Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
Quercetin Promote the Chemosensitivity in Organoids Derived from Patients with Breast Cancer
Authors Meng S, Cao Y, Lu L, Li X, Sun S, Jiang F, Lu J, Fan D, Han X, Yao T
Received 5 September 2024
Accepted for publication 27 November 2024
Published 20 December 2024 Volume 2024:16 Pages 993—1004
DOI https://doi.org/10.2147/BCTT.S494901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Pooja Advani
Shengwen Meng,1 Yifan Cao,1 Lei Lu,1 Xuanhe Li,1 Siyu Sun,1 Fangqian Jiang,1 Jianfei Lu,2 Dongwei Fan,3 Xinxin Han,1 Tingjing Yao1
1Department of Surgical Oncology, The Fourth Ward of Breast and Thyroid, the First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui Province, People’s Republic of China; 2Department of Breast and Thyroid Surgery, Bengbu First People’s Hospital, Bengbu, Anhui Province, People’s Republic of China; 3Department of General Surgery, Affiliated Hospital of West Anhui Health Vocational College, Luan, Anhui Province, People’s Republic of China
Correspondence: Tingjing Yao, Department of Surgical Oncology, The Fourth Ward of Breast and Thyroid, The First Affiliated Hospital of Bengbu Medical College, 287 Changhuai Road of Bengbu, Anhui Province, 233099, People’s Republic of China, Email [email protected]
Aim: The study aimed to culture organoids from tissues of patients with breast cancer (BC) and use the organoids to measure the sensitivity to quercetin and its combination with chemotherapeutic agents.
Methods: Four patient-derived organoids (PDOs) of BC were cultured. The proliferative activity and morphology of PDOs were evaluated on different generations and after resuscitation. H&E and immunohistochemical (IHC) staining were used to identify the pathological changes and the expression of biomarkers. The sensitivity to quercetin and chemotherapeutic agents and their combinations were evaluated using adenosine triphosphate (ATP) viability assays.
Results: We successfully obtained all PDOs from BC tissues. PDOs preserved their activity and morphology during generation passage. In addition, the pathological changes and expression patterns of estrogen receptor (ER), human epidermal growth factor receptor (HER2), and Ki67 of each PDO were consistent with their original tissues. All four PDOs were highly sensitive to quercetin, and their IC50 values were less than 22 μM. PDOs showed better sensitivity to docetaxel and epirubicin hydrochloride, but less sensitivity to cis-platinum. Combination with quercetin promoted the sensitivity to three chemotherapeutic agents. In particular, the IC50 value of cis-platinum greatly decreased.
Conclusion: We successfully established PDOs from patients with BC and demonstrated that quercetin can promote the sensitivity of chemotherapeutic agents in these PDOs.
Keywords: quercetin, breast cancer, patient-derived organoids, drug sensitivity
Introduction
Breast cancer (BC) is the most common malignancy among women worldwide,1 which undermines their physical and mental health.2,3 Notably, the incidence of BC exceeded that of lung cancer and accounted for 11.7% of new cancer cases in 2020 according to the latest report by the International Agency for Research on Cancer (IARC).4 Although screening programs and new treatments have greatly improved the survival rates of women with BC,5 chemotherapy remains the most commonly used treatment for BC. However, recurrent or metastatic BC is often resistant to chemotherapeutic drugs.6 Hence, exploring medicines that can improve the effectiveness of chemotherapy for BC is crucial.
