Back to Journals » Clinical Ophthalmology » Volume 19
Relationship Between Intraocular Pressure-Lowering Effects and Alterations in Anterior Segment Scleral Birefringence Following Micropulse Cyclophotocoagulation
Authors Nemoto H , Honjo M, Okamoto M, Tominaga S, Yamanari M, Aoyama Y, Arai T, Ishiyama Y, Sugimoto K, Sakata R, Saito H, Fujishiro T, Aihara M
Received 7 April 2025
Accepted for publication 23 June 2025
Published 9 July 2025 Volume 2025:19 Pages 2179—2188
DOI https://doi.org/10.2147/OPTH.S530136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hotaka Nemoto,1 Megumi Honjo,1 Michiaki Okamoto,2 Sou Tominaga,2 Masahiro Yamanari,2 Yurika Aoyama,1 Takahiro Arai,1 Yukako Ishiyama,1 Koichiro Sugimoto,1 Rei Sakata,1 Hitomi Saito,1 Takashi Fujishiro,1 Makoto Aihara1
1Department of Ophthalmology, University of Tokyo Graduate School of Medicine, Tokyo, Japan; 2Tomey Corporation, Nagoya, Japan
Correspondence: Megumi Honjo, Department of Ophthalmology, University of Tokyo Graduate School of Medicine, 7-3-1 Hongo Bunkyo-ku, Tokyo, 113-8655, Japan, Tel +81-3-3815-5411, Fax +81-3-3817-0798, Email [email protected]
Purpose: To assess the changes in anterior segment scleral birefringence (ASSB), a marker of collagen-related fibrotic responses, following micropulse cyclophotocoagulation (MP-CPC) using polarization-sensitive optical coherence tomography (PS-OCT).
Patients and Methods: Eighteen eyes of sixteen patients with glaucoma who underwent MP-CPC at the University of Tokyo Hospital in Japan from July 2022 to August 2023 were included. The procedure utilized a Cyclo G6 glaucoma laser system (IRIDEX, Mountain View, CA, USA) with laser power set at 2,500 mW and an 80-second duration per hemisphere. Clinical outcomes and ASSB were assessed for 6 months. The principal assessment was the evaluation of post-procedure ASSB. Anterior segment images were captured before MP-CPC, and at 1 week, 1 month, 3 months, and 6 months after MP-CPC, using PS-OCT (ROCTIA; Tomey Corporation, Nagoya, Japan) to assess the ASSB. We also assessed the correlation between intraocular pressure (IOP) reduction and changes in birefringence.
Results: The IOP was significantly lower after than before MP-CPC (pretreatment: 24.8 ± 1.8, 1 week: 13.9 ± 1.1, 1 month: 17.4 ± 1.1, 3 months: 20.1 ± 1.7, and 6 months: 19.0 ± 1.6 mmHg). ASSB increased significantly after treatment (pretreatment: 1.00 ± 0.08, 1 week: 1.20 ± 0.11, 1 month: 1.27 ± 0.06, 3 months: 1.42 ± 0.07, and 6 months: 1.26 ± 0.15), suggesting post-laser collagen remodeling in the sclera. Notably, a significant positive correlation was found between the rate of IOP reduction and the degree of birefringence elevation at 3 and 6 months post-procedure.
Conclusion: We observed a significant increase in ASSB after MP-CPC, and this increase was positively correlated with IOP reduction. These findings point to the potential mechanism underlying IOP reduction and emphasize the clinical relevance of the birefringence value.
