Back to Journals » Journal of Inflammation Research » Volume 17
Retrospective Analysis of Aseptic Meningitis in Kikuchi–Fujimoto Disease
Received 19 July 2024
Accepted for publication 31 October 2024
Published 21 November 2024 Volume 2024:17 Pages 9319—9324
DOI https://doi.org/10.2147/JIR.S480056
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam Bachstetter
Ran Cheng,1,* Fei Lin,1,* Ming Lu2
1Department of Infectious Diseases, Peking University Third Hospital, Beijing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ming Lu, Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing, People’s Republic of China, Email [email protected]
Introduction and Objectives: Kikuchi–Fujimoto disease (KFD) is self-limiting, has an unknown origin, and predominantly affects the lymph nodes. KFD with aseptic meningitis is rare and diagnostically challenging. This retrospective observational study aimed to elucidate the clinical features and treatment outcomes of KFD, particularly in cases with aseptic meningitis.
Methods: We conducted this retrospective study to describe KFD to determine the characteristics of the disease, with a particular focus on cases involving aseptic meningitis.
Results: Our study comprised 103 patients (33 men, 70 women) diagnosed with KFD at Peking University Third Hospital between January 2013 and March 2024. Diagnosis was based on histological examination of lymph node biopsies. The mean age was 25 (range: 16– 66) years. Clinical manifestations included fever (100%), cervical pain (79.6%), fatigue (49.5%), headache (44.7%), myalgia (26.2%), and hepatosplenomegaly (23.3%). Biological signs included leukopenia (66.0%) and elevated lactate dehydrogenase (> 250 U/L, 83.5%) and ferritin (> 300 ng/mL, 44.6%) levels. Forty-three cases improved with nonsteroidal anti-inflammatory drugs (NSAIDs) as monotherapy, whereas 24 required corticosteroid therapy. Four of the 46 patients with headache underwent cerebrospinal fluid analysis, confirming aseptic meningitis. Notably, all four responded well to nonsteroidal anti-inflammatory drugs.
Conclusion: Our findings highlight the features and outcomes of KFD, particularly its association with aseptic meningitis, which has a favorable prognosis in the absence of corticosteroid therapy.
Keywords: Kikuchi–Fujimoto disease, aseptic meningitis, histiocytic necrotizing lymphadenitis, self-limiting
Introduction
Kikuchi–Fujimoto disease (KFD), also known as histiocytic necrotizing lymphadenitis, was first reported in Japan in 1972 and has a higher prevalence among Asians.1,2 KFD predominantly affects young to middle-aged individuals but can occur at any age.3–5 While most reports indicate a female predominance, some studies from Asian countries suggest a closer male-to-female ratio.6,7 Despite extensive research, the exact cause of KFD remains elusive, with viral infections or autoimmune processes being proposed as potential triggers.8,9
KFD manifests as a benign condition characterized by lymphadenopathy and often presents with systemic symptoms such as fever, sore throat, rash, weight loss, and hepatosplenomegaly.3,10 Laboratory investigations may reveal elevated C-reactive protein (CRP) levels, a high erythrocyte sedimentation rate, leukopenia, and anemia in some cases.11 Rare neurological complications include cerebellar ataxia and mononeuritis multiplex;12 aseptic meningitis is also rare but less so, occurring in 2.8–9.8% of KFD cases.13,14
Despite sporadic case reports and limited case series on aseptic meningitis in KFD, comprehensive summaries from China are lacking. This retrospective study aimed to assess the clinical and laboratory characteristics of KFD, with a particular focus on cases involving aseptic meningitis. Our overall goal is to raise awareness of this potential complication and emphasize the need for further research and clinical vigilance in managing KFD.
Patients and Methods
Patients
This retrospective study enrolled patients diagnosed with KFD at Peking University Third Hospital between January 1, 2013 and March 31, 2024. The inclusion criteria were age ≥16 years and histopathologic lesions consistent with the Kikuchi criteria. Patients with a suspected or confirmed alternative diagnosis, such as lymphoma or infectious adenitis, were excluded.
