Back to Journals » Journal of Multidisciplinary Healthcare » Volume 18
Robot-Assisted Surgery for Endometrial Cancer Using KangDuo versus Da Vinci Systems: A Retrospective Comparative Study
Authors Liu T, Ma L, Wang Y, Wang B, Xu Y, Gao Y, Yu G, Gao J , Chen J
Received 18 March 2025
Accepted for publication 18 June 2025
Published 7 July 2025 Volume 2025:18 Pages 3891—3900
DOI https://doi.org/10.2147/JMDH.S525579
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Pavani Rangachari
Tianbo Liu,* Li Ma,* Yan Wang, Bo Wang, Yue Xu, Ying Gao, Ge Yu, Jialiang Gao, Jie Chen
Department of Gynecological Oncology, Harbin Medical University Cancer Hospital, Harbin, 150081, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Chen, Email [email protected]
Background: With the advancement of medical technology, robotic-assisted surgery has emerged as a promising approach for the management of endometrial cancer (EC). This retrospective comparative study aimed to evaluate the efficacy, safety, and functional outcomes of two robotic systems—Kangduo (KD-RAS) and Da Vinci (DV-RAS)—in the treatment of EC.
Methods: This study included patients with stage T1 EC who underwent robotic-assisted surgery using either the Kangduo or Da Vinci system at Harbin Medical University Cancer Hospital. A comprehensive statistical analysis was conducted on their perioperative clinical data, encompassing preoperative, intraoperative, and postoperative parameters.
Results: A total of 211 patients were enrolled in this study, including 125 in the KD-RAS group and 86 in the DV-RAS group. The surgical success rate was 100% in both groups, with no significant differences observed in preoperative baseline characteristics (P > 0.05). There were also no significant differences between the two groups in terms of blood loss, transfusion requirements, or Clavien-Dindo grade I/II complications (P > 0.05). However, the KD-RAS group exhibited longer operation time, console time, time to first flatus, and length of hospital stay compared to the DV-RAS group (P < 0.05). Notably, both total hospitalization costs and surgical expenses were significantly lower in the KD-RAS group than in the DV-RAS group (P < 0.05).
Conclusion: The Kangduo robotic system demonstrates comparable efficacy and equivalent safety profiles to the Da Vinci system, supporting its non-inferiority in clinical performance for the treatment of early-stage endometrial cancer.
Keywords: endometrial cancer, Kangduo robotic system, Da Vinci robotic system, efficacy, safety
Introduction
Endometrial cancer (EC) accounts for approximately 90% of all malignant tumors of the uterine corpus, making it one of the most common malignancies among women, with an increasing incidence year by year.1,2 Among the histopathological subtypes of EC, endometrioid carcinoma is the most prevalent, representing 75–80% of cases.3 For patients with EC, particularly those diagnosed at an early stage, surgery remains the primary treatment modality. The standard procedure involves extrafascial total hysterectomy combined with bilateral salpingo-oophorectomy, often accompanied by lymph node dissection when suspicious findings are present.4,5
Surgical approaches include both open and minimally invasive techniques. In recent years, minimally invasive surgery has gained popularity due to its advantages such as shorter hospital stays, reduced pain and blood loss, and faster recovery, thereby improving both surgical and quality-of-life outcomes for EC patients.6 Although laparoscopy is currently the most widely used minimally invasive method, it presents certain limitations, including a restricted field of view, technical challenges in suturing, and ergonomic disadvantages.7
The advent of robotic-assisted surgery (RAS) aims to address these shortcomings. RAS is a novel surgical technique wherein surgeons operate through a robotic system that controls instruments mounted on robotic arms to perform complex procedures. It offers advantages such as enhanced precision, minimal invasiveness, greater flexibility, and reduced surgeon fatigue. These features make RAS particularly suitable for gynecologic oncology procedures, especially those involving complex anatomical environments, such as paraaortic lymphadenectomy, radical hysterectomy, and surgeries in obese patients.8
Since the Da Vinci® robotic-assisted surgery (DV-RAS) system received CE certification in Europe in 1999, RAS has entered a phase of rapid development. In parallel, China has developed its own robotic surgical platform, the Kangduo Surgical Robot-01 (KD-SR-01®), which comprises an open surgical console, three robotic arms, and a high-definition 3D imaging system (Figure 1). To date, the Kangduo robotic-assisted surgery (KD-RAS) system has been clinically applied in urology,9–11 and several comparative studies have been conducted between KD-RAS and DV-RAS.12–14 However, evidence regarding its application in gynecologic oncology remains limited.
 |
Figure 1 The KD-SR-01® surgical robot system. |
Therefore, this study was designed to compare the effectiveness, safety, and functional outcomes of KD-RAS and DV-RAS across the preoperative, intraoperative, and postoperative phases in patients undergoing surgery for endometrial cancer.
