicebio, Wuhan, China). Stained sections were examined under the Nikon Eclipse E100 microscope, and the area of positive DAB staining was quantified using Image J software.
Immunofluorescence (IF) Analysis
IF staining of Lgr5, Lyz1, ChgA and Muc2 in intestine tissue sections were performed. The samples were incubated with respective Lgr5, Lyz1, ChgA and Muc2 antibodies, followed by HRP-linked secondary antibodies. CY3-Tyramide (TSA) was subsequently applied for staining. After labeling the cell nuclei with DAPI, slides were examined using the Nikon Eclipse C1 laser scanning confocal microscope (Nikon Instruments Inc., Tokyo, Japan).
TUNEL Cells Apoptosis Assay
The TUNEL assay was performed as we previously reported.20 The paraffin sections underwent treatment with DNase-free protease K for 30 min after dewaxing, followed by TUNEL staining according to the manufacturers’ instructions. Subsequently, a Nikon Eclipse C1 microscope was utilized for observation, and the number of TUNEL-positive cells was counted.
EdU Cell Proliferation Assay
Organoids were exposed to 10 μM of EdU for 2 h. Afterward, organoids were harvested. Subsequently, cell fixation, permeabilization, and EdU detection were performed according to manufacturers’ instructions.
RNA-Sequencing Analysis
The RNA-sequencing analysis of organoids involved the extraction of RNA from harvested samples, followed by library preparation and quality assessment. Briefly, High-quality RNA samples (total RNA ≥ 1 μg, concentration ≥ 35 ng/μL, OD260/280 ≥ 1.8, OD260/230 ≥ 1.0, RNA integrity number ≥ 8.0) were selected for constructing eukaryotic transcriptome libraries using the Illumina TruSeq™ RNA sample prep Kit. mRNA was isolated through magnetic bead-based selection and base pairing, followed by random fragmentation into fragments of approximately 300 bp. cDNA synthesis was performed using mRNA as a template, followed by adapter ligation. Transcriptome sequencing was conducted using an Illumina NovaSeq 6000 system (Illumina, USA). Principal component analysis (PCA) was utilized to compare gene expression levels across different experimental groups. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was employed to compare the signaling pathways between the model group and the treatment group.
Statistical Analysis
GraphPad Prism 9.0 was used for statistical analysis. All assay data are shown as the mean ± SD. One-way ANOVA with the follow-up Tukey’s test was used for multiple comparisons. A level of p < 0.05 was considered of statistically difference.
Results
Rov Protects Against DSS-Induced Colitis
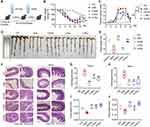
The therapeutic effect of Rov (12.5 and 25 mg/kg) was assessed on a mouse colitis model induced by DSS. The mice were grouped and administered with drugs as illustrated in Figure 1A. Except for the Ctrl group, a gradual decrease in body weight was observed in the remaining four groups of mice after DSS challenge. Particularly, mice in the DSS group exhibited a rapid decrease in body weight, accompanied by noticeable diarrhea and bloody stools, indicating successful construction of the colitis model. Starting from day 8, the body weights of L-Rov and H-Rov groups gradually increased as in the 5’ASA group, suggesting that Rov could attenuate the weight loss in mice with acute colitis (Figure 1B). The DAI based on indicators such as weight loss, stool consistency, and rectal bleeding, provided a preliminary assessment of the mice’s condition. As depicted in Figure 1C, the DAI scores of mice in the DSS group were significantly higher than those in the Ctrl group and the Rov-treated group. It is worth noting that the DAI scores in the H-Rov group gradually decreased and became similar to those in the Ctrl group. This suggests that Rov could delay the onset of acute colitis to some extent. The colon lengths depicted in Figure 1D clearly demonstrated that the colon length in the DSS group was shorter than that in the Ctrl group. Further analysis revealed that the average colon length in the H-Rov group was significantly longer than that in the DSS and L-Rov groups (Figure 1E). Therefore, it is believed that an appropriate dose of Rov can partially restore the colon length in mice with acute colitis, indicating effective treatment of acute colitis. The histological analysis of colon and ileum tissues stained with H&E (Figure 1F) revealed that the mucosal epithelium of mice in the Ctrl group was intact, with a normal number and structure of crypts. In contrast, mice in the DSS group exhibited severe pathological changes in the colon and ileum, including infiltration of inflammatory cells into crypts and mucosa, leading to mucosal thickening and shedding, exacerbated infiltration of inflammatory cells, reduced goblet cells, and shortened glandular bundles. The extent of damage in the Rov and 5’ASA groups was significantly reduced, with reduced infiltration of inflammatory cells and relatively intact intestinal epithelium with visible crypt structures. The histopathological scores of colon and ileum tissues (Figure 1G and H) showed that the histological scores in the Rov group were lower than those in the DSS group and closer to those in the Ctrl group, indicating significant reduction of intestinal damage. Additionally, an analysis of crypt depth and villus length in the ileum (Figure 1I and J) revealed that both parameters were lower in DSS group compared to other groups, suggesting that Rov could alleviate intestinal damage induced by DSS-mediated acute colitis.