Diagnostic Criteria
The diagnosis of KFD was based on histopathological criteria, including well-circumscribed focal lesions in the lymph node cortex or paracortex, severe necrosis with karyorrhexis or apoptosis, absence of large numbers of polymorphonuclear neutrophils or eosinophils, occasional histiocytes predominantly around areas of necrosis, and lymphoid hyperplasia. Immunohistochemical analysis aided in ruling out malignant lymphoma, which is characterized by the predominance of T cells (mostly CD8+) and histiocytes expressing myeloperoxidase and CD68 antigens.6
The diagnosis of aseptic meningitis was based on the presence of lymphocytic pleocytosis and elevated protein levels with normal glucose levels on cerebrospinal fluid (CSF) analysis. Additional laboratory tests, including viral serology and polymerase chain reaction (PCR), were performed to rule out other causes of aseptic meningitis.
Ethical Considerations
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the institutional review board of Peking University Third Hospital, Beijing, China. Informed consent was waived owing to the retrospective nature of the study. Data were analyzed anonymously.
Statistical Analysis
Statistical analyses were conducted using SPSS for Windows (version 22.0; IBM Corp., Armonk, NY). Continuous variables with a normal distribution are reported as mean ± standard deviation and non-normal variables as median and interquartile range. Categorical variables are expressed as frequencies (percentages).
Results
Patient Characteristics
A total of 103 patients treated between January 1, 2013 and March 31, 2024 met the criteria for inclusion in our analysis. The baseline characteristics of the patients are presented in Table 1. Among the patients, 32.1% were men and 67.9% were women. The mean age of the patients was 27.5 ± 8.65 years, with 73.8% (76/103) younger than 30 years.
 |
Table 1 Baseline Characteristics of Patients with Kikuchi-Fujimoto Disease |
The most common symptoms were fever (100%) and cervical pain (79.6%), followed by fatigue (49.5%), headache (44.7%), and myalgia (26.2%). Cervical lymphadenomegaly was present in 99 (96.1%) cases, axillary involvement in 39 (37.9%), and generalized lymphadenomegaly (≥3 parts) in nine (8.7%). Splenomegaly was present in 24 (23.3%) patients. Erythematous rashes and joint pain were rare.
The primary biological abnormalities are listed in Table 2. The levels of an acute-phase reactant (CRP >10 mg/L), lactate dehydrogenase (LDH, >250 U/L), and ferritin titers (>300 ng/mL) were elevated in 28.2%, 83.5%, and 44.7% of the patients, respectively. Leukopenia (66.0%), anemia (13.6%), and thrombocytopenia (9.7%) were also observed.
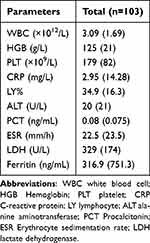 |
Table 2 Baseline Biochemical Parameter of Patients with Kikuchi-Fujimoto Disease |
All patients improved after treatment, with regression of clinical symptoms and normalization of biological parameters. The treatments included nonsteroidal anti-inflammatory drugs (NSAIDs) in 43 (41.7%) patients and a corticosteroid (0.5–1 mg/kg prednisone, 14–60 days) in the 24 (23.3%) patients with significant systemic manifestations. The remaining 36 (35.0%) patients received various treatments (eg, antibiotics, acyclovir, ganciclovir) as monotherapy or combination therapy. No patient died during the study.