Keywords: polarization-sensitive optical coherence tomography, PS-OCT, micropulse cyclophotocoagulation, MP-CPC, birefringence
Introduction
Patients with glaucoma, a progressive disease leading to irreversible visual impairment through visual field defects,1,2 often benefit from intraocular pressure (IOP)-lowering therapy.3,4 Continuous-wave cyclophotocoagulation (CPC), which has long been used as an IOP-lowering laser treatment, suppresses aqueous humor production by destruction of the ciliary processes.5 However, its use is limited because of severe adverse effects, including postoperative intraocular inflammation, decreased vision, scleral melting, hypotony, and phthisis bulbi.6
By contrast, the recently introduced micropulse CPC (MP-CPC) technique, which employs an 810-nm wavelength micropulse diode laser with P3 probe (Cyclo G6; IRIDEX Laser Systems, Mountain View, CA, USA), has gained popularity because of its lower complication rate.7,8 Whereas continuous-wave CPC primarily achieves its therapeutic effect through the destruction of pigmented tissues in the ciliary body via absorbed laser energy, micropulse mode delivers short diode-laser pulses interspersed with pauses.9,10 This approach theoretically minimizes heat-induced tissue damage by facilitating tissue cooling during laser pauses. MP-CPC has demonstrated both safety and efficacy, even in patients with advanced refractory glaucoma and in earlier stages of the disease.7,8,11,12
In a previous study, we observed fibrotic reactions in the conjunctiva and sclera after MP-CPC in rabbit eyes and reported as a potential mechanism of IOP reduction.13 Cases of scleral thinning or persistent conjunctival hyperemia have also been reported with the clinical use of MP-CPC,14,15 indicating possible reactions in the conjunctiva and sclera. However, the details of these phenomena remain unclear.
Optical coherence tomography (OCT) is a noninvasive imaging technique commonly employed in daily practice to visualize tissue structures. It is widely used to evaluate intraocular structures such as the retina, choroid, and optic nerve, and anterior segment OCT is used to examine structures such as the cornea, conjunctiva, and sclera.
Polarization-sensitive OCT (PS-OCT) is the functional extension of OCT, which provides additional contrast to characterize the tissue polarization properties.16,17 Birefringence is an optical phenomenon where anisotropic materials exhibit different refractive indices along different polarization axes, leading to a phase retardation between orthogonally polarized light components. In biological tissues, this is often caused by organized structures such as collagen fibers. PS-OCT has emerged as a potent noninvasive technique for investigating and potentially diagnosing postoperative fibrosis or collagen alterations in the sclera.18,19 Therefore, we hypothesized that PS-OCT-based anterior segment scleral birefringence (ASSB) measurements can be used to detect collagen remodeling, including fibrotic reactions, following MP-CPC.
In this study, we used PS-OCT to investigate changes in birefringence in the sclera of patients who underwent MP-CPC, and we assessed the association of these changes with the outcome of the procedure.
Materials and Methods
Patients
This prospective observational study involved 18 eyes of 16 patients with glaucoma who underwent MP-CPC at the University of Tokyo Hospital in Japan from July 2022 to August 2023, with a maximum follow-up period of 6 months. We excluded patients who did not undergo PS-OCT imaging at least twice within the 6-month post-MP-CPC period, required additional MP-CPC or surgery because of elevated IOP within 6 months, or had a history of prior MP-CPC treatment. This observational study was conducted in accordance with the principles of the Declaration of Helsinki, and the institutional review board approved the study protocol (approved number 2217). The ethics committee waived the requirement for informed consent regarding the use of patients’ medical record data in accordance with the regulations of the Japanese Guidelines for Epidemiologic Study issued by the Japanese government. Clinical trial registration was not required because of the observational nature of the study. All participants were at least 18 years of age. Informed consent was obtained from all patients prior to the procedures. We anonymized all acquired PS-OCT images and medical data. IOP was measured using a calibrated Goldmann applanation tonometer. The use of a combination of topical glaucoma drugs was scored as 2 points, and the use of oral acetazolamide was scored as 1 point.
Surgical Technique
MP-CPC was performed as reported previously using the Cyclo G6 device (IRIDEX Laser Systems).20 Briefly, sub-tenon anesthesia with 2.5–4.0 mL of 2% lidocaine hydrochloride was administered before treatment. Hydroxyethyl cellulose gel (Scopisol Solution for Eyes; Senju Pharmaceutical Co., Ltd., Osaka, Japan) was applied to the conjunctiva and placed within the fluid channel of the MicroPulse P3 probe (IRIDEX Laser Systems).20 The laser power was adjusted to 2,500 mW with an 80-second duration per hemisphere (total of 160 seconds; 20 seconds per sweep) and a 31.3% duty cycle. The procedure was performed with the laser directed toward the pars plana. Areas at the 3- and 9-o’clock positions, where the ciliary neurovascular bundle is located, were avoided. Laser irradiation was performed in a back-and-forth manner for 20 seconds per pass. Postoperatively, topical betamethasone sodium phosphate 0.1% eye drops were used four times daily for 1 week. MP-CPC was performed by several surgeons.