Previous reports have shown that quercetin is a potential alternative or complementary medicine for BC.7 As a natural dietary substance, quercetin is relatively less harmful to normal cells and is regarded as an excellent anti-tumor medicine.8 Recently, quercetin was demonstrated to promote chemosensitivity in BC.9 Specifically, quercetin interferes with the cell cycle of BC cells, including BC cell apoptosis through mitochondrial dysfunction, and inhibits BC cell invasiveness and migration. However, most studies focused on cells or mice and cannot fully reflect the real patient situation. Recent studies have successfully developed patient-derived organoids (PDOs) as a promising and innovative model for studying human cancers.10,11 PDOs can recapitulate the architectural and biological characteristics of their parental tumors.12,13 Importantly, these organoids can be derived from tumor tissues within a short timeframe with a high success rate, further emphasizing their potential as a reliable model for cancer research.14,15
In the present study, we measured the response of BC PDOs to quercetin and its combination with commonly used chemotherapeutic drugs. Our findings support that quercetin can enhance the chemosensitivity of BC, which will further support quercetin’s enhancement of chemotherapy sensitivity in breast cancer, showing promising potential for clinical application and providing new drug or combination options for chemotherapy-resistant patients.
Materials and Methods
Sample Collection
We obtained four BC samples from invasive ductal carcinoma of four patients with BC at the First Affiliated Hospital of Bengbu Medical College (China). The pathological characteristics of these BC patients are shown in Table 1. All patients signed informed consent forms. The study was approved by the Institutional Review Board of Bengbu Medical College (Anhui, China), and this study was complied with the Declaration of Helsinki. Tumor samples were pathologically confirmed.
 |
Table 1 The Characteristic of the Four BC Patients |
Organoid Culture
According to the method previously reported,16 we transferred fresh surgical samples (tumor tissues and adjacent non-tumor tissues) into a preservation solution (advance DMEM F12, Glutamax (100x), HEPES (100x), P/S (100x), all obtained from Gibco). Then, the samples were transferred to the laboratory at 4 °C for primary cell isolation. The samples were divided into three portions. One was used for tissue embedding, one was preserved in −80 °C freezer after rapid freezing in liquid nitrogen, and one was used for organoid construction. For organoid construction, the tissue samples were transferred to a 6 cm culture dish containing pre-chilled PBS (phosphate-buffered saline), and two rounds of washing were done. Photographs were taken to document the appearance of the tissue before each wash. Then, the tissue was transferred to another 6 cm culture dish containing advance DMEM F12 for further washing. The necrotic tissue and adipose tissue were removed, and the tissue was cut into 0.5-~1 mm3 pieces. Then, the samples were transferred to 15 mL centrifuge tubes, and 10 mL of advance DMEM F12, Glutamax (100x), HEPLES (100x), P/S (100x), Y-27632 (10 μM, Sigma), collagenase NB4 (0.4 pzu/mL, MineBio), and nuclease (10 u/mL) were added. The mixture in the 15 mL centrifuge tube was placed in a shaker (37 °C, 100 rpm) and digested for 30 minutes to 2 hours. They were observed under an inverted microscope every 20 minutes. After observing cell clusters containing 5–10 cells, 2% FBS was added to terminate the digestion. The digested tissue suspension was filtered through a 100 μm mesh filter and then centrifuged at 300 g for 5 minutes. If there were more red blood cells, 1 mL of red blood cell lysis solution was added to lyse them at 300 g for 3 minutes. Then, the supernatant was removed, 1 mL of Advance DMEM F12 was added to resuspend the pellet, and centrifuged twice at 300 g for 3 minutes. The remaining cell pellet in extracellular matrix gel (ECM gel) was resuspended at a ratio of >7:3 (gel: pellet), mixed well, and then placed on ice. The suspension was spread into a 24-well plate, and 20–30 μL of gel droplet was added to each well. The culture plate was placed in a 37°C, 5% CO2 cell culture incubator and allowed to solidify for 30 minutes. The culture plate was taken from the incubator, and 500 μL of breast cancer organoid culture medium was added to each well. The medium included Advanced DMEM/F12 1x, penicillin/streptomycin, glutamax 100x, HEPES 10 mm, nicotinamide (Sigma) 5 mm, N-acetylcysteine (Sigma) 1.25 mm, B27 (Gibco) 1x, SB202190 (Cell signaling Technology), 500 nM, Y-27632 (Sigma) 5 uM, A83-01 (Sigma) 500 nM, EGF (Organregen) 5 ng/mL, FGF10 (Peprotech) 20 ng/mL, neuregulin 1 (Peprotech) 50 ng/mL, and R-spondin1 (Organregen) 500 ng/l. The medium was replaced every 3–4 days. The status of the organoids was observed using an inverted microscope, and photographs were taken.
