Back to Journals » Journal of Inflammation Research » Volume 17
Recombinant Humanized Collagen Type XVII Promotes Oral Ulcer Healing via Anti-Inflammation and Accelerate Tissue Healing
Authors Hao Y, Zhao B, Wu D, Ge X , Han J
Received 20 May 2024
Accepted for publication 11 July 2024
Published 24 July 2024 Volume 2024:17 Pages 4993—5004
DOI https://doi.org/10.2147/JIR.S470649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yukai Hao,1 Baoling Zhao,1 Dongchao Wu,1 Xuejun Ge,1 Jianmin Han2
1Department of Endodontics, Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials, Shanxi Medical University School and Hospital of Stomatology, Taiyuan, 030001, People’s Republic of China; 2Central Laboratory, Peking University School and Hospital of Stomatology, National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing Key Laboratory of Digital Stomatology, Beijing, 100081, People’s Republic of China
Correspondence: Xuejun Ge, The Department of Endodontics, Shanxi Medical University School and Hospital ofStomatology, 63 Xinjian South Road, Yingze District, Taiyuan, 030001, People’s Republic of China, Tel +8613834568537, Email [email protected] Jianmin Han, Central Laboratory, Peking University School and Hospital of Stomatology, 22 Zhongguan Cun South Street, Beijing, 100081, People’s Republic of China, Tel +8613810459668, Email [email protected]
Introduction: Recombinant humanized collagen, as a novel biomaterial, exhibits multiple excellent biological functions, such as inhibition of inflammation, promotion of cell proliferation and vascular proliferation, and promotion of tissue healing. However, there is a lack of conclusive evidence regarding the specific role of recombinant humanized collagen type 17 (rhCol 17) in oral ulcer healing. This study explored whether rhCol 17 could promote the proliferation of human gingival fibroblasts (HGFs) and inhibit its inflammation, and whether it could promote the healing of oral ulcers in rats by inhibiting inflammation and accelerating tissue healing.
Methods: At the cellular level, we investigated the effect of rhCol 17 on the proliferation of (HGFs) by CCK8; HGFs were mixed with lipopolysaccharide (LPS) to investigate the effect of rhCol 17 on HGFs in an inflammatory state. Eighteen adult male Sprague-Dawley rats were randomly distributed into three groups: blank control group, carbomer group (carbomer sprayed only), and rhCol 17 group (carbomer containing rhCol 17 sprayed), 1 time/day. The samples were collected at D3 and D5. At completion, histological staining and PCR were carried out to study its effect on the healing of oral ulcers in rats.
Results: Through cellular experiments, we found that rhCol 17 possesses good biocompatibility and anti-inflammatory properties, and can effectively promote the proliferation and migration of HGFs, as well as significantly reduce the inflammation level of the cells. The animal experimental results showed that rhCol 17 could significantly reduce the inflammation level, promote collagen deposition and angiogenesis at the ulcer site, thus effectively accelerating the healing of oral ulcers in rats.
Conclusion: In summary, the collagen sprays containing rhCol 17 have excellent anti-inflammatory effects and could accelerate tissue healing and are expected to provide a new effective treatment for patients with recurrent oral ulcers.
Keywords: oral ulcer, inflammation, wound healing, recombinant humanized collagen
Introduction
Oral ulcers are common ulcerative lesions that occurring in the oral mucosa and the most common diseases in stomatology, with a very high incidence in the population, and the prevalence in the general population ranges from 5% to 25%.1,2 There are many causes of oral ulcers, such as local irritation, trauma, stress, malnutrition or radiotherapy and chemotherapy.3,4 Oral ulcers are a significant complication for patients undergoing head and neck chemoradiotherapy, with an incidence of oral mucositis as high as 100%.5 About two-thirds of patients will develop severe ulcerative mucositis, which affects patients’ quality of life seriously.6 Oral ulcers are characterized by inflammatory and ulcerative lesions involving the stimulation of various inflammatory cells, metabolic enzymes, and cytokines.7 Taylor et al studied the peripheral blood of patients with RAU during attacks and intermittent periods and found that the content of TNF-α produced by white blood cells in patients was significantly increased during attacks.8 Shen et al also found that the levels of IL-6 and TNF-α in serum of RAS patients were significantly increased, indicating that the occurrence and development of oral ulcers were related to the level of inflammatory response.9
Despite the fact that the common condition of oral ulcers causes considerable distress to many people, there is no treatment with significant efficacy. Currently, there are two kinds of intervention measures for oral ulcers. The first category includes physical interventions, which utilize film-forming materials such as sprays and patches to physically isolate the ulcer site to alleviate symptoms.10,11 The second category encompasses pharmacological interventions, which include the use of local anesthetics, anti-inflammatory drugs, and medications that promote tissue healing, which are designed to treat oral ulcers by blocking pain transmission, decreasing inflammation levels, and promoting tissue repair. However, the therapeutic efficacy of these medications is similar to that of physical interventions and does not significantly reduce the healing time of ulcers.12,13 Due to the limited efficacy of medications and the need for frequent use, patients’ medical compliance tends to be low. Therefore, it is necessary to develop a drug that can remain on the ulcer surface for an extended period, effectively relieve pain and promote the healing of oral ulcers by reducing inflammation and promoting tissue repair.
