Back to Journals » Journal of Inflammation Research » Volume 17
Quercetin Attenuates MRGPRX2-Mediated Mast Cell Degranulation via the MyD88/IKK/NF-κB and PI3K/AKT/ Rac1/Cdc42 Pathway
Authors Zhao C, Ding Y, Huang Y, Wang C, Guo B, Zhang T
Received 27 June 2024
Accepted for publication 22 August 2024
Published 7 October 2024 Volume 2024:17 Pages 7099—7110
DOI https://doi.org/10.2147/JIR.S480644
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Chenrui Zhao,1,2,* Yuanyuan Ding,2,* Yihan Huang,2 Chao Wang,2 Bin Guo,1 Tao Zhang2
1Department of Anesthesiology, Xi’an Honghui Hospital, Xi’an Jiaotong University, Xi’an, 710054, People’s Republic of China; 2College of Pharmacy, Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Guo, Department of Anesthesiology, Xi’an Honghui Hospital, Xi’an Jiaotong University, Xi’an, 710054, People’s Republic of China, Email [email protected]
Background: CMRF35-like molecule-1 (CLM-1) is a receptor of the CD300 family that inhibits MRGPRX2-mediated mast cell degranulation. Understanding the role and mechanism of CLM-1 agonist has significant implications for the treatment of allergic disease. Quercetin is a natural small molecule compound derived from plants and vegetables that has been shown to prevent histamine release by immune cells.
Objective: This study aims to examine the inhibitory effects of quercetin on MRGPRX2-mediated mast cell degranulation via CLM-1.
Results: We found that C48/80 stimulation resulted in significantly increased release of β-hexosaminidase, histamine and Ca2+ in CLM-1-knockdown LAD2 cells than in NC-LAD2 cells. Surface plasmon resonance (SPR) and molecular docking analyses revealed high-affinity binding between quercetin and CLM-1 (KD = 2.962× 10− 5 mol/L) mediated by the formation of hydrogen bonds. In addition, quercetin can selectively bind to CLM-1 on mast cells, leading to SHP-1 phosphorylation and subsequent inhibition of downstream MyD88/IKK/NF-κB signaling. Furthermore, activation of CLM-1 modulated the surface expression of MRGPRX2 by inhibiting F-actin, leading to internalization of the MRGPRX2 receptor via the PI3K/AKT/ Rac1/Cdc42 pathway.
Conclusion: Quercetin is a promising treatment for allergic diseases by acting as a CLM-1 agonist that inhibits MRGPRX2-mediated mast cell degranulation.
Keywords: quercetin, CLM-1, inhibition, MRGPRX2, mast cell degranulation
Graphical Abstract:

Introduction
Mast cells (MCs) are key effector cells responsible for initiating inflammatory responses and are widely distributed around capillaries within various anatomical sites,1 including the skin, respiratory tract, and gastrointestinal tract. Due to their distinctive spatial distribution, MCs function not only as the first line of defense against external pathogens but also as the trigger of allergic diseases.2 When MCs become hyperactivated, they release various bioactive substances into the surrounding tissue milieu, subsequently precipitating allergic reactions and ultimately allergic disorders.3 MCs are typically activated through antigen/IgE/FcεRI cross-linking, a key process in the regulation of hypersensitivity.4 Recent research has unveiled that not all individuals experiencing allergic reactions exhibit elevated IgE levels, and conventional treatments may not invariably yield the desired efficacy.
The Mas-related G protein-coupled receptor 2 (MRGPRX2) has been reported to activate MCs through a non-IgE-mediated pathway.5 Recently, significant targets linked to pseudoallergic reactions have been found to be extensively expressed in human dendritic cells, basophils, and mast cells.6 In 2015, researchers from Johns Hopkins University in the United States demonstrated that MRGPRX2 on mast cells can be directly activated by the endogenous ligand substance P, thereby triggering an anaphylactoid reaction.5 Activation of MRGPRX2 by either exogenous agents (eg, C48/80) or endogenous ligands (eg, substance P) resulted in a rapid increase of intracellular Ca2+ levels within mast cells, leading to mast cell activation and subsequent onset of allergic symptoms.7,8 Therefore, the inhibition of MRGPRX2 activation stands as a pivotal treatment strategy for inflammatory responses.