Notably, the protective effect of Rov (12.5 and 25 mg/kg) was generally smaller than that of 5’ASA regarding weight loss attenuation, DAI score and histopathological alterations in colon. Whereas, the impact of Rov (particularly for the H-Rov group) on histopathological alterations in ileum, crypt depth and villus length of ileum was comparable to that of 5’ASA group.
Rov Protects Against DSS-Induced Gut Inflammation and Tight Conjunction Despair
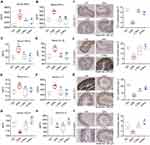
Given the potential of epithelial damage to trigger inflammatory responses,21 this study conducted further examinations on associated inflammatory indicators. MPO is a peroxidase located in lysosomal azurophilic granules of neutrophils and released upon stimulation of the immune system.22 MPO release is important for mediating oxidative stress through generating reactive oxygen species.23 Elevating MPO level positively correlates with the neutrophil infiltration in gut, thereby measuring as a biomarker of inflammation.24 Therefore, the levels of MPO in mouse serum were assessed, revealing significantly lower levels in the Rov-treated groups compared to the DSS group (Figure 2A). The findings indicate that Rov holds promise in alleviating oxidative reactions within intestinal cells, thus attenuating tissue damage induced by intestinal inflammation and ultimately providing intestinal protection. To delve deeper into the pivotal role of Rov in modulating the expression of inflammatory factors, ELISA assays were employed to detect both pro-inflammatory factors, including IFN-γ, TNF-α, IL-1β, IL-6, IL-17, and anti-inflammatory factors such as TGF-β1 and IL-4. The levels of pro-inflammatory factors in the L-Rov and H-Rov groups were significantly lower than those in the DSS group (Figure 2B–F). In contrast, the levels of TGF-β1 and IL-4 were higher in the Rov group compared to the DSS group (Figure 2G and H). In summary, Rov demonstrated a significant reduction in the expression of MPO and pro-inflammatory factors in mice with acute colitis, while concurrently enhancing the expression of anti-inflammatory factors, indicating its potent anti-inflammatory effects.
One of the pathophysiological characteristics of IBD is the disruption of the epithelial barrier, which is essential to maintain intestinal permeability.25 Studies have demonstrated characteristic alterations in intestinal permeability among patients with enteritis.26 Additionally, the epithelial cell framework, comprising ZO-1 and occludin, is pivotal in safeguarding and regulating the permeability of the intestinal mucosa.27 The absence of ZO-1 and occludin results in structural alterations between cells, heightening intestinal permeability. This facilitates the invasion of bacteria and viruses, thereby compromising the protective function of the intestinal mucosa.28,29 IHC was utilized to assess the levels of ZO-1 and occludin (Figure 2I–L). In both ileal and colonic tissues, the expression of ZO-1 and occludin significantly decreased in the DSS group, whereas it notably increased in the Rov group, nearly reaching levels observed in the control group. The results indicate that Rov effectively prevents the reduction of ZO-1 and occludin caused by DSS-induced acute colitis, potentially restoring intestinal barrier function.