Collagen is a protein abundant in animals and is the main component of the extracellular matrix, accounting for about one-third of the protein content of the human body.14 Collagen possesses excellent biological functions, such as good biocompatibility and biodegradability, and has been widely used in biomedical and regenerative materials. For example, collagen type 17 (Col 17) significantly promotes skin wound healing. Jacków et al found that Col 17 promotes wound re-epithelialization and accelerates wound healing by establishing a functional non- desiccated Col 17 mouse model and investigating stable skin adhesion and wound regeneration.15 Most collagen used in clinical applications is isolated from animals, posing biosafety risks such as allergic reactions and potential pathogen contamination.16,17 In contrast, recombinant humanized collagen, produced through DNA recombination and protein engineering techniques, can minimize these safety concerns. Compared with collagen of animal origin, recombinant humanized collagen not only avoids the risk of transmitting animal viruses, but also has a very low immunogenicity, thus offering a good safety profile and a broad clinical application prospect.18 Recombinant humanized collagen (RHC), as a new material, has been widely used in medical industry. RCH has beneficial biological functions, such as inhibiting inflammation, promoting cell proliferation and vascular hyperplasia, and promoting tissue healing.19–21 Although there are no studies on the relationship between oral ulcers and collagen type 17, and our preliminary experiments have demonstrated that rhCol 17 has the function of inhibiting inflammation and promoting cell proliferation, we plan to explore whether rh Col 17 can promote the healing of oral ulcers.
Due to the scour effect of oral saliva, drug dressings applied to the oral cavity are inevitably affected. Therefore, we chose Carbomer, a material with high adhesion, as the drug loading system. Carbomer, an acrylic polymer, is a crucial rheological regulator and thickener widely used in mucosal adhesion preparations.22 After thickening, it can adhere to the oral mucosal surface for a long time, forming a thin film that prolongs the drug release and action time and plays a physical isolation role.23 Here, it is planned to produce a dressing that can be used to cover oral ulcer wounds and promote ulcer healing. Firstly, we prepared a pH-sensitive and sprayable Carbomer spray. Secondly, recombinant human collagen type 17 (rh Col 17) was dissolved in carbomer spray to prepare the medication-loaded dressing. Finally, we used this spray to treat oral ulcer in SD rats, and the effect and mechanism of rh Col 17 in oral ulcer healing were observed.
Materials and Methods
Materials
Carbomer 971P (Lubrizol), glycerin (Analytical grade), Xylitol, Potassium hydroxide, IKA Agitator, PH meter, rh Col 17 (Shanxi Jinbo), Human gingival fibroblasts (Wuhan Procell), Lipopolysaccharide (LPS), Sprague Dawley male rat, pentobarbital sodium, glacial acetic acid, normal saline, periodontal probe, digital camera (Canon), eosin dye, Mason Kit, RNA extraction Kit (Animal Total RNA isolation Kit), reverse transcription kit (Prime Script RT) Master Mix), qPCR SYBR Green Master Mix.