CMRF35-like molecule-1 (CLM-1), also known as leukocyte single immunoglobulin-like receptor 3 (LMIR3), is an important inhibitory receptor of the CD300 family9 highly expressed on mast cells.10,11 This receptor contains two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and a immunoreceptor tyrosine-based switch motif (ITSM), which mediate the activation of immune cells by recruiting phosphatases.12 SHP-1 and SHP-2 are two members of the SH2 domain-containing nonreceptor protein tyrosine phosphatases (PTPs) that dephosphorylate FcεRIγ and the Y-residues of immunoreceptor tyrosine-based activation motif (ITAM), thereby regulating FcεRI signaling and inhibiting mast cell activation.13 In addition, CLM-1 not only exhibits inhibitory activity but also activates apoptotic cell engulfment via recruitment of p85α in the PI3K pathway.14 Ceramide is an exogenous ligand for mouse CLM-1 (mCD300f) and human CLM-1 (hCD300f). The interaction between CLM-1 and ceramide facilitates a negative regulatory effect on FcεRI-mediated MC activation.15 Notably, recent findings have indicated that CLM-1 also suppresses IgE-independent pseudoallergic responses through MRGPRX2/MRGPRB2.16 Furthermore, the absence of CLM-1 has been shown to exacerbate MC-dependent allergic reactions, airway inflammation, and dermatitis in mice.17 These studies collectively suggest that CLM-1 may represent a promising therapeutic target for MC-dependent allergic reactions. Nonetheless, the precise mechanism by which CLM-1 negatively modulates MRGPRX2-induced MC activation and allergic reactions remains unclear.
Flavonoids generally refer to compounds consisting of two benzene rings (ring A and ring B) containing phenolic hydroxyl groups, which are connected by a central three-carbon atom (C6-C3-C6 unit). Flavonoids include apigenin, baicalein, puerarin, quercetin, hesperidin, myricetin, kaempferol, etc,18 which have various physiological activities such as antioxidant, anti-inflammatory, hypoglycemic, hepatoprotective, antibacterial, antiviral, and antitumor effects. Flavonoids also exert anti-inflammatory effects by inhibiting the activity of nuclear factor κb (NF-κB) and TNF-α or activating adenosine 5’-monophosphate-activated protein kinase (AMPK). Kaempferol exerts anti-inflammatory effects by inhibiting the activity of NF-κB and TNF-α.19 Quercetin is a flavonoid compound derived from fruits and vegetables, such as apples and onions, with a wide range of biological activities.20 Studies have indicated that quercetin possesses robust and long-lasting anti-inflammatory properties21 and exerts immunosuppressive effects on dendritic cells.22 Existing research also shows that that quercetin exhibit both anti-inflammatory and immune-enhancing properties.23,24 Nevertheless, further exploration is warranted to identify the direct target and specific molecular mechanism of quercetin in the context of human MC LAD2 cell activation.
In the present study, we investigated the inhibitory effect of quercetin on MRGPRX2-mediated MC activation through CLM-1 and elucidated the potential mechanism of quercetin as an exogenous ligand for CLM-1. Furthermore, we examined the signaling pathways elicited by CLM-1 activation and the mechanisms involved in modulating MRGPRX2.
Materials and Methods
Pharmaceuticals and Reagents
Quercetin was purchased from Baoji chenguang (Baoji, China), ceramide was obtained from Macklin (Shanghai, China), and also purified to≥98%. Compounds 48/80 (C48/80), p-nitrophenyl N-acetyl-b-D-glucosamide (β-hexosamine) and Triton X-100 were purchased from Sigma-Aldrich Co., LLC. (Shanghai, China). All aqueous solutions are prepared with ultrapure water before use.
TM buffer consists 120 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 10 mmol/L HEPES, 5.5 mmol/L glucose, and 5 mmol/L BSA. Stop buffer composed of 0.1 mol/L Na2CO3 and 0.1 mol/L NaHCO3.
CIB buffer consists of 125 mmol/L NaCl, 3 mmol/L KCl, 2.5 mmol/L CaCl2, 0.6 mmol/L MgCl2, 10 mmol/L HEPES, 20 mmol/L glucose, 1.2 mmol/L NaHCO3, 20 mmol/L sucrose, pH 7.4. Fluorescent incubation buffer consisting of 0.5 μL Fluo-3, 2 μL Pluronic F-127 and 997.5 μL CIB.