H&E Staining
The tissue samples of BC and organoids were prepared into paraffin sections. We baked the slices in an oven at 60 ° C for 1 hour. The slices were immersed in xylene twice for 5 minutes. Subsequently, the sections were sequentially immersed in 100%, 100%, 95%, 90%, 80%, and 75% ethanol solutions for 3 minutes each. Then, the slices were immersed in distilled water. For H&E staining, the slices were incubated with hematoxylin staining solution for 30–90 seconds. Then, the sections were slowly rinsed with tap water. The slices were differentiated using 0.05% hydrochloric acid in 70% ethanol solution for 3 seconds, then rinsed with tap water. Thereafter, the slices were incubated with eosin solution for 1–3 minutes to achieve the desired color intensity. Finally, the slices were sequentially immersed in 80% ethanol for 30 seconds, 90% ethanol for 1 minute, 95% ethanol for 2 minutes, 100% ethanol for 2 minutes, 100% ethanol for another 2 minutes, and xylene for 2 minutes. Air-dried stained slices were sealed by neutral gum.
Immunohistochemical Staining
The paraffin sections of BC tissues and organoids were baked in an oven at 60 °C for 1 hour. Then, the sections were put into xylene twice. Subsequently, the sections were immersed in 100%, 100%, 95%, 90%, 80%, and 75% ethanol solutions for 3 minutes each. Then, the sections were rinsed with PBS twice, 5 minutes each time. The sections were removed and transferred to a container filled with citrate antigen retrieval solution. The container was placed in a pressure cooker, and pressure was maintained for 20 minutes. Afterward, the sections were cooled at room temperature for approximately 40 minutes. The sections were rinsed with PBST twice, 5 minutes each time. Next, the sections of tissues or organoids were incubated at room temperature for 15 minutes. Then, the sections were rinsed with PBS twice, 5 minutes each time. The sections were blocked with 5% BSA for 30 minutes at room temperature. The confining liquid was removed, and the following primary antibodies were added: (ER: sc-8002, SANTA; HER2: sc-33684, SANTA; Ki67: 550609, BD, USA; CK7 (Cytokeratin 7): sc-23876, SANTA; CK20 (Cytokeratin 20): sc-271183, SANTA. All the primary antibodies were diluted at 1:250 by 3% BSA and incubated at 4°C overnight. The sections were rinsed with PBST three times 10 minutes each time. The secondary antibody (Goat Anti-Mouse IgG Antibody (H+L) Biotinylated: BA-9200, Vector Laboratories, diluted at 1:400 by 1% BSA) was added and incubated for 1 hour. The samples were then rinsed with PBS three times, 10 minutes each time. Horseradish peroxidase-conjugated streptavidin was added and incubated at room temperature for 1 hour. Then, the samples were rinsed with PBS three times, 10 minutes each time. DAB (3, 3’-diaminobenzidine) staining solution was added, and the stained samples were observed under a microscope.