PS-OCT Measurement
We captured anterior segment images before MP-CPC, and at 1 week, 1 month, 3 months, and 6 months after MP-CPC, using PS-OCT (ROCTIA; Tomey Corporation, Nagoya, Japan). The details of the system and the processing algorithm to calculate birefringence were described previously.16,17 The standard deviation of birefringence measurements in a healthy retina was less than 0.33 deg/µm. Since the OCT signal intensity in the sclera is greater than in the retina, the accuracy of birefringence measurements in the sclera is higher than that. Because this PS-OCT device has a default scanning system for retinal imaging, we used an attachment lens for anterior segment imaging, which had a lateral resolution of 35 µm. The maximum imaging depth of the PS-OCT system used in this study is approximately 4 mm, allowing sufficient penetration to visualize anterior segment structures including the sclera. During imaging, the eye was adjusted to an upward position to ensure comprehensive visualization of the cornea, angle, and lower scleral area within a single field of view.
Analysis of Birefringence
Birefringence was visualized using a customized color map exhibiting monotonically increasing brightness. Gamma correction was applied to an open-source colormap called “viridis” to enhance the visibility of low birefringence. We performed an RBG-to-CIELAB conversion on the exported images using ImageJ software (National Institutes of Health, Bethesda, MD, USA) to create a brightness (L) image. We then standardized all images by rotating them to orient the sclera horizontally. Finally, we applied a consistent threshold value to binarize the images. This threshold was determined based on analysis of multiple birefringence maps, selecting the value that best distinguished between regions of high and low birefringence.
Within these processed images, we placed a region of interest in the sclera, measuring 3,000 μm in width and 500 μm in height and located 3 mm from the angle (the laser irradiation site of MP-CPC). After cropping this area, we calculated the number of pixels exhibiting a birefringence value of 255, which we defined as high birefringence. Standardization was achieved by dividing each number of thresholded pixels at each time point by the mean value of number of thresholded pixels before treatment. The repeatability of the measurement was assessed by calculating the coefficient of variation (CV) from three repeated measurements on each sample. The average CV across all samples was 6.5%, indicating high measurement precision.
Statistical Analysis
Statistical analyses were performed using R software (version 4.2.2; R Development Core Team, Austria, Vienna). Values for line graphs and bar graphs are expressed as mean ± standard error of the mean (SEM). Differences between pre- and post-treatment values were analyzed using a mixed linear model. We assessed the correlation between two variables using Spearman’s rank correlation coefficient. A p-value of < 0.05 was considered statistically significant.
Results
Patient Characteristics
The patients’ characteristics are shown in Table 1. We analyzed 18 eyes of 16 patients after MP-CPC. The patients’ average age was 69.8 years (standard deviation [SD], 4.0 years), and 15 eyes (83%) belonged to male patients. The average pretreatment IOP was 24.8 mmHg (SD, 1.8), and the average pretreatment medication score was 4.6 (SD, 0.2). The glaucoma subtypes were primary open-angle glaucoma (9 eyes), exfoliation glaucoma (3 eyes), and secondary glaucoma (6 eyes). We found no significant correlation between pretreatment IOP and ASSB (r = −0.23, p = 0.36 according to Spearman’s rank correlation coefficient test).
 |
Table 1 Patient Demographics and Characteristics (18 Eyes of 16 Patients) |
IOP Reduction and Changes in Medication Scores Following MP-CPC
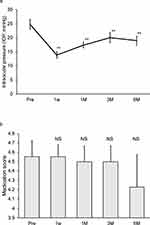
Following MP-CPC, statistically significant IOP reductions were observed at 1 week, 1 month, 3 months, and 6 months compared with the pretreatment IOP (pretreatment: 24.8 ± 1.8, 1 week: 13.9 ± 1.1, 1 month: 17.4 ± 1.1, 3 months: 20.1 ± 1.7, and 6 months: 19.0 ± 1.6 mmHg) (Figure 1a). The medication scores after MP-CPC showed no statistically significant differences at any time point (pretreatment: 4.6 ± 0.2, 1 week: 4.6 ± 0.1, 1 month: 4.5 ± 0.2, 3 months: 4.5 ± 0.2, and 6 months: 4.2 ± 0.3) (Figure 1b).