Rov Inhibits DSS-Induced Intestinal Epithelial Apoptosis
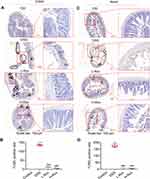
Intestinal epithelial cells (IECs) play a pivotal role in maintaining the integrity of the gut barrier system, a critical defense mechanism against luminal pathogens and toxins. Their apoptosis is intricately linked to the initiation and progression of mucosal inflammation.30 Therefore, TUNEL staining was used to evaluate the apoptotic status of IECs in the colon and ileum of mice. As depicted in Figure 3, a notable increase in apoptosis of IECs was observed in the DSS-induced acute colitis model in mice, corroborating findings from previous studies.31 Remarkably, both the Ctrl group and Rov treatment group exhibited significantly lower counts of apoptotic cells in the colon and ileum compared to the DSS group (Figure 3). These findings underscore the potential of Rov in attenuating the apoptosis of colonic and ileal epithelial cells, thereby offering a protective effect against intestinal injury associated with acute colitis.
Rov Restores Intestinal Despair by Increasing the Number of Lgr5+, Lyz1+ and Muc2+ Cells in Intestines
Previous research has indicated a close association between increased apoptosis of IECs and the disruption of intestinal barrier.32 Lgr5 is a significant marker for ISCs, offering vital cellular resources to maintain the stability of the intestinal epithelial barrier. Paneth cells play pivotal roles in regulating the balance of gut microbiota by secreting antimicrobial peptides and other regulatory molecules. Therefore, Lyz1, as one of the markers for Paneth cells, serves to evaluate the activity and function of these cells, offering insights into their involvement in both intestinal health and diseases. Furthermore, Muc2 acts as a protective barrier that covers the epithelial surface of the intestine, shielding it from physical and chemical damage caused by luminal contents, such as digestive enzymes and pathogens. This barrier helps prevent direct contact between harmful agents and the underlying epithelial cells, thus reducing the risk of injury and inflammation. Lastly, ChgA is a marker for enteroendocrine cells, which secrete hormones and growth factors crucial for maintaining intestinal immunity and metabolic balance. Therefore, immunofluorescence staining was employed to detect Lgr5+, Lyz1+, Muc2+, and ChgA+ in the small intestine and colon of mice to assess the health status of the intestinal mucosa. Although the differences in ChgA+ among groups were not significant, the quantities of Lgr5+, Lyz1+, and Muc2+ in the small intestine and colon of both L-Rov group and H-Rov group were notably higher compared to those in the DSS group (Figure 4 and Supplementary Figure 1). These findings imply that Rov has the potential to stimulate ISCs and safeguard mucosal layer cells, thereby bestowing a protective influence on intestinal crypts. Consequently, the study posits that Rov might mitigate the harm and apoptosis of IECs in cases of acute colitis.
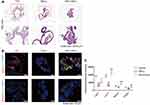
Rov Promotes the Growth of Intestinal Organoids and Protects Against TNF-α-Induced Organoid Injury
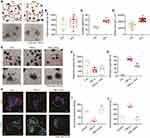
To evaluate the influence of Rov on intestinal organoids, images were meticulously captured using an optical microscope, and subsequent manual counting was conducted to analyze and quantify several parameters, including the number, surface area, and budding rate of intestinal organoids. Impressively, Rov (1.0 μM) exhibited a substantial augmentation in the surface area of intestinal organoids along with a notable enhancement in their budding rate, while maintaining a consistent overall number of organoids (Figure 5A–D). These findings underscore Rov’s significant capacity to effectively stimulate the growth and morphological development of organoids under physiological conditions.
In a TNF-α-induced organoid inflammatory injury model,19 the regenerative potential of Rov under pathological conditions was investigated. Following 24 h of exposure to 500 ng/mL TNF-α, approximately 80% of the organoids exhibited damage, characterized by shrinkage, darkening, and loss of budding morphology (Figure 5E). Nonetheless, organoids co-cultured with Rov and TNF-α exhibited a significant reduction in the damage rate to around 50%, although the change in the number of organoids was not significant (Figure 5F and G). Moreover, EdU staining and TUNEL assays were conducted on organoids from both the model and treatment groups to assess cell proliferation and apoptosis. It was observed that Rov treatment ameliorated cell loss (Figure 5H and I), indicative of the initiation of regeneration. Additionally, Rov mitigated TNF-α-induced cell apoptosis (Figure 5J). These results underscore the promising potential of Rov in promoting cell proliferation, suppressing cell apoptosis, and thereby facilitating the restoration of intestinal functionality under inflammatory conditions.
