Back to Journals » Journal of Inflammation Research » Volume 17
Pustular Psoriasis Induced by Dupilumab: A Case Report
Authors Liu Y , Liu L , Zhou H, Chen G, Wen C, Wu R
Received 1 May 2024
Accepted for publication 7 September 2024
Published 16 September 2024 Volume 2024:17 Pages 6389—6394
DOI https://doi.org/10.2147/JIR.S476297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yuetong Liu,1,* Lanxin Liu,1,* Hongmei Zhou,2 Guangfang Chen,2 Changhui Wen,2 Ran Wu2
1Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550002, People’s Republic of China; 2Department of Dermatology, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ran Wu, Department of Dermatology, The first affiliated hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550001, People’s Republic of China, Email [email protected]
Abstract: Atopic dermatitis (AD) and psoriasis (Pso) are both recurrent inflammatory diseases. Recently, it has been reported that a small number of AD patients experience immune drift after receiving dupilumab treatment, leading to a transition from eczema-like lesions to psoriasis-like lesions. Here, we report a case of pustular psoriasis caused by dupilumab treatment for atopic dermatitis. This case underscores the diagnostic challenges associated with immune drift related to biological agents while also highlighting the shortened time frame for immune drift onset compared to previous reports.
Keywords: immune drift, dupilumab, atopic dermatitis, pustular psoriasis
Introduction
Currently, there have been reports of a few atopic dermatitis patients going from eczema-like lesions to psoriasis-like ones after treatment with dupilumab. This change is due to an immune shift. Here, we report a case of pustular psoriasis caused by dupilumab treatment for atopic dermatitis. It raises awareness among doctors about the possible development of psoriasis in patients treated with dupilumab.
Case Presentation
The patient was a 3-year-and-6-month-old boy presented to our department on November 15, 2023, with a history of “recurrent red bumps, dry and itchy skin for 3+ years, worsening over the past 2+ months”. His symptoms began more than 3 years ago with the presence of red, itchy bumps and dry skin without any clear cause. However, he did not receive systematic treatment at that time. His condition worsened 2+ months prior. After consulting several hospitals, the patient was diagnosed with severe atopic dermatitis. Despite treatment, his skin lesions only slightly improved. Since then, he experienced recurrent symptoms and was admitted to our department. The patient presented with redness and papules scattered across the body. He also had dry skin, itching, scattered pinpoint lesions, and line scratches. The patient’s SCORAD score was 38 (mild: 0–24; moderate: 25–50; severe >50) (Figure 1A). The child had a history of allergic rhinitis and no family history of atopic dermatitis or psoriasis. Allergen screening (conducted at other hospital) revealed a TIgE of 92.28, with a reference value of 0.0–60.0 IU/mL, and he tested positive for allergies to house dust mites, milk, eggs, and wheat flour. After discussing the patient’s condition with his family and obtaining full consent, the patient received a 200 mg dupilumab injection. The next day, his condition worsened, with red plaques and pustules appearing all over his body. On December 1, 2023, the patient returned for a follow-up visit. His parents reported that one day after receiving the first dupilumab injection, he developed congestion in both eyes and a fever. His highest body temperature was 38.9 °C. Additionally, his skin lesions changed from redness and pimples to plaques and pustules (Figure 1B). Dermatoscopy indicated a tendency toward psoriasis (Figure 2A). Reflective confocal laser scanning microscopy suggested the possibility of psoriasis-like dermatitis (Figure 2B). Biopsies of the right abdominal skin revealed epidermal hyperkeratosis and parakeratosis, abscess formation in the stratum corneum, local crust formation, acanthosis thickening with mild spongy edema, and chronic inflammatory cell infiltration around the small vessels in the superficial dermis (Figure 2C). He was diagnosed with moderate atopic dermatitis on the basis of the clinical manifestations and auxiliary examinations. With the consent of the patient’s family, dupilumab treatment was suspended. We administered oral sulfoxamycin 0.5 g/d, halomethasone, tacrolimus, and hydrocortisone butyrate ointment. The pustules subsided within 1 week (Figure 3A), but the plaque pustules recurred. After a month of continuous treatment, the patient’s condition gradually stabilized, and the eruptions and pustules gradually subsided over his entire body. On February 20, 2024, the patient returned to our hospital for a follow-up visit. On February 14, 2024, he had received a second subcutaneous injection of dupilumab (200 mg). One day after the injection, he again developed scattered eruptions and pustules all over his body, but this time without fever or conjunctival congestion in either eye. The treatment plan remained essentially the same as before. After one month of stepped treatment, the pustules disappeared, and the patient’s skin lesions gradually recovered to a mild atopic dermatitis state (Figure 3B).