Methods
Preparation and Characterization of Collagen Sprays
Prepare carbomer 971P solutions at different mass concentrations, Using KOH adjusts the pH of the solution to 4.0–6.5, and measure its viscosity with Brook Field R/S Plus rheometer (20 rpm). Selecting 1wt% carbomer solution, 1wt%, 1.5wt% and 2wt% of RH Col 17 were added to measure the change in viscosity and different concentrations of potassium dihydrogen phosphate were added to measure the change in pH and viscosity of the solution. Prepare carbomer solutions, adjust the PH value to the appropriate viscosity, add a small amount of ink to it. Take a clear glass sample bottle with a volume of 1mL, spray 0.1g into the bottom of 1mL white transparent glass bottle, stand for about 1 min, add 1mL normal saline, cover the lid and stand for 60 min, turn the glass bottle upside down, observe whether there is any carbomer residue at the bottom of the bottle and calculate the relative residual rate of residue. Select 1wt% of solution and measure the transmittance at different pH values after addition of rhCol 17 using a spectrophotometer.
Proliferation and Migration of HGF Cells
The effect of prepared rhCol17 on cell proliferation and migration ability was observed by incubating rhCol17 solution with HGF cells in vitro. The rhCol17 solution was obtained by dissolving 10 mg rhCol17 in 1 mL of DMEM cell culture medium. HGF cells were inoculated in 96-well plates, and after 24 h cells were attached to the wall, Different concentrations of rhCol17 solution, low concentration (L-rhCol 17 1mg/mL), middle concentration (M-rhCol 17 2mg/mL), and high concentration (H-rhCol 17 3mg/mL) were added to the above well plates and co-cultivated for 24, 48, and 72 hours. Finally, the cells were stained with CCK8 assay to observe the proliferation effect. The cells were divided into four groups for cell scratching experiments, blank control, collagen group (L-rhCol 17. M-rhCol 17. H-rhCol 17), three replicate wells in each group, inoculated into six-well plate at 10^6/well. 1–2 day cell confluence rate of 80–90% to start the experiment). The culture solution was removed in the ultra-clean bench, washed twice with PBS, and one well was taken for counting. After remaining well was scratched with a 200 μL pipette tip, the cells were treated with different concentrations of collagen solution. Immediately after 12, 24, and 48 hours of incubation, microscope images were taken to observe cell migration. We denoted the scratch area of 0h as S0, the scratch area after that as Sn. The cell migration rate is calculated according to the following formula: Cell migration rate = (Sn-S0)/S0* 100%. The experiment was repeated three times and representative images were selected.
Anti-Inflammatory Assay in vitro
The effect produced by the prepared rhCol17 on cells in an inflammatory state was observed by co-incubating the rhCol17 solution with LPS-treated HGF cells.24 HGF was inoculated into 6-well plates (10^6 cells/well) and the experiment was started 24h later. The experiment was divided into 5 groups, blank control group, LPS-PG, LPS-PG+1mg/mL rhCol 17, LPS-PG+2mg/mL rhCol 17, LPS-PG+3mg/mL rhCol 17. 10uL of LPS was added to each group except the blank control group, and the RNA of cells in each group was extracted at 12h and 24h, respectively, and the levels of the inflammatory factors between different groups were detected by RT-PCR (IL-1, IL-6, IL −8, TNF-α, SOD-1) between different groups, and the experiment was repeated three times. Table 1 includes a list of the tested genes and specific primers.
 |
Table 1 Primer Sequences of HGF Cells for Reverse Transcription and Quantification RT-PCR |
Establish Ulcer Models and Group
Select 18,150–180g Sprague Dawley male rats and feed them adaptively for three days. Use 1% pentobarbital sodium (0.4–0.5mL/100g) to anesthetize the rat, and fully expose the buccal mucosa on both sides. A 5-mm diameter filter paper was cut and soaked in 50% glacial acetic acid for 5 seconds, then the buccal mucosa was pressed rapidly for 40 seconds, and the excess glacial acetic acid was wiped away with a cotton swab dipped in saline.25 It was observed that the mucosa at the pressed site of the rats was damaged and turned bright red. After 24 hours, observe the local redness, swelling and ulceration of the treated buccal mucosa, indicating that the modeling is successful, which is recorded as D0. After successful modeling, the rats were randomly divided into 3 groups: blank group, carbomer group and collagen group (carbomer + rhCol 17). Medication was started after grouping, and each time the medication could completely cover the ulcer, 1 time/day. The dose in the collagen group was 1 mg/mL.
All rats were obtained from the Laboratory Animal Center of Peking University according to the International Guiding Principles for Animal Research (1985). Before surgery, the rats were acclimatized in cages in an animal husbandry facility, fed a standard laboratory diet, and housed in a room temperature and humidity environment. The study was approved by the Ethics Committee of the Peking University Health Science Center, Beijing, China (Approval No.: LA2020514).