Rov Reduces Epithelial Apoptosis in Intestinal Organoids and Promotes Lgr5+ Stem Cell Mediated Repair of TNF-α-Induced Injury
To investigate the influence of Rov on cells within damaged organoids, H&E staining and IF staining were employed to assess the control group, model group (TNF-α group), and treatment group (TNF-α + Rov group). Results from H&E staining revealed that organoids in the control group exhibited well-preserved morphology with tightly packed cells, while those in the TNF-α group displayed diminished or shrunken buds, with a notable scarcity of cells (Figure 6A). Furthermore, IF staining was conducted to evaluate the epithelial cell levels in different groups. Assessment of epithelial cell markers including Lgr5+, Lyz1+, Muc2+, and ChgA+ (Figure 6B) demonstrated a downregulation in expression within the TNF-α group. However, in organoids subjected to Rov treatment, there was a remarkable upregulation in the expression of these IEC markers, particularly evident in Lyz1+ and Muc2+ (Figure 6C). Taken together, the findings suggest that Rov holds the potential to mitigate inflammation-induced damage to ISCs, promote IECs differentiation, and uphold the integrity of intestinal epithelial function.
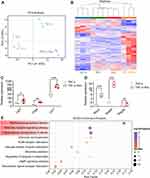
Regulation of Genes and Signaling Pathways by Rov in the Intestinal Epithelium
To unravel the intricate dynamics of gene expression and transcriptional processes within cells, RNA sequencing analysis was conducted on organoids, identifying a comprehensive dataset of 28,111 genes and 95,760 transcripts. PCA was employed to dissect the gene expression profiles across different experimental groups, revealing significant distinctions between the TNF-α-induced model group and the Ctrl, Rov, and TNF-α+Rov treatment groups (Figure 7A). These findings suggest the capacity of Rov treatment to induce nuanced alterations in gene expression patterns, gradually steering them towards a profile reminiscent of normal organoids. The heatmap representation of differentially expressed genes among the TNF-α, Rov, Ctrl, and TNF-α+Rov groups (Supplementary Table 1) delved deeper into genes associated with intestinal epithelial cells and intestinal stem cells within the organoids (Figure 7B). Rov treatment elicited a significant upregulation in the expression of crucial intestinal epithelial markers, including Lgr5, ChgA, and Lyz1 (Figure 7C). Moreover, genes linked with goblet cells, such as Muc2, Defa5, and the antimicrobial peptide Reg3a, exhibited substantial enhancement following Rov treatment (Figure 7D). These insights align closely with in vivo observations, underscoring Rov’s potential in reinstating intestinal mucosal functionality. Furthermore, KEGG pathway enrichment analysis of differentially expressed genes between the TNF-α+Rov and TNF-α groups (Figure 7E) revealed significant enrichment in multiple pathways after Rov treatment, including staphylococcus aureus infection, NOD-like receptor signaling, and transcriptional misregulation in cancer. With a closer look, the main altered genes in these signaling pathways mainly included the defensins (Defa) (Supplementary Table 2), which were secreted mostly by Paneth cells and other epithelial. Further verification by qPCR of Defa5 indicated that its relative expression in both cultured organoids and ileum tissues of colitis mice was significantly increased after Rov treatment (Supplementary Figure 2). This result indicate that Rov restores epithelial injury via promoting growth of epithelial populations.