Organoids Sensitivity Test
After digestion, high-quality organoids were passed through a 70 μm filter to remove large debris. The sensitivity test was based on the previous study.17,18 The organoids were washed once with a basic culture medium, and a cell number was counted. A 96-well plate was prepared, and 5000 cells were added per well. The desired volume of cell suspension was taken, centrifuged, and the cells were resuspended in an appropriate volume. The cells were added to wells, and then 3 μL of matrigel was added to each well. The culture medium was added to the plate, and high-resolution images were captured. Every three days, the organoids were observed, and the medium was replaced. Once the organoids reached a size of approximately 50 μm, the following drugs were added for treatment: epirubicin hydrochloride (Epiru, 0 μM, 0.037 μM, 0.111 μM, 0.333 μM, 1.5 μM, and 3 μM), docetaxel (DTX, 0 μM, 0.004 μM, 0.012 μM, 0.036 μM, 0.108 μM, 0.324 μM, and 0 μM, 0.00010883 μM, 0.000325 μM, 0.0013 μM, 0.004 μM, and 0.012 μM specific in No.010), cis-platinum (cisplatin, 0 μM, 1.5 μM, 4.5 μM, 13.5 μM, 40.5 μM, and 121.5 μM) and quercetin (all purchased from MCE). For treatment, six concentrations of each drug were used based on our preliminary experiments, and the sensitivity to each drug was represented by the concentration inhibiting 50% of cells (IC50). PBS was used as the positive control, while plates without drugs were used as negative controls. After three days of treatment, the medium was replaced, and fresh doses of the drugs were added. ATP viability assay was used to assess the viability and activity of organoids after six days of treatment.
ATP Viability Assay
We used the CellTiter-Glo®3D Cell Viability Assay (G9681, promega) for measurement. In brief, the organoids were left at room temperature for 10 minutes before adding the lysis agent to DMEM/F12 in a 1:1 proportion. Then, the organoids were placed in the microplate reader for vigorous linear shaking (1000 rpm) for 5 minutes to ensure complete cell lysis. After keeping the samples at room temperature for 20 minutes, we measured the chemiluminescent value of each well of organoids. The following formula was used to calculate cell activity:
Cell activity (%) = [X (dosing test well) - P (positive control)]/[N (negative control) - P (positive control)] x100%. X represents the chemiluminescent values of wells treated with drug solutions after adding the ATP reagent. N represents the chemiluminescent values of wells after adding the ATP reagent in the absence of drug solutions. P represents the chemiluminescent values of wells treated with 1x PBS, which results in complete cell apoptosis after adding the ATP reagent.
Statistical Analysis
All data for drug sensitivity were repeated at least three times. Data are presented as Mean ± SD. GraphPad Prism 8.0 was used for statistical analysis. We supplemented the value of cell viability as 1 at x = 0, and converted the drug concentration value x to X = log10(x). We obtained the simulation curve using nonlinear regression (curve fit) and the following equation “log (inhibitor) vs response - variable slope (four parameters)”. We automatically removed any outlier values to obtain the “cell viability-log10 (drug concentration)” curve for organoids and the corresponding IC50 of drugs. The CompuSyn software was used to perform synergistic quantification. The two drugs exhibited a synergistic effect when CI < 1, exhibited an additive effect when CI = 1, and exhibited an antagonistic effect when CI > 1.
Results
We successfully cultured all four BC organoids and found that the organoids were successfully resuscitated after freezing (Figure 1). These organoids had irregularly round, dense vesicular structures, and varying sizes. In addition, we found no change in organoid morphology from passage 0 to 5 (Figure 2). The organoids continued to proliferate according to imaging.
 |
Figure 1 Photographs of cultured PDTOs before resuscitation and after thawing. |
 |
Figure 2 Photographs of cultured PDTOs after each passage. |
We further compared the histological characteristics of organoids with those of original BC tissues using H&E staining. The results showed that the characteristics of BC organoids were similar to those of original BC tissues (Figure 3), with similar growth patterns and cellular and nuclear atypia. The expression of estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2), the common biomarkers for BC, and tumor biomarkers Ki-67, CK7, and CK20 were also compared to their matched tissues. We found that the organoids retained the expression of ER, HER2, and Ki-67 in all samples. The No.010 PDTOs were positive for CK7 (an adenoma marker) expression while negative for CK20 expression (Figure 4).