Materials and Methods
Study Patients
From March 2024 to December 2024, we retrospectively analyzed 211 EC patients aged 18–85 years undergoing robot-assisted surgery (RAS) at Harbin Medical University Cancer Hospital, with 125 cases in the KD-RAS group and 86 in the DV-RAS group. All participating surgeons had ≥50 prior DV system procedures and completed standardized KD system training (structured courses, simulation training, and proctored surgeries). Each surgeon performed comparable numbers of procedures on both platforms to minimize operator-dependent bias. The study protocol received approval from the Ethics Committee of Harbin Medical University Cancer Hospital (Approval No. KY2021-46). All experimental protocols were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Inclusion criteria: (1) meeting surgical indications; (2) histologically confirmed endometrioid carcinoma; (3) FIGO stage T1;15 (4) voluntary RAS treatment.
Exclusion criteria: (1) prior adjuvant therapy (endocrine/radiotherapy/chemotherapy/targeted); (2) confirmed distant metastasis; (3) contraindications for general anesthesia; (4) severe comorbidities; (5) incomplete follow-up.
Data Collection
We systematically collected preoperative, intraoperative, and postoperative data through electronic medical records. Preoperative parameters included: demographic characteristics (age, BMI), gynecological history (reproductive/menopausal status), comorbidities (hypertension/diabetes/cardiovascular diseases), and laboratory indices (CA125, WBC, Neu, HGB, ALT, AST, albumin).
Intraoperative parameters comprised: docking time (robot-to-table positioning completion), console time (docking-to-undocking duration),11 estimated blood loss, transfusion requirements, total operation time, and lymphadenectomy details.
Postoperative parameters encompassed: pathological characteristics (tumor size, histologic grade, myometrial invasion, LVSI, nodal metastasis), FIGO staging, clinical outcomes (complications graded by Clavien-Dindo,16 ICU stay, first flatus, hospitalization duration), cost analysis (total/surgical expenses), and laboratory indices.
Statistical Analysis
A non-inferiority margin of Δ=15% was predetermined based on historical EBL differences in minimally invasive gynecologic surgery. Categorical variables were expressed as n(%) and analyzed using χ²-test. Continuous variables following normal distribution were presented as mean±SD with Student’s t-test; non-normal variables used median[IQR] with Mann–Whitney U-test. Statistical power was calculated post hoc using G*Power 3.1, achieving 80% power to detect inter-group differences at α=0.05. Analyses were performed using SPSS 26.0 and GraphPad Prism 8, with p<0.05 considered statistically significant.
Results
Baseline Characteristics
The study roadmap is shown in Figure 2. Among the 211 patients, there were 125 patients in the KD-RAS group (59.2%) and 86 patients in the DV-RAS group (40.8%).
 |
Figure 2 The study roadmap. |
All procedures were completed without conversion to open or laparoscopic surgery, and no intraoperative complications occurred in either group. As presented in Table 1, the median age of EC patients in both groups was 55 years (range: 28–77 for KD-RAS; 36–77 for DV-RAS). Most patients had early-stage disease, with 107 (85.6%) in the KD-RAS group and 72 (83.7%) in the DV-RAS group diagnosed as FIGO stage IA. No statistically significant differences were observed between the two groups in terms of demographic characteristics, nutritional status, menopause status, parity, surgical history, comorbidities, tumor size, or serum CA125 levels (P > 0.05).
 |
Table 1 Baseline Demographic and Clinicopathological Characteristics of EC Patients Undergoing RAS |
Pathological Outcomes
As shown in Table 2, no significant differences were found between the two groups regarding histologic grade, depth of myometrial invasion, lymphovascular space involvement (LVSI), number of resected lymph nodes, or lymph node metastasis (P > 0.05). However, a significantly higher proportion of patients underwent lymph node dissection in the DV-RAS group compared to the KD-RAS group (89.6% vs 76.0%, P = 0.013).
 |
Table 2 Pathological Outcomes of EC Patients Undergoing RAS |
Perioperative Outcomes
As displayed in Table 3, all procedures were completed without conversion to open or laparoscopic surgery. The docking time was similar between the KD-RAS and DV-RAS groups (2.4 min vs 2.2 min, P = 0.086). However, the KD-RAS group had a longer operation time (111.1 min vs 95.8 min, P = 0.001) and console time (70.3 min vs 56.6 min, P = 0.001) compared to the DV-RAS group. There were no significant differences in estimated blood loss (EBL) and blood transfusion rates between the two groups (P > 0.05). Additionally, the trend of console time for EC patients using the KD-RAS system showed a decreasing tendency over time (Figure 3).
 |
Table 3 Perioperative Data of EC Patients Undergoing RAS |
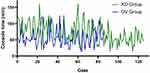 |
Figure 3 The curve of console time in two groups as the number of cases increases. |
Follow-up Outcomes
The overall complication rates were comparable between the groups. Clavien-Dindo grade I complications occurred in 97.6% (122/125) of the KD-RAS group and 96.5% (83/86) of the DV-RAS group. Grade II complications were observed in 2.4% (3/125) and 3.5% (3/86) of the KD-RAS and DV-RAS groups, respectively. No major complications (Clavien-Dindo grade ≥ III) were reported. ICU stay times were also comparable between the groups. No reoperations or deaths occurred during hospitalization. Interestingly, the mean time to first flatus in the DV-RAS group was significantly shorter at 2.9 hours compared to 3.0 hours in the KD-RAS group (P < 0.001). Similarly, the length of hospital stay was significantly shorter in the DV-RAS group (4.4 days) than in the KD-RAS group (5.0 days, P = 0.001). The dynamic change curves indicated no significant difference between the two groups in the change of blood indexes before and after surgery (Figure 4). The hospitalization and surgical expenses of the KD-RAS group were significantly lower than those of the DV-RAS group (P < 0.001 for both) (Tables 4 and 5).