Discussion
In recent years, natural products have attracted significant attention from researchers owing to their safety profile and promising potential in treating IBD. Rov, a significant active component found in Rhodiola spp. (especially the Rhodiola rosea), has been previously reported with anti-oxidant, anti-fatigue, anti-tumor and immunomodulatory effects.17 In several chronic conditions including the idiopathic pulmonary fibrosis, sepsis, non-alcoholic fatty liver diseases and ischemia–reperfusion injury, supplementation of Rov has resulted in remarkable therapeutic effects.17 Notably, Rov has shown remarkable efficacy in treating radiation-mediated intestinal damage.11 It was found that rosavin significantly improved the cell viability of the irradiated IEC-6 cells.11 Park et al in 2018 demonstrated that Rov together with zinc, prebiotics and 12 probiotics could effectively alleviate acute colitis in mice.12 While these discoveries suggest that Rov may hold the potential to alleviate IBD, there are still no direct evidence yet. Therefore, in-depth research into the therapeutic effects of Rov on IBD and its impact on the intestinal epithelial system is crucial for the development of novel therapeutic products.
The DSS-induced acute colitis mouse model was employed to simulate human enteritis in the study. Rov administration significantly delayed weight loss in DSS-induced mice, restored colon length, and reduced DAI scores. Notably, levels of pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-1β, IL-6, IL-17,33,34 along with MPO, a marker of neutrophil activation, showed significant reductions in mouse serum following Rov treatment, while anti-inflammatory factors TGF-β1 and IL-4 levels increased.35 These findings indicate that Rov, akin to RCE, possesses potent anti-inflammatory properties. ZO-1 and occludin serve as essential components of the epithelial cell scaffold, crucial for maintaining intestinal permeability.29 During acute enteritis, the intestinal mucosa is susceptible to damage, which allows toxins to enter the bloodstream, triggering inflammatory responses,36 and resulting in apoptosis of IECs.37 Rov intake stimulates the secretion of antimicrobial peptides, thereby fortifying the intestinal epithelium. Mice treated with Rov displayed intact IECs with minimal apoptotic cells and significantly increased expression of ZO-1 and occludin proteins, suggesting Rov can mitigate epithelial damage and restore intestinal barrier function. More importantly, in intestine of colitis mice, Rov stimulated Lgr5+ ISCs and safeguard mucosal layer cells (Lyz1+, ChgA+, and Muc2+ cells), thereby promoting epithelial regeneration and bestowing a protective influence on intestinal crypts.
Due to the robust utility of intestinal organoids in studying the onset and progression of intestinal diseases, this study established an in vitro intestinal organoid model to investigate the protective mechanism of Rov on the intestinal epithelial barrier. Subsequently, TNF-α-induced small intestinal organoids were utilized to mimic epithelial damage observed in patients with acute enteritis. Intestinal organoids are primarily established in vitro due to the presence of ISCs in the crypts.14 As Lgr5+ ISCs are much less in colon than that in ileum, in the present study, small intestine organoids were used. Under physiological conditions, Rov directly promoted the budding of small intestinal organoids, with ISCs and Paneth cells located at the bud tips.38 Even under TNF-α-induced pathological conditions, damaged small intestinal organoids remained repairable, promoting the differentiation of damaged epithelial cells and the proliferation of ISCs. Further analysis of organoid gene sequencing revealed that Rov not only upregulated the expression of Lgr5, Lyz1, ChgA, and Muc2 but also upregulated the expression of crucial genes for cellular proliferation and other life activities, such as Defa5 and Reg3.39,40 Thus, Rov promotes epithelial cell differentiation and protects the intestinal epithelial barrier function damaged by TNF-α. However, further research is necessitated to validate the related pathways and molecular targets mediated by Rov. Moreover, the current study did not investigate the toxicological profile of Rov in mice, which necessitated future research.
Conclusion
In summary, both an in vivo model of acute colitis and an in vitro model of intestinal organoids induced by TNF-α were developed to investigate the anti-inflammatory effects of Rov in IBD and elucidate its potential mechanisms of action. These results indicate that Rov has a significant alleviating effect and epithelial protection on colitis. Additionally, the establishment of intestinal organoids provides a feasible strategy for the preclinical screening and personalized drug therapy of natural products like Rov.
Abbreviations
CD, Crohn’s disease; DSS, dextran sulfate sodium salt; ELISA, enzyme-linked immunosorbent assay; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; ISC, intestinal stem cell; MPO, myeloperoxidase; Rov, rosavin; UC, ulcerative colitis; ZO-1, zonula occludens 1.