Cell Lines
LAD2 cells were friendly provided by Kirshenbaum and D. Metcalfe (NIH, USA) and cultured in StemPro-34 medium supplemented with StemPro ® supplements, penicillin (1:100), streptomycin (1:100), 2 mm glutamine and 100 ng/mL human stem cell factor. The medium is normally replaced every 7 days, and the cell growth environment is 5% CO2 at 37 °C.5
β-Hexosamine Release Rate
LAD2 cells were inoculated in 96-well plate,100 μL per well while final concentration was 1×106 cells/mL. Only TM buffer was set as blank group and C48/80 group was set as control group.In order to inhibit C48/80 stimulation, quercetin or ceramide (100 μmol/L) was given to cells and were incubated for 30 min. And then, except for the blank group, other groups were incubated to C48/80 (final concentration of 30 μg/mL) for 30 min.
After centrifugation at 2000 rpm for 5 min, 50 μL supernatant were taken from each well. Then, a 0.1% Triton X-100 was added to blank group to lysis cells for 5 min, and 50 μL of the supernatant after lysis was taken. At last, 50 μL of β-hexosaminidase (1 μg/L) was added to the supernatant for 90 min at 37°C. The samples were measured at 405 nm after plus of the stop buffer.
Histamine Release Assay
LAD2 cells were inoculated in 96-well plate, 100 μL per well while final concentration was 1×106 cells/mL and centrifuged at 2,000 rpm for 5 min. Add 50 μL of TM buffer containing quercetin or ceramide (100 μmol/L) and incubate for 30 min in an incubator at 37°C, only TM buffer for blank and control group as well. After, add 50 μL of TM solution containing C48/80 (final concentration of 30 μg/mL) to each well except the blank group and incubate again for 30 min. After 2000 rpm centrifugation for 5 min, 50 μL of supernatant was taken from each well, 100 μL of histamine internal standard was added and mixed fully, and LC-MS was used for detection and analysis.
Calcium Influx Experiment in Cells
Quercetin and ceramide were diluted to 100 μmol/L with Calcium imaging buffer (CIB) while blank and control group only with CIB, then incubated all groups in a cell incubator for 30 min. After that, LAD2 cells were washed twice CIB and finally plated into the new plate 50 μL each well. The cells were taken under blue light using a fluorescence microscope (Nikon, Ti-U, Japan). The shooting condition was 1 shot per second for 2 min.
siRNA Transfection of LAD2 Cells
Specific knockout was achieved using siRNA targeting CLM-1 and non-targeted siRNA as negative controls (NC). The SMART double-stranded siRNAs for CLM-1 and non-specific siRNAs is from Shanghai GenePharma Co., Ltd. (Shanghai, China). The siRNA sequence is as follows:
Forward:5’-CGGUCCAACAACAGUGAAUTT-3’; reverse:5’-AUUCACUGUUGUUGGACCGTT-3’ for CLM-1; and forward:5’-UUCUCCGAACGUGUCACGUTT-3’: reverse:5’-ACGUGACACGUUCGGAGAATT-3’ for the control.
During transfection, according to the manufacturer ‘s instructions, GP-transfect-Mate transfection reagent Shanghai GenePharma Co., Ltd. (Shanghai, China) was used to deliver siRNA at a final concentration of 20 nM, and the expression of CLM-1 was inhibited after 36 h of cell incubation. The effectiveness of transfected siRNA expression was confirmed by western blot and RT-PCR experiments.
Western Blot
A total of 1×106 LAD2 cells were inoculated into the well plate, then discard the medium and wash twice with ice PBS. The cells were placed in RIPA buffer containing 10% protease inhibitor and phosphatase inhibitor, next placed them on ice for 30 min and centrifuged at 13500g for 15 min to remove insoluble protein lysates. The BCA protein quantification kit (Shanghai Epizyme Biomedical Technology Co., Ltd., Shanghai, China) was used to get evaluation of the protein concentration according to the instructions, and the protein was denatured in the cell lysate with 5 × sample buffer at 100 °C for 5 min. The SDS-PAGE we bought from Shanghai Epizyme Biomedical Technology Co., Ltd. (Shanghai, China) was used to separate the equal amount of protein by 10% gel and transferred to polyvinylidene fluoride membrane (Micro-nano Membrane Technology Co., Ltd., Hangzhou, Zhejiang), and 5% skim milk was used for it. The Tris hydrochloride buffer containing Tween-20 was blocked at room temperature for 2 h, stirring continuously. Then the membrane was incubated with primary antibody at 4°C overnight.