Changes in ASSB Following MP-CPC
The pretreatment PS-OCT images displayed regions of high birefringence in the band of extracanalicular limbal lamina21 surrounding the trabecular meshwork as well as multiple islands of thresholded high birefringence within the sclera (Figure 2a). The size of the thresholded high birefringence in the band of extracanalicular limbal lamina did not change significantly after MP-CPC. We observed an increase in ASSB at the laser-irradiated area at 1 week, 1 month, 3 months, and 6 months after MP-CPC (Figure 2b). Analysis of the sclera in 18 eyes revealed statistically significant increases in ASSB at all timepoint compared with the pretreatment values (pretreatment: 1.00 ± 0.08, 1 week: 1.20 ± 0.11, 1 month: 1.27 ± 0.06, 3 months: 1.42 ± 0.07, and 6 months: 1.26 ± 0.15) (Figure 2c). Birefringence within the sclera increased gradually, peaking at 3 months.
Correlation Between the Rate of IOP Reduction and Rate of Birefringence Increase
We examined the correlation between the rate of IOP reduction and the rate of birefringence increase in the sclera at 1 week, 1 month, 3 months, and 6 months after MP-CPC. At 1 week and 1 month, no significant correlation was observed. At 3 and 6 months, however, we found a significant positive correlation between the rate of IOP reduction and the rate of birefringence increase (1 week: r = 0, p = 1; 1 month: r = −0.17, p = 0.51; 3 months: r = 0.55, p = 0.03; 6 months: r = 0.63, p = 0.04) (Figure 3a–d).
Discussion
MP-CPC has gained popularity as an effective and safe IOP-lowering glaucoma treatment. However, the precise mechanism of IOP reduction and the impact of MP-CPC on the anterior eye tissue have remained unclear. In this study, we used PS-OCT to analyze changes in ASSB following MP-CPC. Our findings revealed a significant increase in birefringence in the laser-irradiated scleral area after MP-CPC (Figure 2). Moreover, a statistically significant positive correlation between the rate of IOP reduction and the rate of birefringence increase was observed at 3 and 6 months after the procedure (Figure 3a–d).
Birefringence is a phenomenon characterized by phase retardation when light waves penetrate birefringent materials.19 Materials with dense structures and high orientation exhibit elevated birefringence. Collagen, known for its highly oriented structure, is a representative birefringent material in living organisms.22 PS-OCT has been used to visualize various types of fibrosis-related scarring, such as dermal fibrosis, joint or tendon fibrosis, and gastrointestinal and lung fibrosis.22–26 In ocular tissues, PS-OCT has been used to analyze birefringence in fibrous tissues that contain collagens with specific polarization properties, especially the cornea, sclera, and angle structures.21,27,28 Liu et al22 recently demonstrated that the posterior scleral birefringence value was higher in myopic eyes and showed a positive correlation with longer axial length, especially in pathologic myopia. In eyes affected by high myopia, the myopic posterior sclera exhibits alterations in the arrangement of scleral collagen and reductions in interwoven fibers.29,30 These reports collectively suggest that higher birefringence can be associated with changes in collagen fiber microstructure as well as collagen fiber reorganization, which is consistent with the results of the present study.
Our prior research demonstrated fibrotic responses in subconjunctival and scleral tissues of rabbits following MP-CPC, even at relatively low laser power.13 Other studies have also revealed a fibrotic response of the subconjunctival tissue following MP-CPC.9 Indeed, an impact of MP-CPC on the conjunctiva and sclera is reasonable considering the reports of scleral thinning and persistent conjunctival hyperemia in real-world clinical practice following MP-CPC.14,15 Given the predominance of collagen in the sclera, the observed increase in birefringence following MP-CPC may have resulted from fibrotic reactions induced by MP-CPC or thermal energy generated by laser energy absorption, leading to collagen degeneration or reconstruction in the sclera.
The observed trends in IOP reduction and medication scores after MP-CPC in our study were similar to those in a previous report from our institution and are consistent with previous reports from other institutions.7,20,31,32 This suggests that the MP-CPC procedures in the present study were conducted appropriately and highlights the possible importance of a sustained increase in ASSB for effective IOP reduction.