Analysis of Aseptic Meningitis in KFD
We identified four patients who developed aseptic meningitis during the course of their illness. The clinical features of these patients are summarized in Table 3. All four patients were female and had no remarkable medical or family history. They presented to the fever emergency department of our hospital with fever and headache and were subsequently hospitalized for further examination and treatment. Upon admission, no signs of meningeal irritation were observed. Brain magnetic resonance imaging was performed in three patients and computed tomography in one patient. CSF analysis showed negative findings for bacteria, fungi, and Mycobacterium tuberculosis (Table 3). Tests for paraneoplastic autoantibodies (anti-Hu, anti-Yo, and anti-Ri) also yielded negative results. KFD was confirmed via lymph node biopsy (Figure 1). The diagnosis of aseptic meningitis was based on CSF, and the patients’ condition improved within 1 month of NSAID treatment.
 |
Table 3 Demographical, Laboratory and Radiological Findings of 4 Cases of Aseptic Meningitis Associated with KFD |
Discussion
KFD is a benign, self-limited lymphadenitis that can occur at all ages (range: 5–75 years); however, in most case series, it predominantly affected young women. KFD is most common in Japan, with sporadic instances in various other countries.3–5 Similar to findings in Japan, our study confirmed the female predominance of KFD, as well as its preferential involvement during the third decade of life, in China.
The dominant clinical features of KFD include lymphadenopathy, fever, fatigue, and splenomegaly, along with leukopenia, anemia, thrombocytopenia, and elevated CRP and LDH levels. Lymph node involvement is mainly cervical and usually painful. The lymph nodes are firm, mobile, and sometimes very large (2–4 cm in diameter), but never ulcerating.6,15,16 Systemic signs, predominantly fever, were observed in all patients in our study, with fever of unknown origin being the primary reason for admission to our hospital. Laboratory abnormalities are inconsistent and nonspecific; inflammatory syndrome and leukopenia are classically reported, as observed in our patients.
Extranodal manifestations of KFD are rare and include neurological conditions such as aseptic meningitis, mononeuritis multiplex, and acute cerebellar ataxia. Aseptic meningitis is the most common neurological complication, occurring in 2.8–9.8% of KFD cases.13 Its diagnosis relies on clinical features and, in most cases, the presence of lymphocytic pleocytosis and elevated protein but normal glucose levels in CSF samples.13 Additional laboratory tests, such as viral serology and PCR, may aid in ruling out other causes of aseptic meningitis.
A meta-analysis of 244 patients with KFD revealed neurological impairments in 11 (45%) patients, including aseptic meningitis (eight patients), polyneuritis, and acute cerebellar ataxia.11 The results of our review support these findings. We identified four cases of aseptic meningitis ultimately diagnosed as KFD based on the pathological findings of cervical lymph node biopsies. All four patients presented with fever and headache. While previous reports have described the development of meningitis in patients with KFD,13 our case series highlights patients presenting primarily with aseptic meningitis, emphasizing the importance of considering aseptic meningitis when neurological symptoms are evident. Therefore, for patients with fever accompanied by headache of unknown etiology, a thorough examination for lymphadenopathy may be helpful, while also considering KFD as a differential diagnosis.
The treatment of aseptic meningitis in KFD typically relies on standard therapies (eg, NSAIDs and corticosteroids) for underlying conditions.17 Antiviral agents are administered if a viral etiology is suspected. The duration of treatment varies based on symptom severity and treatment response. In our case series and literature review, all symptoms, including aseptic meningitis, were resolved in all patients within a few weeks of treatment initiation.
Despite its significant findings, this study has two limitations. First, it was a single-center, retrospective study. Second, aseptic meningitis associated with mild KFD may resolve spontaneously before a comprehensive examination can be conducted.
Conclusion
This study updates the clinical presentation of KFD, which is common among young individuals. Clinicians must recognize this condition to prevent misdiagnosis, which could lead to unnecessary treatments. Although rare, aseptic meningitis in KFD has been documented in several reports. Its treatment involves standard therapies such as NSAIDs and corticosteroids, with antiviral agents if a viral etiology is suspected. Further research is required to fully determine the pathogenesis of and optimal management protocol for aseptic meningitis in KFD.
Abbreviations
CSF, cerebrospinal fluid; CRP, C-reactive protein; KFD, Kikuchi-–Fujimoto disease; LDH, lactate dehydrogenase; NSAID, nonsteroidal anti-inflammatory drug; PCR, polymerase chain reaction.