Primary antibody mainly includes the followings: anti-GAPDH (1: 5000, Signalway Antibody), anti-MRGPRX2 (1:1000, Abcam), anti-CLM-1 (1:1000, proteintech), anti-SHP-1 (#ab227503, 1:1000, Sino-Biological), anti-phosphorylated-SHP-1 (#ab692169, 1:1000, Abcam), anti-AKT (1:1000, CST), anti-phosphorylated-AKT (1:1000, CST), anti-PI3K (1:1000, CST), anti- MyD88(1:1000, Abcam), anti-NF-κB (1:1000, CST, anti-IKKα/β (1:1000, Abcam anti-phosphorylated-IKKα/β (1:1000, Abcam), anti-IκB (1:1000, CST), anti-phosphorylated-IκB (1:1000, CST), anti-Rac1/Cdc42 (1:1000, CST).
On the second day, wash the membrane with TBST 5 times, 5 min each time. Add secondary antibody was for incubation at room temperature for 2 h, and the membrane was washed with TBST for 5 times, 5 min each time again. Finally, the protein level was displayed by enhanced chemiluminescence (ECL) kit and analyzed by Image Pro Plus 5.1 software.
RT-PCR
Place the cells in a 1.5 mL Eppendorf tube, wash it three times with ice-cold PBS buffer, add 1 mL of Trizol solution to each well and let it stand at room temperature for 10 minutes. Subsequently, perform high-speed centrifugation at 12,000 g for 10 minutes at 4°C, and then pipette 1 mL of Trizol into a new Eppendorf tube. Add 0.2 mL of chloroform to each tube, shake vigorously for 15 seconds, let it stand at room temperature for 3 minutes, and then centrifuge at 12,000 g for 15 minutes at 4°C. Pipette the upper aqueous phase into a new Eppendorf tube, taking 400 μL from each sample. Add 400 μL of isopropanol solution, gently invert to mix, and let it precipitate at room temperature for 10 minutes. After centrifugation, a white gelatinous precipitate visible at the bottom of the tube is RNA. Wash it twice with 0.8 mL of 75% alcohol and once with pure alcohol. Add 20 μL of DEPC water to each tube to dissolve the RNA. Proceed with genome removal and reverse transcription according to the steps and procedures outlined in the reverse transcription kit. Subsequently, prepare the PCR reaction mixture according to the PCR systems outlined, place it in a PCR machine, and set the program to perform the PCR reaction.
Immunofluorescence
LAD2 was connected to 2×106 per hole in the orifice plate. The blank group was not treated, the control group was added with C48/80 (final concentration 30 μg/mL), and the administration group was added with quercetin or ceramide (final concentration 100 μmol/L) and C48/80 (final concentration 30 μg/mL). The growth conditions were incubated for 24 h. The medium was discarded and washed with PBS 3 times, 5 min each time. Add 4% paraformaldehyde fixed for 10 min, and PBS washed 3 times each 5 min. At room temperature, 0.5% Triton X-100 was added for 5 min, and PBS was washed three times for 5 min each time. The cells were blocked with 5% BSA for 30 min and washed twice with PBS for 5 min each time. The cells were resuspended on the slide and placed at 65 °C for 30 min. Phalloidin dye TRITC Phalloidn (Yisheng Biotechnology Co., Ltd., Shanghai) diluted with 1:200 PBST was added to the PBST solution for 30 min at room temperature and washed twice with PBST for 5 min each time. DAPI (BOSTER) staining was added dropwise for 5 min, PBST was washed twice for 5 min each time, and 10 μL anti-fluorescence attenuation sealing agent was added dropwise to cover the cover glass. Super-resolution Confocal Microscope (Leica TCS SP8 STED 3 ×) was used to photograph the F-actin skeleton under red light and the nucleus under blue light.
Surface Plasmon Resonance
In order to analyze surface plasmon resonance (SPR), CML-1 (R&D Systems, Inc, Minneapolis, MN) protein (50 μg/mL) was immobilized on sensor chip CM5 (Cytiva Sweden AB, Uppsala, Sweden) by capture coupling. The interaction between CLM-1 and quercetin was detected by Biacore T200 (General electric medical system, Fairfield, CT) at 25°C. The quercetin sample was prepared by PBSP-5% dimethyl sulfoxide (DMSO), and the mobile phase was PBSP-5% DMSO.
Molecular Docking Analysis
Molecular docking aims to search for active small molecules with binding potency to protein receptors based on the complementary laws of geometry, energy and chemical environment.25 According to the minimum binding free energy principle, the scoring function ranks the binding ability between ligands and receptors, which represents the bioactivity of small molecules. This high-throughput technique performs at a virtual level without wasting solvent and monomer components and has been extensively applied to screen bioactive molecules from herbal medicines.26
The crystal structure of CLM-1 based on those reported by Dr. Orchard RC (PDB ID: 5FFL)27 was downloaded from the RCSB PDB database. The structure of quercetin was minimized using Chemi-office software were applied to perform the molecular docking model.