Interestingly, a positive correlation between the rate of IOP reduction and the increase in birefringence was observed at 3 and 6 months postoperatively (Figure 3c and d), but not at 1 week. One possible explanation is that the structural changes in the sclera responsible for birefringence alterations—such as collagen fiber reorganization or degeneration—may require more time to manifest than the immediate IOP-lowering effects of MP-CPC. Notably, the peak increase in ASSB occurred 3 months after MP-CPC, aligning with the gradual progression of fibrotic responses (Figure 2c). This time lag suggests that tissue remodeling is a gradual process, and the birefringence signal may reflect more sustained or cumulative changes in the extracellular matrix rather than acute pressure changes alone. Another possible explanation involves individual-patient variations may exist in the absorption of laser energy within the sclera, its conversion to thermal energy, and the subsequent scleral reactions. In clinical practice, MP-CPC does not uniformly yield identical IOP-lowering effects in all patients; instead, there are individual differences in both the efficacy and extent of IOP reduction.7,20,31,32 Consequently, significant changes in the polarization characteristics of the sclera may be more likely to occur when MP-CPC achieves a substantial IOP reduction. Moreover, MP-CPC might not be optimally executed in patients with limited IOP reduction. Factors such as improper laser positioning, variations in probe angles among patients, or complications (eg, application of insufficient force during probe placement or the occurrence of sub-tenon anesthesia-induced conjunctival hemorrhage that absorbs the laser power) may lead to both milder collagen degeneration in the sclera and limited IOP reduction. In our study, multiple surgeons performed MP-CPC. Additionally, patients with glaucoma often exhibit ptosis and eyelid hardening due to topical prostaglandin analog treatment. These factors can further contribute to the variability in MP-CPC techniques across patients.33,34 The increase in ASSB observed in this study may serve as a valuable tool for assessing the effectiveness of MP-CPC as well as a highly plausible explanation for the mechanisms of IOP reduction by this treatment.
This study had three main limitations. First, all patients were Japanese. Research has revealed variations in melanin content in the uvea among different racial groups. Caucasian eyes allow slightly more effective laser penetration of the sclera than Asian eyes, perhaps because there is less scattering and absorption caused by lower levels of pigmentation.22,35,36 It is conceivable that the Japanese population has higher melanin content, potentially resulting in greater laser absorption during MP-CPC. Further investigations involving diverse racial groups are warranted. Second, we excluded patients requiring additional surgery or repeat MP-CPC during the 6-month observation period because we aimed to evaluate the effect of only one MP-CPC procedure. These exclusions may have introduced bias because such refractive cases are likely indicative of poor IOP reduction efficacy. Further studies are needed to explore the response in these cases. Third, the long-term effects of MP-CPC were not fully evaluated in this study. Further longitudinal studies are needed.
In conclusion, our findings indicate an increase in ASSB following MP-CPC, with a positive correlation between the rate of birefringence increase and the rate of IOP reduction at the intermediate time point after MP-CPC. These results suggest a possible mechanism for IOP reduction and highlight the clinical significance of birefringence measurements.
Acknowledgments
The authors thank Tomey Corporation for their significant contribution to the PS-OCT image analysis. The authors also thank George Marcellino, PhD, the late vice president of IRIDEX, for his generous support of this study. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/ANMLVn. This paper has been uploaded to ResearchSquare as a preprint: https://www.researchsquare.com/article/rs-4343501/v1.
Funding
This work was financially supported by grants from the Japan Society for the Promotion of Science (JSPS-KAKENHI 23H03058, 20H03839, 25K20176, and 22K09807).
Disclosure
Michiaki Okamoto, Sou Tominaga, and Masahiro Yamanari are employees of Tomey Corporation. In addition, Dr Masahiro Yamanari has patents JP 6463051 B2, US 9593936, EP 2995245 B1, JP 6542178 B2, and JP 7332131 B2 issued to Tomey Corporation. Professor Makoto Aihara reports grants, personal fees, and/or non-financial support from Santen Pharmaceutical Co.Ltd, Senju Pharmaceutical Co.Ltd, Otsuka Pharmaceutical Co.Ltd, Kowa Pharmaceutical Co.Ltd, Crewt medical systems, Alcon Japan, HOYA, Viatris Pharmaceutical Co.Ltd, TOMEY, Rohto Nitten, and Sato Pharmaceutical Co.Ltd, outside the submitted work. The remaining authors declare no conflicts of interest in this work.