Ethical Considerations
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the institutional review board of Peking University Third Hospital, Beijing, China. Informed consent was waived due to the retrospective nature of the study. Data were analyzed anonymously.
Acknowledgments
We thank all the physicians and technicians who contributed to this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytosis: a clinicopathological study. Nippon Ketsueki Gakkai Zasshi. 1972;53:379–380.
2. Fujimoto Y. Cervical sub-acute necrotizing lymphadenitis. A new clinicopathologial entity. Naika. 1972;30:920–927.
3. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004;122(1):141–152. doi:10.1309/YF081L4TKYWVYVPQ
4. Pepe F, Disma S, Teodoro C, Pepe P, Magro G. Kikuchi-Fujimoto disease: a clinicopathologic update. Pathologica. 2016;108(3):120–129.
5. Hutchinson CB, Wang E. Kikuchi-Fujimoto disease. Arch Pathol Lab Med. 2010;134(2):289–293. doi:10.5858/134.2.289
6. Kuo TT. Kikuchi’s disease (histiocytic necrotizing lymphadenitis). A clinicopathologic study of 79 cases with an analysis of histologic subtypes, immunohistology, and DNA ploidy. Am J Surg Pathol. 1995;19(7):798–809. doi:10.1097/00000478-199507000-00008
7. Lin HC, Su CY, Huang CC, Hwang CF, Chien CY. Kikuchi’s disease: a review and analysis of 61 cases. Otolaryngol--Head Neck Surg. 2003;128(5):650–653. doi:10.1016/S0194-59980223291-X
8. Rosado FG, Tang YW, Hasserjian RP, McClain CM, Wang B, Mosse CA. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Human Pathol. 2013;44(2):255–259. doi:10.1016/j.humpath.2012.05.016
9. Chong Y, Kang CS. Causative agents of Kikuchi-Fujimoto disease (histiocytic necrotizing lymphadenitis): a meta-analysis. Int J Pediatric Otorhinolaryngol. 2014;78(11):1890–1897. doi:10.1016/j.ijporl.2014.08.019
10. Perry AM, Choi SM. Kikuchi-Fujimoto Disease: a Review. Arch Pathol Lab Med. 2018;142(11):1341–1346. doi:10.5858/arpa.2018-0219-RA
11. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26(1):50–54. doi:10.1007/s10067-006-0230-5
12. Moon JS, Il Kim G, Koo YH, et al. Kinetic tremor and cerebellar ataxia as initial manifestations of Kikuchi-Fujimoto’s disease. J Neurol Sci. 2009;277(1–2):181–183. doi:10.1016/j.jns.2008.10.021
13. Sato Y, Kuno H, Oizumi K. Histiocytic necrotizing lymphadenitis (Kikuchi’s disease) with aseptic meningitis. J Neurol Sci. 1999;163(2):187–191. doi:10.1016/S0022-510X(99)00037-4
14. Nakamura I, Imamura A, Yanagisawa N, Suganuma A, Ajisawa A. Medical study of 69 cases diagnosed as Kikuchi’s disease. Kansenshogaku Zasshi J Japanese Assoc Infectious Dis. 2009;83(4):363–368. doi:10.11150/kansenshogakuzasshi.83.363
15. Norris AH, Krasinskas AM, Salhany KE, Gluckman SJ. Kikuchi-Fujimoto disease: a benign cause of fever and lymphadenopathy. Am j Med. 1996;101(4):401–405. doi:10.1016/S0002-9343(96)00231-8
16. Frikha F, Marzouk S, Frigui M, et al. Kikuchi-Fujimoto’s disease and connective tissue disease: a report of three cases. Rev Med Interne. 2008;29(2):129–134. doi:10.1016/j.revmed.2007.07.012
17. Noursadeghi M, Aqel N, Pasvol G. Kikuchi’s disease: a rare cause of meningitis? Clinical infectious diseases: an official publication of the infectious diseases society of America. Clin Infectious Dis. 2005;41(8):e80–82. doi:10.1086/444563
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