Using SYBYL-X 2.0 software, pre-processing steps such as repairing missing atoms, fixing amino acid residue termini, and removing water molecules are performed on CLM-1. The pre-formed three-dimensional small molecule model is opened, and undergoes a series of processes including adding hydrogen atoms, generating different conformations, and calculating charges. Subsequently, the docking space is defined, and the extracted ligand substructure function is utilized to identify the active site or ligand binding site of the protein, while simultaneously determining the linking space for the small molecule. The software automatically selects the optimal docking mode to perform docking simulations, ultimately yielding the hydrogen bond sites and groups involved in the binding of quercetin to the CLM-1 protein.
Levels of MRGPRX2 on the Cell Membrane and in the Cell Membrane
After incubating quercetin or ceramide (100 μmol/L) in LAD2 cells for 24 hours and simultaneously subjecting them to C48/80 stimulation, membrane protein and cytoplasmic protein were separated and extracted following the instructions provided by the membrane protein extraction kit (Bestbio, Nanjing, China, Lot: BB-3111-100T). The content of MRGPRX2 was subsequently determined using established research methods.28
Statistical Analysis
The data were expressed as mean ± standard error (SEM) and analyzed by analysis of variance (ANOVA). Two-tailed test was used for comparison between the two groups. P < 0.05 was considered statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001).
Results
Binding of Quercetin to CLM-1 Negatively Regulates MRGPRX2-Induced MC Activation
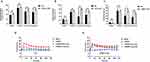
Design two CLM-1 siRNA knockdown sites for transfection, and select the most suitable knockdown site for subsequent experiments by detecting the mRNA and protein expression levels in LAD2 cells after transfection with these knockdown sites (Figure S1). Knockdown of CLM-1 expression in LAD2 cells resulted in similar levels of β-hexosaminidase release, histamine and calcium influx induced by C48/80 compared to NC-LAD2 cells (Figure 1A and C–E). However, ceramide or quercetin pretreatment significantly increased β-hexosaminidase release, histamine and calcium influx in CLM-1-knockdown LAD2 cells than in NC-LAD2 cells (Figure 1A and C–E). Pretreatment with anti-human CLM-1 antibody blocked ceramide- or quercetin-mediated suppression of degranulation (Figure 1B). These results show that CLM-1 downregulation significantly abrogates the inhibitory effect of quercetin on MC degranulation.
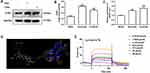
Western blot and RT-PCR showed that both quercetin and ceramide upregulated CLM-1 expression (Figure 2A–C). Molecular docking indicated that quercetin and CLM-1 formed hydrogen bonds at GLN37 (2.71 Å), ARG61 (2.45 Å), TYR288 (2.01 Å), SER83 (2.39 and 2.05 Å), and ASN60 (2.38 and 2.02 Å) (Figure 2D). Surface plasmon resonance (SPR) assay revealed strong and dose-dependent binding of quercetin to CLM-1 (KD = 2.962×10−5 mol/L) (Figure 2E).
Furthermore, our data demonstrated that quercetin significantly upregulated CLM-1 mRNA and protein expression (Figure 3D–E) but downregulated MRGPRX2 mRNA and protein expression in C48/80-treated LAD2 cells (Figure 3A–C). Collectively, these data show that the combination of quercetin and CLM-1 has a negative regulatory effect on MRGPRX2-mediated MC activation.
Quercetin Downregulates MyD88 Expression Through SHP-1 Dephosphorylation
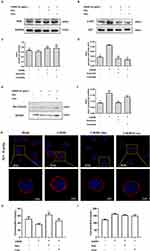
We found that quercetin enhanced SHP-1 phosphorylation in the CLM-1 signaling pathway (Figure 4A and B) but suppressed MyD88-mediated MC degranulation (Figure 4A and C) by downregulating downstream IKK/IκB phosphorylation (Figure 4A, and F). Ultimately, this cascade of events led to the downregulation of NF-κB (Figure 4A and F), a key player of inflammation.
Quercetin Attenuates Membrane MRGPRX2 Internalization Through Inhibition of F-Actin
The binding of ceramide or quercetin to CLM-1 triggered the recruitment of PI3K (Figure 5A and C), a central signaling enzyme involved in various cellular processes. This process in turn inhibited downstream AKT phosphorylation (Figure 5B and D), Rac1 and Cdc42 expression (Figure 5E and F), and ultimately F-actin expression (Figure 5G), an important cytoskeletal protein that participates in processes such as cell movement and endocytosis.