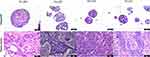 |
Figure 3 H&E staining of cultured PDTOs and original tissues. |
 |
Figure 4 Immunohistochemical staining of ER, HER2, Ki-67, CK7, and CK20 on cultured PDTOs and original tissues. |
To confirm that these organoids can serve as effective models for measuring the effects of drugs, we perform drug sensitivity tests. Firstly, different concentrations (5 μM, 10 μM, 20 μM, 40 μM, and 80 μM) of quercetin were used to treat PDTOs, and we found that all four PDTOs were inhibited by quercetin (Figure 5). Specifically, the BC sample No.010 negative for ER and PR and with no lymphatic metastasis was more sensitive to quercetin compared to other samples. The sample No.011 with strong ER expression and metastatic nodules had the maximum IC50 value. The values of IC50 were 12.12 μM (No.003), 18.19 μM (No.007), 7.562 μM (No.010), and 21.77 μM (No.011), respectively. The results showed that all of the four PDTOs were less sensitive to cisplatin and more sensitive to DTX and Epriu (Figure 6 and Table 2). The sensitivity ranking of samples No.003, No.010, and No.011 was consistent for the three chemotherapeutic agents. DTX was the most sensitive, followed by Epriu. Cisplatin had the highest IC50 value. No. 007 PDTO was slightly more sensitive to Epriu than DTX, and No. 007 PDTO showed the least sensitivity to cisplatin.
 |
Table 2 The IC50 Values of Quercetin and Chemotherapeutics and Their Combinations for Each PDOs |
 |
Figure 5 The sensitivity of PDTOs to quercetin using ATP viability assay. |
 |
Figure 6 The sensitivity of PDTOs to docetaxel, epirubicin, and cis-platinum using ATP viability assay. |
To demonstrate that quercetin can enhance the sensitivity of chemotherapeutic agents, we measured the effects of the combination of the three chemotherapeutic drugs with quercetin on PDTOs. The concentration of quercetin was set at 10 μM in each combination, and the concentration gradient of the three chemotherapeutic drugs remained consistent. We found that the IC50 values of all three chemotherapy drugs, especially DTX and cisplatin, significantly decreased after being combined with quercetin (Figure 7 and Table 2). The IC50 value of Epriu only decreased by 30–60% in all four PDTOs. In conclusion, we found that the combination of quercetin with the three chemotherapy drugs can greatly promote their effect compared to their single use.
 |
Figure 7 The sensitivity of PDTOs to the combination of the three common chemotherapeutic agents and quercetin. |
The effects of the combinations are presented in Table 3. We found that different chemotherapeutic agents had a synergistic effect with quercetin.
 |
Table 3 The Synergistic Quantification Between Quercetin and Chemotherapeutics |
Discussion
We successfully established four BC organoids obtained from patients and demonstrated that these PDTOs can maintain their morphological features and vitality after cryopreservation, resuscitation, or continuous passage. We further measured the sensitivity to quercetin, three common chemotherapeutic drugs, and their combinations, using these PDTOs. We obtained the IC50 values of these drugs and their combinations. We also measured the sensitivity ranking of different PDTOs to these drugs. Importantly, we found that combination with quercetin greatly promoted the DTX sensitivity in No.007 patients with lymphatic metastasis and low expression of ER and PR receptors when compared with other patients. Meanwhile, quercetin increases the Epriu sensitivity of the No.003 and No.010 patients by about two times but did not seem to be significant in patient No.011 patients with metastatic nodules. These results demonstrated that combination with quercetin can significantly improve the sensitivity of BC to common chemotherapy drugs and are possibly affected by different types of BC and chemotherapy agents.
We also demonstrated that the combination with quercetin improving the sensitivity of BC was dependent on the synergistic effect. Previous studies have proposed that the synergistic effect of quercetin with cisplatin on cervical cancer cells was in specific concentrations, which is consistent with our results.19 Meanwhile, we also found that the antagonistic effect occurred between quercetin and other chemotherapeutic combinations, depending on the type of drugs, concentration differences, and the source of the PDTOs being tested. Therefore, seeking the synergistic effect of quercetin combined with chemotherapy drugs is also important for the treatment of tumor patients, and the in vitro drug combination simulation study is of great significance.