Measurement of Oral Ulcer Size
Starting from D0, rat ulcers photos were taken daily with a Canon digital camera. A calibrated periodontal probe was placed on the edge of the ulcers for comparison. Each photo was analyzed by Image analysis software (Image J). The ulcer area was measured by visual examination and macro quantitative analysis. The degree of ulcer healing is calculated according to the following formula: Ulcer healing degree = (A0-An)/A0 * 100%, where A0 is the original ulcer area at day D0 and An is the area of the ulcer that has not healed every day after D0 day.
Histological Evaluation of the Healing of Oral Ulcers in Rats
Each group randomly selected 6 sites at D3 and D5, and the mucosal tissues of the 3 sites were immediately fixed with 10% formalin for 24 h at room temperature. The specimens were then embedded in paraffin, fixed, sliced, and placed on slides for hematoxylin and eosin (HE) staining. Select 5 fields of view from each slide, photographed at 400x magnification to count the number of polymorphonuclear cells (PMN), monocytes (MN), and neovascularization (excluding mature blood vessels and granulation tissue). Masson three-color staining was used to evaluate collagen deposition in sections of each group. The darker blue color represented the deposition of new collagen. Each slide was taken with 5 fields of view at 100x magnification. The slide images were calibrated with the image J software and the total collagen area was measured.
Cytological Evaluation of the Healing of Oral Ulcers in Rats
Mucosal tissue for molecular biological evaluation and storage in liquid nitrogen and transfer to −80 refrigerator. Take about 15g of mucosal tissue and lyse the tissue using the Animal Total RNA isolation Kit to obtain about 50μL of RNA. Reverse transcription was performed using cDNA synthesis kit, using 1000ng RNA per 20μL system. Using cDNA as template, quantitative PCR was performed on real-time fluorescence quantitative PCR system to analyze the expression levels of inflammatory cytokines IL-1, IL-6, TNF-α, TGF-β, growth-related cytokines EGF, VEGF and other genes. Table 2 includes a list of the tested genes and specific primers.
 |
Table 2 Primer Sequences of Rats for Reverse Transcription and Quantification RT-PCR |
Statistical Analysis
All experimental results are expressed as the mean ± standard deviation (SD) of at least three independent experiments. Statistical analyses were performed using conventional one-way ANOVA for multiple groups by using GraphPad Prism 8. No significant difference (ns), *P < 0.05, **P < 0.01, ***P < 0.001 indicated different variability, respectively.
Results
Results of Collagen Preparation and Characterization
The soluble had a large effect on the viscosity of the carbomer solution (Figure 1A), which decreased exponentially when the ions were just introduced. There were residues at the bottom of the bottles with different concentrations of solutions, the higher the concentration, the more residues were found (Figure 1B), indicating that the higher concentration of carbomer had better film-forming and adhesion properties after the increase of the PH value. When the PH was increased to about 5, rhCol 17 could be completely dissolved in the solution (Figure 1C). Therefore, we set the pH value to about 5 to ensure that collagen could be completely dissolved, and the viscosity range was set about 2600mPas to ensure good spray performance. Due to the limitation of viscosity range, high concentration of carbomer often needs to add more soluble ions to ensure its spraying performance, which will affect the solubility of rhCol 17, so we set the concentration range of carbomer to about 1.2 wt%.
Results of HGF Cell Proliferation and Migration
The results of CCK8 detection revealed rhCol 17could promote cell proliferation (Figure 2A), compared with the blank group, there was no significant difference in cell proliferation between the groups at 24 h and 48 h. At 72 h, there was a significant growth trend of HGF cells in the low concentration collagen group, with the best growth trend was observed in M-rhCol 17 group (P<0.001), followed by L-rhCol 17 group (P<0.01) (Figure 2A). There was no significant difference in the rate of cell scratch closure between the groups at 24 h compared with the blank control group (Figure 2B). At 48 h, the percentage of scratch closure area was as high as about 75% in L-rhCol 17 group (P<0.001) and the rate of scratch closure was 64% in the in M-rhCol 17 group (P<0.01), while the rate of scratch closure in the other groups was about 50%, (Figure 2C).