Receptor internalization is a key mechanism governing MRGPRX2 regulation and is influenced by F-actin. To ascertain whether CLM-1 activation enhances MRGPRX2 internalization, we stimulated cells with C48/80 and collected membrane and cytoplasmic proteins separately. Our data revealed that treatment with ceramide or quercetin increased the membrane content but decreased the cytoplasmic content of MRGPRX2 (Figure 5H and I). Altogether, these findings indicate that CLM-1 agonists may potentially suppress MC degranulation by inhibiting MGRPRX2 internalization.
Discussion
MCs play a vital role in the first line of defense in the host. However, due to their localization around the microvasculature of the skin and mucous membranes,29 MCs are easily activated by foreign substances, leading to various inflammatory responses.4 MC activation is a complex process and can be classified as IgE-dependent and non-IgE-dependent. In recent years, MRGPRX2 has been identified as a crucial receptor for non-IgE-dependent activation of MCs. MC activation through MRGPRX2 leads to pseudo-allergic reactions characterized by itching, pain, and inflammation.30,31 It has also been associated with the pathogenesis of allergic diseases such as asthma and urticaria.32,33 Therefore, identifying drug candidates that inhibit MRGPRX2-mediated MC activation has significant implications for the treatment of allergic diseases.
CLM-1 is an inhibitory receptor of the CD300 family that plays an important immunoregulatory role in various inflammatory and allergic responses. CLM-1 acts as a negative regulator of Toll-like receptor 4 and inhibits lipopolysaccharide-induced skin inflammation.34 In addition, CLM-1 expression has been reported to affect the biological functions of leukocytes.35 Given that the activation of CLM-1 suppresses MC degranulation, it presents as a potential target for treating food allergies.36 Previous studies have shown that the binding of ceramide to CLM-1 inhibits pseudo-allergies mediated by MRGPRX2.16,37 Therefore, further investigation of the functional effects and mechanisms of CLM-1 and MRGPRX2 will offer new insights to the treatment of various MC-associated diseases.
Consistent with previous findings,38 our data also confirmed that the binding of quercetin to CLM-1 significantly upregulated CLM-1 expression and downregulated MRGPRX2 expression in C48/80-treated LAD2 cells. Though, the exact target and mechanism of quercetin in inhibiting MRGPRX2-mediated MC activation will need to be further clarified.
Apart from the discovery of ceramide as an endogenous ligand for CLM-1, few other exogenous CLM-1 ligands have also been reported. The inhibitory activity of CLM-1 was recently found to be mediated by the interaction of intracellular ITIMs with SHP-1.9 In addition, CLM-1 regulates MyD88/TRIF-mediated TLR signaling through the activation of SHP-1 and SHP-2, thereby dampening the production of inflammatory mediators.10,39 Our study showed that the exogenous CLM-1 ligand quercetin plays a key role in regulating MRGPRX2-mediated MC activation by facilitating downstream SHP-1 phosphorylation and inhibiting MyD88/IKK/NF-κB signaling.
The inhibitory effect of CLM-1 has been proven to be mediated by the PI3K and NF-κB pathways.14 CLM-1-PI3K association leads to activation of downstream Rac/Cdc42 GTPase and mediates changes of F-actin.40 Our findings suggest that CLM-1 acts on PI3K signaling by modulating AKT phosphorylation and inhibiting downstream expression of the cell membrane structural proteins Rac1 and Cdc42. Receptor internalization is a critical mechanism regulated by GPCR, which is influenced by F-actin.41 Immunofluorescence staining further corroborated that ceramide or quercetin treatment inhibited F-actin, a crucial process involved in various cellular activities such as cell movement and receptor internalization. Immunofluorescence staining further confirmed that ceramide or quercetin treatment inhibits F-actin. Our subsequent analysis of MRGPRX2 levels on the cell membrane surface and in the cytoplasm after ceramide treatment revealed that quercetin/CLM-1 binding increased the content of MRGPRX2 on the cell membrane, once again indicating that it may inhibit receptor internalization. However, receptor internalization is a highly intricate process and further in-depth research is warranted to confirm the specific mechanisms involved.
Additionally, our study showed that the absence of ceramide did affect CLM-1 inhibition of MRGPRX2 induced mast cell degranulation. This indicates that the inhibitory function of CLM-1 must be mediated through its binding with the corresponding ligand or agonist. Aside from ceramide, no other exogenous ligands for CLM-1 have been reported thus far, underscoring the significance of identifying quercetin as a potential agonist for CLM-1.