References
1. Aihara M, Ropo A, Lu F, et al. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64(4):398–406. doi:10.1007/s10384-020-00748-x
2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
3. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi:10.1016/S0140-6736(04)16257-0
4. Kwon YH, Fingert JH, Kuehn MH, Alward WLM. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. doi:10.1056/NEJMra0804630
5. Hennis HL, Stewart WC. Semiconductor diode laser transscleral cyclophotocoagulation in patients with glaucoma. Am J Ophthalmol. 1992;113(1):81–85. doi:10.1016/s0002-9394(14)75758-7
6. Ramli N, Htoon HM, Ho CL, Aung T, Perera S. Risk factors for hypotony after transscleral diode cyclophotocoagulation. J Glaucoma. 2012;21(3):169–173. doi:10.1097/IJG.0b013e318207091a
7. Tan AM, Chockalingam M, Aquino MC, Lim ZIL, See JLS, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38(3):266–272. doi:10.1111/j.1442-9071.2010.02238.x
8. Aquino MCD, Barton K, Tan AMWT, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43(1):40–46. doi:10.1111/ceo.12360
9. Tan NYQ, Ang M, Chan ASY, et al. Transscleral cyclophotocoagulation and its histological effects on the conjunctiva. Sci Rep. 2019;9(1):18703. doi:10.1038/s41598-019-55102-0
10. Berger JW. Thermal modelling of micropulsed diode laser retinal photocoagulation. Lasers Surg Med. 1997;20(4):409–415. doi:10.1002/(sici)1096-9101
11. Abdelrahman AM, El Sayed YM. Micropulse versus continuous wave transscleral cyclophotocoagulation in refractory pediatric glaucoma. J Glaucoma. 2018;27(10):900–905. doi:10.1097/IJG.0000000000001053
12. Yelenskiy A, Gillette TB, Arosemena A, et al. Patient outcomes following micropulse transscleral cyclophotocoagulation: intermediate-term results. J Glaucoma. 2018;27(10):920–925. doi:10.1097/IJG.0000000000001023
13. Nemoto H, Honjo M, Okamoto M, Sugimoto K, Aihara M. Potential mechanisms of intraocular pressure reduction by micropulse transscleral cyclophotocoagulation in rabbit eyes. Invest Ophthalmol Vis Sci. 2022;63(6):3. doi:10.1167/iovs.63.6.3
14. Bolek B, Wylęgała A, Wylęgała E. Microcyclophotocoagulation in glaucoma treatment: a medium-term follow-up study. J Clin Med. 2023;12(13):4342. doi:10.3390/jcm12134342
15. Pandey A, Sanghi S, Chaudhary S. Blue sclera as a complication of micropulse transscleral laser therapy. J Glaucoma. 2021;30(11):1011–1014. doi:10.1097/IJG.0000000000001948
16. Yamanari M, Mase M, Obata R, et al. Melanin concentration and depolarization metrics measurement by polarization-sensitive optical coherence tomography. Sci Rep. 2020;10(1):19513. doi:10.1038/s41598-020-76397-4
17. Yamanari M, Tsuda S, Kokubun T, et al. Estimation of Jones matrix, birefringence and entropy using Cloude-Pottier decomposition in polarization-sensitive optical coherence tomography. Biomed Opt Express. 2016;7(9):3551–3573. doi:10.1364/BOE.7.003551
18. Gräfe MGO, van de Kreeke JA, Willemse J, et al. Subretinal fibrosis detection using polarization sensitive optical coherence tomography. Transl Vis Sci Technol. 2020;9(4):13. doi:10.1167/tvst.9.4.13
19. Fukuda S, Fujita A, Kasaragod D, et al. Comparison of intensity, phase retardation, and local birefringence images for filtering blebs using polarization-sensitive optical coherence tomography. Sci Rep. 2018;8(1):7519. doi:10.1038/s41598-018-25884-w
20. Akiyama T, Fujishiro T, Sugimoto K, et al. Short-term outcomes of micropulse transscleral laser therapy using the revised delivery probe in refractory glaucoma. Jpn J Ophthalmol. 2022;66(6):549–558. doi:10.1007/s10384-022-00938-9