Results of in vitro Anti-Inflammatory Experiments
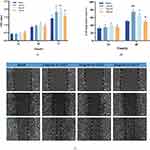
After 12 hours of LPS treatment, the levels of pro-inflammatory factors such as IL-1, IL-6, IL-8, TNF-α and SOD-1 in the LPS group were significantly higher than those in the control group, indicating that LPS could promote inflammation in HGF cells and the inflammatory cell model was successfully established. After adding rhCol 17 to LPS cells in an inflammatory state for 12 h, the expression levels of IL-1β, IL-6, IL-8 and SOD-1 were significantly decreased, and the decrease level of TNF-α was lower. 24 h later, the expression level of TNF-α was significantly decreased, and the decrease level of other inflammatory factors was not significant (Figure 3).
Therapeutic Results of rhCol 17 on Oral Ulcer in SD Rats
Both buccal mucosa of SD rats were treated with 50% glacial acetic acid, and the treated sites were observed 24 hours later. The results showed that the mucosa is red and swollen, widely red, covered by a large false film, yellow and white, and the modeling is successful. Visual observation showed that the ulcer area of rats in each group did not change much on the first and second day. From the third day, the ulcer area in the collagen group turned mostly pink, resembling surrounding healthy tissue, while the other groups still had a false membrane with clearly defined edges (Figure 4A). By calculating the ulcer area, it was found that the ulcer healing rate of the collagen group was significantly higher than that of the other groups at D5, and the ulcer healing rate was as high as 93%, while the healing rate of the blank group and the carbomer group were 67% and 79% (P<0.001), respectively, which was statistically significant (Figure 4D).
Histological Effects of the Healing of Oral Ulcers in Rats
The results of HE staining of mucosal tissue showed that all groups had defects in the oral mucosal epithelium, the blank group and the carbomer group had larger defects than the collagen group on D3. In the blank and carbomer groups, there were more inflammatory cells such as monocytes and polymorphonuclear cells in the epithelial layer and intrinsic layer, and fewer new blood vessels; while the collagen group had relatively fewer inflammatory cells and more new blood vessels. On D5, the defect of mucosal epithelium was healed in all groups, and the collagen group had the thickest epithelial thicknesses (Figure 4B). The new blood vessels increased significantly and the inflammatory cells in the epithelial layer and intrinsic layer were further reduced in the collagen group. In the other two groups, there were still more inflammatory cells in the intrinsic layer, less neovascularization in the intrinsic layer. The blank group had the worst level of inflammation with only a small amount of capillaries. The results of Masson staining showed that the collagen group had darker neocollagenous deposits on D3 compared to the other groups. On D5, the defective mucosal epithelium in the collagen group had healed and had the largest area of neocollagenous deposits (Figure 4C). By calculating the area of collagen deposition, we found that the area of collagen deposition was greater in the collagen group compared to the blank control group at D3 and D5, up to 19% and 31% respectively, and the difference was statistically significant. These signs indicate that rh Col 17 can significantly promote collagen deposition to accelerate oral ulcer healing.
Cytological Effects of the Healing of Oral Ulcers in Rats
The results of quantitative PCR revealed that rhCol 17 could inhibit inflammation levels and increase growth factor levels at oral ulcers (Figure 5). On D3, the expression levels of IL-1, IL-6 in the collagen group were significantly lower than those in the other two groups, but there was no significant difference in the decrease of TNF-α (Figure 5A). And the expression levels of TGF-β, EGF and VEGF were significantly higher than those two other groups (Figure 5B). On D5, the differences in the expression levels of inflammatory factors between the groups were reduced (Figure 5C), whereas the collagen group still showed significant differences in the expression levels of growth factors compared with the other two groups (Figure 5D). The results indicated that rhCol 17 has a good effect of inhibiting inflammation and promoting tissue growth.
Discussion
Oral ulcers, a common disease of the oral mucosa, are typically characterized by disruption of the mucosal epithelial integrity. The surface is covered with yellowish white false membrane, surrounded by hyperemia and edema.1 The occurrence and development of oral ulcers are accompanied by changes in inflammation levels and tissue destruction and repair. Ulcer healing requires a series of complex physiological processes, including the reduction of inflammation, cell proliferation, differentiation and migration, neovascularization, collagen deposition and re-epithelialization.26,27 In the early stage of ulcer formation, an inflammatory response occurs in the traumatic area, and white blood cells, platelets and inflammatory mediators will gather in the ulcer area to trigger an inflammatory response. At this time, the accumulated inflammatory cells participate in the phagocytic process, promoting the clearance of damaged tissue and the initiation of wound repair, which is essential for wound healing. When the ulcer begins to heal, a prolonged and violent inflammatory response can adversely affect healing process.28
Carbomer viscosity is affected by soluble ions.29 The presence of soluble ions in saliva will reduce its viscosity. The solubility of rhCol 17 in carbomer is greatly affected by PH. We determined the minimum pH for complete dissolution of rhCol 17 in carbomer. Since carbomer is a pH-sensitive polymer and its viscosity changes are most sensitive at PH 4–6, we formulated gels with minimal pH without affecting the solubility of rhCol 17. Therefore, we set the gel PH at around 5. Carbomer has good film formation and adhesion,30 which makes carbomer isolate the stimulation of external factors on ulcer to relieve pain and prolong the time of drug action.