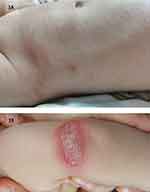 |
Figure 1 (A) A photo of the loin before dupilumab injection. (B) 1 day after injection of dupilumab(left elbow). |
 |
Figure 3 (A) A week after treatment of the left elbow. (B) A month after treatment of the left elbow. |
Discussion
Atopic dermatitis and psoriasis are common chronic inflammatory diseases in dermatology. They are characterized by T-cell immune reactions and abnormal keratinocyte growth.1 The pathogenesis of AD is primarily related to Th2-mediated responses and associated cytokines, including IL-4, IL-13, and IL-31. In contrast, the pathogenesis of Pso is closely associated with the induction of Th1, Th17, and Th22 cells.2
Dupilumab is a fully human IgG4 antibody that blocks the signaling of IL-4 and IL-13 by binding to the IL-4R-α and IL-13R-α-1 receptor subunits. This action leads to the downregulation of downstream receptor signaling in the JAK-STAT pathway, resulting in the inhibition of receptor signaling.3,4 Numerous studies have demonstrated that dupilumab is both effective and safe for the treatment of moderate to severe AD.5,6
When biologics are used to treat psoriasis or atopic dermatitis, Th1/Th2 imbalances may cause a shift in cytokine levels. This shift can sometimes result in the conversion to the opposite skin disease, where psoriasis patients may develop eczema-like skin lesions, and patients with atopic dermatitis may develop psoriasis-like skin lesions. This phenomenon is known as an immune shift. Dupilumab can block Th2 cell pathway genes in AD patients leading to a Th1/Th2 imbalance and causing Th2 cells to change into Th1 and Th17 cells. This transformation can trigger the onset of psoriasis.7
Reports indicate that some biologics can induce an immune shift in patients with AD or Pso.8 These biologics include ustekinumab, ixekizumab, adalimumab, and dupilumab. The results of a retrospective study conducted in Italy showed that in adults, the average time from the start of dupilumab therapy to the onset of psoriasis was 5–6 months (range: 1–30 months).9 Similarly, a study on pediatric patients with atopic dermatitis revealed that dupilumab caused the development of psoriasis-like skin lesions within 8 months of treatment.10 In our patient, however, psoriasis-like skin lesions developed after just one day of dupilumab treatment. To date, there have been no similar reported cases.
Currently, no guidelines or expert consensus exists regarding the treatment of immune shifts caused by biologics. The general approach is to discontinue the biologic and then administer drugs such as glucocorticoids. A study of 14 patients who developed immune shift revealed that for the treatment of psoriasis-like rashes caused by dupilumab, half of the patients chose to stop using dupilumab. Most of these patients required additional treatments, such as topical corticosteroids or other biologics such as omalizumab.11 An Italian literature review found that 38% of new “psoriasis” patients improved after discontinuing dupilumab. Additionally, 50% of patients with relapsed psoriasis patients improved after stopping treatment.9 For the patient in this report, we discontinued dupilumab treatment and administered antibiotics (mupirocin), corticosteroids (fluocinolone acetonide cream, hydrocortisone cream), and immunosuppressants (tacrolimus ointment). The patient’s condition has improved.
Conclusion
When using biologics, to manage immune shift, it is important to review the patient’s family and medical histories before starting treatment. Close follow-up should occur after treatment initiation. If signs of immune shift are detected, timely action should be taken. Dupilumab was introduced recently in China, so there is limited clinical experience with this treatment. Currently, there is no clear explanation for how dupilumab inhibits Th2 cells, nor do we fully understand its impact on the development of psoriasis. Many factors related to immune shift caused by dupilumab remain unknown and need further investigation.
Ethical Approval
Written informed consent was obtained from the patient and the patient’s parents for the publication of all the images and data included in this article. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical review and approval were not required to publish the case details in accordance with the institutional requirements.
Consent to Publish
The patient’s family provided informed consent to publish their case details and any accompanying images.
Funding
This work was supported by Guiyang Science and Technology Project [Tsuke Contract (2019) 9-2-10].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68–73. doi:10.1016/j.coi.2017.08.008
2. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. doi:10.1038/nature05663
3. Gooderham MJ, Hong HC, Eshtiaghi P, Papp KA. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3):S28–S36. doi:10.1016/j.jaad.2017.12.022
4. Seegräber M, Srour J, Walter A, Knop M, Wollenberg A. Dupilumab for treatment of atopic dermatitis. Expert Rev Clin Pharmacol. 2018;11(5):467–474. doi:10.1080/17512433.2018.1449642
5. Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab provides favourable long‐term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open‐label phase IIa study and subsequent Phase III open‐label extension study. Br J Dermatol. 2020;184(5):857–870. doi:10.1111/bjd.19460
6. Xu Y, Guo L, Li Z, Wu S, Jiang X. Efficacy and safety profile of dupilumab for the treatment of atopic dermatitis in children and adolescents: a systematic review and meta-analysis. Pediatr Dermatol. 2023;40(5):841–850. doi:10.1111/pde.15398
7. Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155–172. doi:10.1016/j.jaci.2018.08.022
8. Paolino G, Di Nicola MR, Brianti P, et al. New onset atopic dermatitis and psoriasis in the same patients under biologic treatments: the role of systemic treatments as a possible trigger. Dermatologic Therapy. 2022;35(11):e15814. doi:10.1111/dth.15814
9. Trave I, Salvi I, Burlando M, Cozzani E, Parodi A. “De Novo” psoriasis and relapse of psoriasis induced by dupilumab: three new cases and review of the literature. J Clin Med. 2023;12(19):6291. doi:10.3390/jcm12196291
10. Parker JJ, Sugarman JL, Silverberg NB, et al. Psoriasiform dermatitis during dupilumab treatment for moderate-to-severe atopic dermatitis in children. Pediatr Dermatol. 2021;38(6):1500–1505. doi:10.1111/pde.14820
11. Casale F, Nguyen C, Dobry A, et al. Dupilumab‐associated psoriasis and psoriasiform dermatitis in patients with atopic dermatitis. Australas J Dermatol. 2022;63(3):394–397. doi:10.1111/ajd.13846
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