Quercetin is a flavonol compound that possesses various biological activities. It is widely found in vegetables and fruits that are commonly consumed in daily diet.20 Quercetin exhibits a wide range of biological effects, including anti-cancer activity.21 Quercetin exerts anti-cancer effects by regulating cyclins and promoting cell apoptosis. Quercetin can inhibit cyclin D1 expression by downregulating the Twist gene and increasing p16 and p21 expression, thereby blocking the cell cycle in the G1 phase.22 Previous reports have shown that quercetin can inhibit the proliferation of BC cells by inhibiting inflammation, oxidative stress, and angiogenesis.7 Moreover, Yamamoto et al23 showed that quercetin can restore the sensitivity of MCF7 cells to docetaxel by inhibiting the Lef1 gene and TGF-β signaling pathway. Quercetin enhances the expression of P53 and P57, resulting in cell cycle arrest in the S stage.24 Importantly, we knew that energy metabolism is the key feature of tumor cells; quercetin could decrease the glucose uptake capacity of BC cells by down-regulating the glucose transporter 1 level.25 In addition, Paweł Przybylski’s team showed that quercetin can promote the apoptosis of MCF-7 cells in the condition of glucose deficiency,26 and the glucose deficiency also enhances the sensitivity to DOX-induced apoptosis of BC cells. These explained our results of ATP test and emphasized the important role of energy metabolism in the process of quercetin increasing the sensitivity of BC. Meanwhile, our study also demonstrated that quercetin in combination with DTX can significantly decrease the IC 50 value of DTX in BC PDTOs. Quercetin is a supplement or alternative to docetaxel in the treatment of BC.
In addition, quercetin greatly improved the sensitivity of PDTOs to cisplatin. In fact, it has been reported that quercetin can enhance chemosensitivity in several tumors, including pancreatic cancer,27 prostate cancer,28 and ovarian cancer.29 The mechanism by which quercetin promotes the sensitivity to cisplatin remains unclear, Carmi et al30 reported that flavonoids can restore the sensitivity of ovarian carcinoma to platinum drugs through the ERK1/2 pathways. Li et al31 found that quercetin can increase the sensitivity to doxorubicin and inhibit BC cell proliferation and invasion by upregulating PTEN and downregulating p-Akt. Hence, we speculated that quercetin can enhance the sensitivity to chemotherapeutic drugs through different signaling pathways. Here, we used PDTOs to demonstrate the role of quercetin in inhibiting tumor growth and enhancing the sensitivity to chemotherapeutic agents.
PDTOs have been established from various tumors to help study cancer biology, molecular mechanisms, and drug efficacy in vitro.32 PDTOs provide the optimal drugs for specific patients and reduce the cost of drug testing and side effects. Due to different tumor characteristics, including pathological types, TNM stages, and individual backgrounds, patients present with distinct drug sensitivity when treated with different chemotherapeutic agents. Zhao et al33 showed that HER2-positive and TNBC PDTOs are more sensitive to chemotherapeutic agents than endocrine drugs. Our study also demonstrated that patients with HER2-positive cells were all sensitive to the three chemotherapeutic agents, although small differences existed between them in terms of ER and PR expression, lymph node metastasis, and the presence of nodules. These findings suggest that patients with HER2-positive BC or TNBC may benefit more from chemotherapy. However, to assess the relationship between tumor pathology and optimal drugs, larger sample sizes with different pathological types should be included in future studies.
PDTOs are valuable platforms for drug development, toxicology assessment, and preclinical trials, which can help the potential toxicity and safety of substances.10 Compared to cell lines, organoids have more complex three-dimensional structures that better simulate organ physiological functions and are closer to the actual situation in the body in the fields of building disease models, drug screening and regenerative medicine, although their cultivation and construction are more technically difficult and costly. However, the results of sensitivity analysis using PDTOs can only provide a reference. Due to the complexity of human structure and function, further validation based on animal models and clinical trials is still needed. Our PDTO models confirmed the value of quercetin in the treatment of BC and indicated that quercetin combined with other chemotherapeutic agents can improve chemosensitivity in BC. These are important and valuable for the treatment of BC, this is mainly because advanced BC is significantly more resistant to chemotherapy drugs, hence the development of a natural medicine to enhance the effect is a good supplement for treatment of BC. Moreover, we used PDTOs as drug test models, which greatly enhanced the authenticity and clinical significance of the results compared with previous studies on quercetin at the cell line level. In the future, providing specific quercetin combinations to different patients through PDTOs can also be fully utilized to improve patient medication guidance.