Good biocompatibility is one of the fundamental properties of biomaterials and migration of cells are essential for wound healing and closure.31 In this experiment, we assessed the biocompatibility of rhCol 17 by evaluating the viability of human gingival fibroblast (HGF) cells, which are often affected by oral ulcers. The results of CCK8 indicated that lower concentrations of rhCol 17 (1–2mg/mL) significantly accelerated the proliferation of HGF cell. Additionally, the results of cell scratch assay showed that L-rhCol 17 group can significantly promote the proliferation of HGF cells. In contrast, the cell migration rate did not increase significantly in the H-rhCol 17 group. This difference may be related to the high adhesive properties of collagen, which adheres to the lower part of the well plate, thus affecting proliferation and migration of adherent cells. Then we established an HGF cell inflammation model and treated them with rhCol 17 to observe its anti-inflammatory effects. The results indicated that rhCol 17 exhibited significant anti-inflammatory effects on cells in the inflammatory state at 12h. At 24h, there was no significant difference in mRNA expression levels between the groups. This may be due to the weakened inflammatory stimulation of HGF cells by LPS. These experimental findings demonstrate that rhCol 17 can promote cell proliferation and migration, and can significantly inhibit cellular inflammation, which provides an experimental basis for the subsequent animal experiments.
In addition, we further explored the effects of rhCol 17 on the healing of oral ulcers in rats. By accurately calculating the ulcer healing area using Image J software, we obtained quantitative healing data. The results demonstrated that rats treated with collagen exhibited a significantly higher ulcer healing rate compared to the other groups on D5 which indicate that rhCol 17 positively promotes oral ulcer healing. The healing process of oral ulcers is closely associated with changes in inflammation levels. It has been noted that in the middle and late stages of ulcer healing, there is a significant downward trend in the level of inflammation.32 Similarly, collagen deposition plays a crucial role in the tissue healing process.21 We observed changes in the number of inflammatory cells and new vessels by HE staining and collagen deposition by Masson staining. HE and Masson staining showed that rhCol 17 significantly suppressed tissue inflammation levels, promoted neovascularization, mucosal epithelial healing and facilitated collagen deposition in the middle to late stages of ulcer healing. These findings indicate that rhCol 17 can significantly inhibit the release of inflammatory cells and reduce the level of inflammation at the ulcer in the later stage of wound healing. By promoting neovascularization to enhance blood supply and promoting collagen deposition, rhCol 17 creates favorable conditions for the healing of oral ulcers and accelerates the healing process.
The detection of cytokines is an effective means of assessing oral ulcer healing and the degree of local inflammatory cell infiltration.13 We detected the expression levels of related cytokines by PCR to evaluate the ulcer healing status in each group. The results showed that the level of inflammation was significantly increased in the early stage of ulcer healing, but gradually decreased with tissue repair. During this process, rhCol 17 showed significant anti-inflammatory effects, which could effectively reduce the levels of pro-inflammatory factors and simultaneously elevate the levels of anti-inflammatory factors at the D3 and D5 stages, thus contributing to the reduction of inflammation levels.
In addition, ulcer healing requires the release and regulation of growth promoting factors, as well as an adequate blood supply. The number of new vessels serves as a crucial indicator of the extent of blood supply to the lesion.33 In this process, key growth factors such as EGF and VEGF play an indispensable role.34 EGF promotes wound healing by accelerating the migration and proliferation of fibroblasts. VEGF, on the other hand, promotes capillary proliferation by promoting vascular endothelial cell growth and protein infiltration, thus promoting wound healing. The results of the cytological level of PCR confirmed that rhCol 17 promotes ulcer healing by inhibiting the levels of pro-inflammatory factors and promoting the levels of anti-inflammatory factors and growth factors.