21. Ueno Y, Mori H, Kikuchi K, Yamanari M, Oshika T. Visualization of anterior chamber angle structures with scattering- and polarization-sensitive anterior segment optical coherence tomography. Transl Vis Sci Technol. 2021;10(14):29. doi:10.1167/tvst.10.14.29
22. Liu X, Jiang L, Ke M, et al. Posterior scleral birefringence measured by triple-input polarization-sensitive imaging as a biomarker of myopia progression. Nat Biomed Eng. 2023;7(8):986–1000. doi:10.1038/s41551-023-01062-w
23. Adams DC, Szabari MV, Lagares D, et al. Assessing the progression of systemic sclerosis by monitoring the tissue optic axis using PS-OCT. Sci Rep. 2020;10(1):2561. doi:10.1038/s41598-020-59330-7
24. Jaspers MEH, Feroldi F, Vlig M, de Boer JF, van Zuijlen PPM. In vivo polarization-sensitive optical coherence tomography of human burn scars: birefringence quantification and correspondence with histologically determined collagen density. J Biomed Opt. 2017;22(12):1–8. doi:10.1117/1.JBO.22.12.121712
25. Hariri LP, Adams DC, Wain JC, et al. Reply to wijmans et al.: optical coherence tomography: a valuable novel tool for assessing the alveolar compartment in interstitial lung disease? Am J Respir Crit Care Med. 2018;197(9):1232–1233. doi:10.1164/rccm.201711-2347LE
26. Lee YJ, Park E, Ks P, et al. Quantification method to objectively evaluate the fibrous structural status of tendons based on polarization-sensitive OCT. J Biophotonics. 2022;15(11):e202200065. doi:10.1002/jbio.202200065
27. Willemse J, Gräfe MGO, Verbraak FD, de Boer JF. In vivo 3d determination of peripapillary scleral and retinal layer architecture using polarization-sensitive optical coherence tomography. Transl Vis Sci Technol. 2020;9(11):21. doi:10.1167/tvst.9.11.21
28. Lim Y, Yamanari M, Fukuda S, et al. Birefringence measurement of cornea and anterior segment by office-based polarization-sensitive optical coherence tomography. Biomed Opt Express. 2011;2(8):2392–2402. doi:10.1364/BOE.2.002892
29. Boote C, Sigal IA, Grytz R, Hua Y, Nguyen TD, Girard MJA. Scleral structure and biomechanics. Prog Retin Eye Res. 2020;74:100773. doi:10.1016/j.preteyeres.2019.100773
30. Markov PP, Eliasy A, Pijanka JK, et al. Bulk changes in posterior scleral collagen microstructure in human high myopia. Mol Vis. 2018;24:818–833.
31. Kaba Q, Somani S, Tam E, Yuen D. The effectiveness and safety of micropulse cyclophotocoagulation in the treatment of ocular hypertension and glaucoma. Ophthalmol Glaucoma. 2020;3(3):181–189. doi:10.1016/j.ogla.2020.02.005
32. Wong KYT, Aquino CM, Macasaet AM, Suwandono ME, Chew PTK, Koh VTC. MP3 plus: a modified micropulse transscleral cyclophototherapy technique for the treatment of refractory glaucoma. J Glaucoma. 2020;29(4):264–270. doi:10.1097/IJG.0000000000001443
33. Sakata R, Fujishiro T, Saito H, et al. Recovery of deepening of the upper eyelid sulcus after switching from prostaglandin FP receptor agonists to EP2 receptor agonist: a 3-month prospective analysis. Jpn J Ophthalmol. 2021;65(5):591–597. doi:10.1007/s10384-021-00855-3
34. Sakata R, Shirato S, Miyata K, Aihara M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol. 2013;57(2):179–184. doi:10.1007/s10384-012-0219-3
35. Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986;27(2):145–152.
36. Miura M, Makita S, Yasuno Y, et al. Evaluation of choroidal melanin-containing tissue in healthy Japanese subjects by polarization-sensitive optical coherence tomography. Sci Rep. 2022;12(1):4048. doi:10.1038/s41598-022-07818-9
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.