Chemical Compounds
Rosavin (PubChem ID, CID 9823887).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgment
This work was supported by the Sichuan Science and Technology Program (Grant No. 2023NSFSC0614, 2023NSFSC1848), grant from the University of Macau (Grant No. MYRG2022-00009-ICMS), Science and Technology Program of Luzhou, China (Grant No. 2022-YJY-127), grant from Southwest Medical University, China (Grant No. 2021ZKZD017, 05/00190197), and grant from SCU-Luzhou Platform Construction of Scientific and Technological Innovation, China (Grant No. 2022CDLZ-20).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Zeng J, Li M, Zhao Q, et al. Small molecule inhibitors of RORγt for Th17 regulation in inflammatory and autoimmune diseases. J Pharm Anal. 2023;13(6):545–562. doi:10.1016/j.jpha.2023.05.009
2. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterol. 2017;152(2):313–32. doi:10.1053/j.gastro.2016.10.020
3. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: A review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15(1):39–49. doi:10.1038/nrgastro.2017.136
4. Yue B, Luo X, Yu Z, Mani S, Wang Z, Dou W. Inflammatory bowel disease: A potential result from the collusion between gut microbiota and mucosal immune system. Microorganisms. 2019;7(10). doi:10.3390/microorganisms7100440
5. Bardenbacher M, Ruder B, Britzen-Laurent N, et al. Permeability analyses and three dimensional imaging of interferon gamma-induced barrier disintegration in intestinal organoids. Stem Cell Res. 2019;35:101383. doi:10.1016/j.scr.2019.101383
6. Xiao J, Wang J, Chen Y, Zhou Z, Gao C, Guo Z. Sauchinone ameliorates intestinal inflammation and promotes Th17 cell production of IL-10 via Blimp-1. Biochem Biophys Res Commun. 2020;522(2):435–441. doi:10.1016/j.bbrc.2019.11.122
7. Yang Y, Li M, Liu Q, et al. Starch from pueraria lobata and the amylose fraction alleviates dextran sodium sulfate induced colitis in mice. Carbohydr Polym. 2023;302:120329. doi:10.1016/j.carbpol.2022.120329
8. Yang Y, Wang Y, Zhao L, et al. Chinese herbal medicines for treating ulcerative colitis via regulating gut microbiota-intestinal immunity axis. Chinese Herbal Medicines. 2023;15(2):181–200. doi:10.1016/j.chmed.2023.03.003
9. Tao H, Wu X, Cao J, et al. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med Res Rev. 2019;39(5):1779–1850. doi:10.1002/med.21564
10. Wang Y, Tao H, Huang H, et al. The dietary supplement rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. 2021;12(7):3142–3158. doi:10.1039/D0FO03061A
11. Zhou W, Chen K, Lu Q, et al. The protective effect of rosavin from rhodiola rosea on radiation-induced intestinal injury. Chem Biodivers. 2020;17(12):e2000652. doi:10.1002/cbdv.202000652
12. Park JS, Choi J, Kwon JY, et al. A probiotic complex, rosavin, zinc, and prebiotics ameliorate intestinal inflammation in an acute colitis mouse model. J Transl Med. 2018;16(1):37. doi:10.1186/s12967-018-1410-1
13. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi:10.1038/nature07935
14. Luo H, Li M, Wang F, et al. The role of intestinal stem cell within gut homeostasis: Focusing on its interplay with gut microbiota and the regulating pathways. Int J Bio Sci. 2022;18(13):5185–5206. doi:10.7150/ijbs.72600
15. Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science. 2013;340(6137):1190–1194. doi:10.1126/science.1234852
16. Sato T, Stange D, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and barrett’s epithelium. Gastroenterol. 2011;141(5):1762–1772. doi:10.1053/j.gastro.2011.07.050
17. Wang S, Feng Y, Zheng L, et al. Rosavin: Research advances in extraction and synthesis, pharmacological activities and therapeutic effects on diseases of the characteristic active ingredients of rhodiola rosea L. Molecules. 2023;28(21):7412. doi:10.3390/molecules28217412