Conclusion
In summary, this study demonstrated that rhCol 17 significantly inhibited the level of cellular inflammation and promoted cell proliferation and migration, although higher concentrations of rh Col17 had less effect on cell migration. Additionally, we established a rat oral ulcer model to evaluate the effect of rhCol 17-containing spray on oral ulcer healing. Collagen sprays showed a beneficial effect on oral ulcer healing. This promoting effect was manifested by a decrease in inflammatory cell infiltration, a decrease in IL-1β and IL-6 expression, an increase in neovascularization and collagen deposition, and an increase in TGF-β, EGF, and VEGF expression. It was mainly manifested on the third day after successful ulcer establishment. These foundings suggest that rhCol 17-containing sprays are expected to be a new approach to oral ulcer healing, and that rhCol 17 has great potential for application in the biomedical field.
Acknowledgments
The authors are thankful to Prof. Jianmin Han (Peking University Stomatology and Hospital) for providing financial and equipment support, and I would also acknowledge Prof. Xuejun Ge (Shanxi Medical University School and Hospital of Stomatology) for his training and sporting.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. Number VI recurrent aphthous stomatitis. Oral Dis. 2006;12(1):1–21. doi:10.1111/j.1601-0825.2005.01143.x
2. Ślebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp. 2013;62(3):205–215. doi:10.1007/s00005-013-0261-y
3. Marchini L, Campos MS, Silva AM, et al. Bacterial diversity in aphthous ulcers. Oral Microbiol Immunol. 2007;22(4):225–231. doi:10.1111/j.1399-302X.2006.00345.x
4. Mohammed E, Aboulkhair AG, Tawifq MM. Effect of nano-chitosan and nano-doxycycline gel on healing of induced oral ulcer in rat model: histological and immunohistochemical study. Clin Oral Investig. 2021;26(3):3109–3118. doi:10.1007/s00784-021-04293-w
5. Vilar CJF, Ribeiro SB, de Araujo AA, et al. Effect of gold nanoparticle on 5-fluorouracil-induced experimental oral mucositis in hamsters. Pharmaceutics. 2020;12(4):304. doi:10.3390/pharmaceutics12040304
6. Elting LS, Cooksley C, Chambers M, et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531–1539. doi:10.1002/cncr.11671
7. Wu Z, Lin W, Yuan Q, et al. A genome-wide association analysis: m6A-SNP related to the onset of oral ulcers. Front Immunol. 2022;13(931408). doi:10.3389/fimmu.2022.931408
8. Taylor LJ, Bagg J, Walker DM, et al. Increased production of tumour necrosis factor by peripheral blood leukocytes in patients with recurrent oral aphthous ulceration. J Oral Pathol Med. 1992;21(1):21–25. doi:10.1111/j.1600-0714.1992.tb00963.x
9. Shen C, Ye W, Gong L, et al. Serum interleukin-6, interleukin-17A, and tumor necrosis factor-alpha in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2021;50(4):418–423. doi:10.1111/jop.13158
10. Zhang C, Liu Y, Li W, et al. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: in vitro and in vivo studies. Pharm Dev Technol. 2019;24(1):118–126. doi:10.1080/10837450.2018.1428814
11. Suharyani I, Fouad Abdelwahab Mohammed A, Muchtaridi M, et al. Evolution of drug delivery systems for recurrent aphthous stomatitis. Drug Des Devel Ther. 2021;15:4071–4089. doi:10.2147/dddt.S328371
12. Li T, Bao Q, Shen J, et al. Mucoadhesive in situ forming gel for oral mucositis pain control. Int J Pharm. 2020;580(119238):119238. doi:10.1016/j.ijpharm.2020.119238
13. Miao M, Peng M, Xing Z, et al. Effect of Shuangjinlian mixture on oral ulcer model in rat. Saudi J Biol Sci. 2019;26(4):790–794. doi:10.1016/j.sjbs.2019.02.005
14. Liu D, Liang L, Regenstein JM, et al. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis. Food Chem. 2012;133(4):1441–1448. doi:10.1016/j.foodchem.2012.02.032