Conclusions
In this study, we successfully cultured four PDTOs from patients with BC. We measured the sensitivity to quercetin alone and its combination with three common chemotherapeutic agents and found that quercetin greatly promoted the sensitivity to chemotherapeutic agents on PDTOs from patients with BC.
Patients’ Consent for Publication
All patients signed informed consent forms and agreed to this publication.
Data Sharing Statement
Data supporting the findings of this study can be available from the corresponding author upon a reasonable request.
Ethical Statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (Anhui, China). [2021] No: 215
Acknowledgment
The authors would like to thank the experimental technology platform of Bengbu Medical College and their technicians. The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Funding
This study was funded by the Natural Science Project of Bengbu Medical College (Byycx22049), Natural Science Research Project of Anhui Educational Committee (KJ2020A0577), and the Anhui Provincial Health Research Project (AHWJ2023A30031).
Disclosure
The authors declare no conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Olaogun JG, Agodirin OS. Breast cancer screening: can the iBreastExam bridge the gap? Lancet Glob Health. 2022;10:e461–e2. doi:10.1016/s2214-109x(22)00078-x
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. Ca Cancer J Clinicians. 2021;71:7–33. doi:10.3322/caac.21654
4. Liu J, Zhang W, Cai W, et al. Multi-omics analyses revealed GOLT1B as a potential prognostic gene in breast cancer probably regulating the immune microenvironment. Front Oncol. 2021;11:805273. doi:10.3389/fonc.2021.805273
5. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clinicians. 2021;71:209–249. doi:10.3322/caac.21660
6. Bai X, Ni J, Beretov J, Graham P, Li Y. Triple-negative breast cancer therapeutic resistance: where is the Achilles’ heel? Cancer Lett. 2021;497:100–111. doi:10.1016/j.canlet.2020.10.016
7. Ezzati M, Yousefi B, Velaei K, Safa A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020;248:117463. doi:10.1016/j.lfs.2020.117463
8. Sethi G, Rath P, Chauhan A, et al. Apoptotic mechanisms of quercetin in liver cancer: recent trends and advancements. Pharmaceutics. 2023;15:712. doi:10.3390/pharmaceutics15020712
9. Mawalizadeh F, Mohammadzadeh G, Khedri A, Rashidi M. Quercetin potentiates the chemosensitivity of MCF-7 breast cancer cells to 5-fluorouracil. Mol biol rep. 2021;48:7733–7742. doi:10.1007/s11033-021-06782-3
10. Chen P, Zhang X, Ding R, et al. Patient-Derived Organoids Can Guide Personalized-Therapies for Patients with Advanced Breast Cancer. Adv Sci. 2021; 8:e2101176. doi:10.1002/advs.202101176
11. Guillen KP, Fujita M, Butterfield AJ, et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 2022;3:232–250. doi:10.1038/s43018-022-00337-6
12. Broutier L, Mastrogiovanni G, Verstegen MM, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi:10.1038/nm.4438
13. Clevers H. Modeling development and disease with organoids. cell. 2016;165:1586–1597. doi:10.1016/j.cell.2016.05.082
14. Tiriac H, Belleau P, Engle DD, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Disc. 2018;8:1112–1129. doi:10.1158/2159-8290.cd-18-0349
15. Kim M, Mun H, Sung CO, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:3991. doi:10.1038/s41467-019-11867-6