18. Xin X, Yao D, Zhang K, et al. Protective effects of rosavin on bleomycin-induced pulmonary fibrosis via suppressing fibrotic and inflammatory signaling pathways in mice. Biomed Pharmacother. 2019;115:108870. doi:10.1016/j.biopha.2019.108870
19. Lin Y, Lu Y, Huang Z, et al. Milk-derived small extracellular vesicles promote recovery of intestinal damage by accelerating intestinal stem cell-mediated epithelial regeneration. Mol Nutr Food Res. 2022;66(11):e2100551. doi:10.1002/mnfr.202100551
20. Li H, Wang Y, Shao S, et al. Rabdosia serra alleviates dextran sulfate sodium salt-induced colitis in mice through anti-inflammation, regulating Th17/Treg balance, maintaining intestinal barrier integrity, and modulating gut microbiota. J Pharm Anal. 2022;12(6):824–838. doi:10.1016/j.jpha.2022.08.001
21. Wu X, Huang H, Li M, et al. Excessive consumption of the sugar rich longan fruit promoted the development of nonalcoholic fatty liver disease via mediating gut dysbiosis. Food Front. 2023;4(1):491–510. doi:10.1002/fft2.185
22. Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52. doi:10.1016/j.abb.2018.01.004
23. Chen S, Chen H, Du Q, Shen J. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front Physiol. 2020;11:433. doi:10.3389/fphys.2020.00433
24. Hanning N, De Man JG, De Winter BY. Measuring myeloperoxidase activity as a marker of inflammation in gut tissue samples of mice and rat. Bio-Protocol. 2023;13(13):e4758. doi:10.21769/BioProtoc.4758
25. Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):401–407. doi:10.3748/wjg.14.401
26. Büning C, Geissler N, Prager M, et al. Increased small intestinal permeability in ulcerative colitis: Rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis. 2012;18(10):1932–1939. doi:10.1002/ibd.22909
27. Kuo WT, Zuo L, Odenwald MA, et al. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterol. 2021;161(6):1924–1939. doi:10.1053/j.gastro.2021.08.047
28. Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107(11):686–696. doi:10.17235/reed.2015.3846/2015
29. Lin JC, Wu JQ, Wang F, et al. QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-κB signalling. Cell Prolif. 2019;52(2):e12547. doi:10.1111/cpr.12547
30. Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J Gastroenterol Hepatol. 2002;17(7):758–764. doi:10.1046/j.1440-1746.2002.02791.x
31. Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2002;47(7):1447–1457. doi:10.1023/A:1015931128583
32. Woznicki JA, Saini N, Flood P, et al. TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells. Cell Death Dis. 2021;12(10):864. doi:10.1038/s41419-021-04151-3
33. Hou Q, Ye L, Liu H, et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25(9):1657–1670. doi:10.1038/s41418-018-0070-2
34. Dey R, Joshi AB, Oliveira F, et al. Gut microbes egested during bites of infected sand flies augment severity of leishmaniasis via inflammasome-derived IL-1β. Cell Host Microbe. 2018;23(1):134–143. doi:10.1016/j.chom.2017.12.002
35. Li H, Fan C, Lu H, et al. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm Sin B. 2020;10(3):447–461. doi:10.1016/j.apsb.2019.08.006
36. Liu J, Cai J, Fan P, Zhang N, Cao Y. the abilities of salidroside on ameliorating inflammation, skewing the imbalanced nucleotide oligomerization domain-like receptor family pyrin domain containing 3/autophagy, and maintaining intestinal barrier are profitable in colitis. Front Pharmacol. 2019;10:1385. doi:10.3389/fphar.2019.01385
37. Zhang YS, Xin DE, Wang Z, et al. STAT4 activation by leukemia inhibitory factor confers a therapeutic effect on intestinal inflammation. EMBO j. 2019;38(6). doi:10.15252/embj.201899595.
38. Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi:10.1038/nature09637
39. Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi:10.1146/annurev-immunol-031210-101312
40. Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. doi:10.1016/j.ccr.2009.01.002
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.