15. Jacków J, Schlosser A, Sormunen R, et al. Generation of a functional non-shedding collagen XVII mouse model: relevance of collagen XVII shedding in wound healing. J Invest Dermatol. 2016;136(2):516–525. doi:10.1016/j.jid.2015.10.060
16. Shoseyov O, Posen Y, Grynspan F. Human collagen produced in plants: more than just another molecule. Bioengineered. 2014;5(1):49–52. doi:10.4161/bioe.26002
17. Seror J, Stern M, Zarka R, et al. The potential use of novel plant-derived recombinant human collagen in aesthetic medicine. Plast Reconstr Surg. 2021;148(6S):32S–38S. doi:10.1097/PRS.0000000000008784
18. Song X, Zhu C, Fan D, et al. A novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers. 2017;9(12):638. doi:10.3390/polym9120638
19. Xing M, Fu R, Liu Y, et al. Human-like collagen promotes the healing of acetic acid-induced gastric ulcers in rats by regulating NOS and growth factors. Food Funct. 2020;11(5):4123–4137. doi:10.1039/d0fo00288g
20. Shuai X, Kang N, Li Y, et al. Recombination humanized type III collagen promotes oral ulcer healing. Oral Dis. 2023. doi:10.1111/odi.14540
21. Long LY, Liu W, Li L, et al. Dissolving microneedle-encapsulated drug-loaded nanoparticles and recombinant humanized collagen type III for the treatment of chronic wound via anti-inflammation and enhanced cell proliferation and angiogenesis. Nanoscale. 2022;14(4):1285–1295. doi:10.1039/d1nr07708b
22. Alhusban FA, C P. Seville Carbomer-modified spray-dried respirable powders for pulmonary delivery of salbutamol sulphate. J Microencapsul. 2009;26(5):444–455. doi:10.1080/02652040802456924
23. Singla AK, Chawla M, Singh A. Potential applications of carbomer in oral mucoadhesive controlled drug delivery system: a review. Drug Dev Ind Pharm. 2000;26(9):913–924. doi:10.1081/ddc-100101318
24. Xie Y, Sun M, Xia Y, et al. An RNA-seq screen of P. gingivalis LPS treated human gingival fibroblasts. Arch Oral Biol. 2018;88:77–84. doi:10.1016/j.archoralbio.2018.01.002
25. Hitomi S, Ono K, Yamaguchi K, et al. The traditional Japanese medicine hangeshashinto alleviates oral ulcer-induced pain in a rat model. Arch Oral Biol. 2016;66:30–37. doi:10.1016/j.archoralbio.2016.02.002
26. Khan I, Arany P. Biophysical approaches for oral wound healing: emphasis on photobiomodulation. Adv Wound Care. 2015;4(12):724–737. doi:10.1089/wound.2014.0623
27. Chen L, Arbieva ZH, Guo S, et al. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics. 2010;11(1). doi:10.1186/1471-2164-11-471
28. Koh TJ, A L. DiPietro Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13. doi:10.1017/s1462399411001943
29. Kolarova Raskova Z, Hrabalikova M, Sedlarik V. Effect of sodium salicylate on the viscoelastic properties and stability of polyacrylate-based hydrogels for medical applications. Int J Polym Sci. 2016;2016:1–6. doi:10.1155/2016/5614687
30. Carolina Visser J, Weggemans OAF, Boosman RJ, et al. Increased drug load and polymer compatibility of bilayered orodispersible films. Eur J Pharm Sci. 2017;107:183–190. doi:10.1016/j.ejps.2017.07.010
31. Yang B, Song J, Jiang Y, et al. Injectable adhesive self-healing multicross-linked double-network hydrogel facilitates full-thickness skin wound healing. ACS Appl. Mater. Interfaces. 2020;12(52):57782–57797. doi:10.1021/acsami.0c18948
32. Shang Y, Yao S, Qiao X, et al. Evaluations of marine collagen peptides from tilapia skin on experimental oral ulcer model of mice. Mater Today Commun. 2021;26(101893). doi:10.1016/j.mtcomm.2020.101893
33. Karim AS, Liu A, Lin C, et al. Evolution of ischemia and neovascularization in a murine model of full thickness human wound healing. Wound Repair Regener. 2020;28(6):812–822. doi:10.1111/wrr.12847
34. Fujisawa K, Miyamoto Y, Nagayama M. Basic fibroblast growth factor and epidermal growth factor reverse impaired ulcer healing of the rabbit oral mucosa. J Oral Pathol Med. 2003;32(6):358–366. doi:10.1034/j.1600-0714.2003.t01-1-00111.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.