16. Dekkers JF, van Vliet EJ, Sachs N, et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat Protocols. 2021;16:1936–1965. doi:10.1038/s41596-020-00474-1
17. Lee SH, Hu W, Matulay JT, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173:515–28.e17. doi:10.1016/j.cell.2018.03.017
18. Driehuis E, Kolders S, Spelier S, et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Disc. 2019;9:852–871. doi:10.1158/2159-8290.cd-18-1522
19. Xu W, Xie S, Chen X, Pan S, Qian H, Zhu X. Effects of quercetin on the efficacy of various chemotherapeutic drugs in cervical cancer cells. Drug Des Devel Ther. 2021;15:577–588. doi:10.2147/dddt.s291865
20. Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 2019;20:3177. doi:10.3390/ijms20133177
21. Yarahmadi A, Khademi F, Mostafavi-Pour Z, Zal F. In-vitro analysis of glucose and quercetin effects on m-TOR and Nrf-2 expression in HepG2 cell line (diabetes and cancer connection). Nutr Cancer. 2018;70:770–775. doi:10.1080/01635581.2018.1470654
22. Ranganathan S, Halagowder D, Sivasithambaram ND. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS One. 2015;10:e0141370. doi:10.1371/journal.pone.0141370
23. Prieto-Vila M, Shimomura I, Kogure A, et al. Quercetin Inhibits Lef1 and Resensitizes Docetaxel-Resistant Breast Cancer Cells Molecules 2020;25:2576 doi:10.3390/molecules25112576
24. Chou CC, Yang JS, Lu HF, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharmacal Res. 2010;33:1181–1191. doi:10.1007/s12272-010-0808-y
25. Li LJ, Li GW, Xie Y. Regulatory effects of glabridin and quercetin on energy metabolism of breast cancer cells]. Zhongguo Zhong yao za zhi. 2019;44:3786–3791. doi:10.19540/j.cnki.cjcmm.20190505.401
26. Przybylski P, Lewińska A, Rzeszutek I, et al. Mutation status and glucose availability affect the response to mitochondria-targeted quercetin derivative in breast cancer cells. Cancers. 2023;15:5614. doi:10.3390/cancers15235614
27. Hu Y, Li R, Jin J, Wang Y, Ma R. Quercetin improves pancreatic cancer chemo-sensitivity by regulating oxidative-inflammatory networks. J Food Biochem. 2022;46:e14453. doi:10.1111/jfbc.14453
28. Lu X, Yang F, Chen D, et al. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int J Bio Sci. 2020;16:1121–1134. doi:10.7150/ijbs.41686
29. Maciejczyk A, Surowiak P. Quercetin inhibits proliferation and increases sensitivity of ovarian cancer cells to cisplatin and paclitaxel. Ginekologia polska. 2013;84:590–595. doi:10.17772/gp/1609
30. Koren Carmi Y, Mahmoud H, Khamaisi H, Adawi R, Gopas J, Mahajna J. Flavonoids restore platinum drug sensitivity to ovarian carcinoma cells in a phospho-ERK1/2-dependent fashion. Int J Mol Sci. 2020;21. doi:10.3390/ijms21186533.
31. Li SZ, Qiao SF, Zhang JH, Li K. Quercetin increase the chemosensitivity of breast cancer cells to doxorubicin via PTEN/Akt pathway. Anti-Cancer Agents Med Chem. 2015;15:1185–1189. doi:10.2174/1871520615999150121121708
32. Hill SJ, Decker B, Roberts EA, et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Disc. 2018;8:1404–1421. doi:10.1158/2159-8290.cd-18-0474
33. Pan B, Li X, Zhao D, et al. Optimizing individualized treatment strategy based on breast cancer organoid model. Clin Transl. 2021;11:e380. doi:10.1002/ctm2.380;.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Quercetin: A Natural Ally in Combating Breast Cancer
Wu ZY, Qiu KY, Gai YJ, Wu JH, Zhou BX, Shi QF
International Journal of Nanomedicine 2025, 20:9155-9177
Published Date: 19 July 2025