Conclusion
Quercetin attenuate MRGPRX2-mediated mast cell degranulation. Quercetin represented agonist of CLM-1 downregulate membrane internalization of MRGPRX2 by F-actin participation. Quercetin may serve as drug candidate for therapeutic of allergic disease.
Data Sharing Statement
No data was used for the research described in the article.
Acknowledgment
The work was financially supported by the National Natural Science Foundation of China (grant no: 81872837 and 82204337) and The China Postdoctoral Science Foundation (No. 2023M732822).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Kleinschmidt S, Harder J, Nolte I, Marsilio S, Hewicker-Trautwein M. Phenotypical characterization, distribution and quantification of different mast cell subtypes in transmural biopsies from the gastrointestinal tract of cats with inflammatory bowel disease. Vet Immunol Immunopathol. 2010;137(3–4):190–200. doi:10.1016/j.vetimm.2010.05.005
2. Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4(10):787–799. doi:10.1038/nri1460
3. Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142. doi:10.1038/ni1158
4. Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC. IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol. 2020;11:22. doi:10.3389/fimmu.2020.603050
5. McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–241. doi:10.1038/nature14022
6. Lansu K, Karpiak J, Liu J, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017;13(5):529–536. doi:10.1038/nchembio.2334
7. Babina M, Guhl S, Artuc M, Zuberbier T. Allergic Fc epsilon RI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy. 2018;73(1):256–260. doi:10.1111/all.13301
8. Zhang T, Che DL, Liu R, et al. Typical antimicrobials induce mast cell degranulation and anaphylactoid reactions via MRGPRX2 and its murine homologue MRGPRB2. Eur J Immunol. 2017;47(11):1949–1958. doi:10.1002/eji.201746951
9. Clark GJ, Ju XS, Tate C, Hart DNJ. The CD300 family of molecules are evolutionarily significant regulators of leukocyte functions. Trends Immunol. 2009;30(5):209–217. doi:10.1016/j.it.2009.02.003
10. Kim EJ, Lee SM, Suk K, Lee WH. CD300a and CD300f differentially regulate the MyD88 and TRIF-mediated TLR signalling pathways through activation of SHP-1 and/or SHP-2 in human monocytic cell lines. Immunology. 2012;135(3):226–235. doi:10.1111/j.1365-2567.2011.03528.x
11. Maehara A, Kaitani A, Izawa K, et al. Role of the ceramide-CD300f Interaction in gram-negative bacterial skin infections. J Invest Dermatol. 2018;138(5):1221–1224. doi:10.1016/j.jid.2017.11.025
12. Moshkovits I, Karo-Atar D, Itan M, et al. CD300f associates with IL-4 receptor alpha and amplifies IL-4-induced immune cell responses. Proc Natl Acad Sci USA. 2015;112(28):8708–8713. doi:10.1073/pnas.1507625112
13. Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi:10.1111/j.1600-065X.2008.00742.x
14. Moshkovits I, Shik D, Itan M, et al. CMRF35-like molecule 1 (CLM-1) regulates eosinophil homeostasis by suppressing cellular chemotaxis. Mucosal Immunol. 2014;7(2):292–303. doi:10.1038/mi.2013.47
15. Izawa K, Isobe M, Matsukawa T, et al. Sphingomyelin and ceramide are physiological ligands for human LMIR3/CD300f, inhibiting Fc epsilon RI-mediated mast cell activation. J Allergy Clin Immunol. 2014;133(1):270–273. doi:10.1016/j.jaci.2013.08.008
16. Takamori A, Izawa K, Kaitani A, et al. Identification of inhibitory mechanisms in pseudo-allergy involving Mrgprb2/MRGPRX2-mediated mast cell activation. J Allergy Clin Immunol. 2019;143(3):1231–1235. doi:10.1016/j.jaci.2018.10.034
17. Izawa K, Yamanishi Y, Maehara A, et al. The receptor LMIR3 negatively regulates mast cell activation and allergic responses by binding to extracellular ceramide. Immunity. 2012;37(5):827–839. doi:10.1016/j.immuni.2012.08.018
18. Li X, Geng-Ji -J-J, Quan -Y-Y, et al. Role of potential bioactive metabolites from traditional Chinese medicine for type 2 diabetes mellitus: an overview. Front Pharmacol. 2022;13:1023713.
19. Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11(4):298–344. doi:10.2174/138955711795305335
20. Di Petrillo A, Orru G, Fais A, Fantini MC. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. 2022;36(1):266–278. doi:10.1002/ptr.7309
21. Orsolic N, Knezevic AH, Sver L, Terzic S, Basic I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol. 2004;94(2–3):307–315. doi:10.1016/j.jep.2004.06.006
22. Huang RY, Yu YL, Cheng WC, OuYang CN, Fu E, Chu CL. Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol. 2010;184(12):6815–6821. doi:10.4049/jimmunol.0903991
23. Bureau G, Longpre F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008;86(2):403–410. doi:10.1002/jnr.21503
24. Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol. 2004;24(5):542–552. doi:10.1023/B:JOCI.0000040925.55682.a5
25. Mtemeli FL, Ndlovu J, Mugumbate G, Makwikwi T, Shoko R. Advances in schistosomiasis drug discovery based on natural products. All Life. 2022;15(1):608–623. doi:10.1080/26895293.2022.2080281
26. Qi L, Zhong F, Liu N, et al. Characterization of the anti-AChE potential and alkaloids in rhizoma coptidis from different Coptis species combined with spectrum-effect relationship and molecular docking. Front Plant Sci. 2022;13:1020309. doi:10.3389/fpls.2022.1020309
27. Orchard RC, Wilen CB, Doench JG, et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science. 2016;353(6302):933–936. doi:10.1126/science.aaf1220
28. Ding YY, Li XQ, Gao QP, et al. A paper-based ELISA for rapid sensitive determination of anaphylaxis-related MRGPRX2 in human peripheral blood, anal. Biochem. 2021;633:9.
29. Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13(5):362–375. doi:10.1038/nri3427
30. Kolkhir P, Elieh-Ali-Komi D, Metz M, Siebenhaar F, Maurer M. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat Rev Immunol. 2022;22(5):294–308. doi:10.1038/s41577-021-00622-y
31. Roy S, Ayudhya CCN, Thapaliya M, Deepak V, Ali H. Multifaceted MRGPRX2: new insight into the role of mast cells in health and disease. J Allergy Clin Immunol. 2021;148(2):293–308. doi:10.1016/j.jaci.2021.03.049
32. Shtessel M, Limjunyawong N, Oliver ET, et al. MRGPRX2 activation causes increased skin reactivity in patients with chronic spontaneous urticaria. J Invest Dermatol. 2021;141(3):678–681. doi:10.1016/j.jid.2020.06.030
33. Ali H. Mas-related G protein coupled receptor-X2: a potential new target for modulating mast cell-mediated allergic and inflammatory diseases. J Immunobiol. 2016;1(4). doi:10.4172/2476-1966.1000115
34. Shiba E, Izawa K, Kaitani A, et al. Ceramide-CD300f binding inhibits lipopolysaccharide-induced skin inflammation. J Biol Chem. 2017;292(7):2924–2932. doi:10.1074/jbc.M116.768366
35. Cao Y, Ao TR, Wang XH, Wei WM, Fan J, Tian XH. CD300a and CD300f molecules regulate the function of leukocytes. Int Immunopharmacol. 2021;93:8.
36. Uchida S, Izawa K, Ando T, et al. CD300f is a potential therapeutic target for the treatment of food allergy. Allergy. 2020;75(2):471–474. doi:10.1111/all.14034
37. Che DL, Zheng Y, Hou YJ, Li T, Du XS, Geng SM. Dehydroandrographolide targets CD300f and negatively regulated MRGPRX2-induced pseudo-allergic reaction. Phytother Res. 2022;36(5):2173–2185. doi:10.1002/ptr.7445
38. Ding YY, Che DL, Li CM, et al. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int Immunopharmacol. 2019;66:185–197. doi:10.1016/j.intimp.2018.11.025
39. Lee SM, Kim EJ, Suk K, Lee WH. CD300F blocks both MyD88 and TRIF-mediated TLR signaling through activation of Src homology region 2 domain-containing phosphatase 1. J Immunol. 2011;186(11):6296–6303. doi:10.4049/jimmunol.1002184
40. Tian L, Choi S-C, Murakami Y, et al. p85α recruitment by the CD300f phosphatidylserine receptor mediates apoptotic cell clearance required for autoimmunity suppression. Nat Commun. 2014;5(1). doi:10.1038/ncomms4146
41. Ma D, Sun W, Fu C, et al. GPCR/endocytosis/ERK signaling/S2R is involved in the regulation of the internalization, mitochondria-targeting and -activating properties of human salivary histatin 1. Int J Oral Sci. 2022;14(1):42.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.