 |
Table 4 Postoperative Recovery and Follow-up Outcomes of EC Patients Undergoing RAS |
 |
Table 5 Comparison of Hospitalization and Surgery Expenses |
Discussion
Since the beginning of the 21st century, robotic-assisted surgery (RAS) technology has continuously advanced, with its applications gradually expanding across various surgical fields. The robotic arm in RAS systems offers superior precision compared to the human hand, particularly when operating on delicate structures, and provides enhanced maneuverability in complex surgical scenarios. It also contributes to reduced intraoperative bleeding, lower postoperative infection risk, and improved overall surgical safety and quality. Additionally, RAS has demonstrated clear clinical benefits, including minimizing blood loss during surgery, decreasing postoperative complication rates, and enhancing procedural outcomes.
Among RAS platforms, the da Vinci robotic-assisted surgery (DV-RAS) system is the most widely recognized and clinically adopted. Its efficacy and safety have been extensively validated in multiple studies.17,18 However, its widespread application is somewhat limited by high costs and a steep learning curve. With growing awareness of independent innovation and continuous technological progress, the China-developed KangDuo RAS (KD-RAS) system has emerged as a viable alternative. Designed to meet domestic healthcare demands, KD-RAS also aims to improve patients’ quality of life. Several prospective studies have affirmed the safety and effectiveness of the KD-RAS system, indicating that it is not inherently inferior to the DV-RAS system.11–14,19 Nonetheless, its clinical utility in gynecologic surgery remains underexplored.
This retrospective study demonstrated that the KD-RAS system yielded overall comparable outcomes to DV-RAS across preoperative, intraoperative, and postoperative indicators. Notably, KD-RAS was associated with reduced hospitalization costs, offering a more cost-effective solution without compromising care quality.
In our analysis, both the KD-RAS and DV-RAS groups exhibited similar baseline characteristics (including age, BMI, and FIGO staging), without statistically significant differences, thereby reinforcing the credibility of the comparative findings. All procedures were completed successfully in both groups, with no conversions to conventional laparoscopy or open surgery, indicating a 100% surgical success rate and confirming the feasibility of the KD-RAS system.
Although estimated blood loss (EBL) and transfusion rates were higher in the KD-RAS group, these differences were not statistically significant, suggesting that the KD-RAS system is similarly effective in controlling intraoperative bleeding. Postoperative complication rates were also comparable between the two groups, and no severe complications occurred, supporting the safety of the KD-RAS approach.
Furthermore, laboratory analyses conducted before and after surgery showed consistent physiological trends in both groups (Figure 4), indicating similar systemic responses to the procedures. These findings suggest that both robotic systems have comparable impacts on patients’ physiological homeostasis and support favorable short-term prognostic outcomes.
In our study, the KD-RAS group exhibited longer total operation time (111.1 vs 95.8 min, P = 0.001) and console time (70.3 vs 56.6 min, P = 0.001). Three interrelated factors explain this disparity. First, learning curve effects: despite standardized training including structured courses and simulation drills, the initial 30 KD-RAS cases showed wide variability in operative times (Figure 3), consistent with prior reports indicating that 20–30 cases are typically required to achieve robotic proficiency.12,19 Second, ergonomic adaptation: surgeon feedback highlighted differences in tactile feedback (KD-RAS: 0.5N vs DV-RAS: 0.1N) and wrist articulation range (240° vs 540°), necessitating modified tissue handling techniques. Parallel urologic studies reported 15% lower “instrument maneuverability” scores for KD-RAS,20 findings that align with our observations. Third, team coordination challenges: the open-console design of KD-RAS, while beneficial for teaching, initially introduced communication delays between surgeons and assistants. Notably, docking time did not differ significantly (2.4 vs 2.2 min, P = 0.086), indicating comparable system setup efficiency. However, structured ergonomic or convenience scores (such as grabability or rotatability) were not formally collected during the study period. We recognize this as a limitation and suggest incorporating standardized surgeon-reported usability metrics in future prospective studies.
Notably, sentinel lymph node mapping, though recognized as a standard approach for early-stage endometrial carcinoma, was not routinely implemented during our study period due to limited equipment availability and lack of adequate training during the initial KD-RAS rollout. The higher lymph node dissection rate observed in the DV-RAS group (89.6% vs 76.0%, P = 0.013) likely reflects cautious case selection during early KD-RAS implementation rather than technical superiority. Critical evidence supports this interpretation: equivalent nodal yield was achieved when dissection was performed (10.6 vs 10.7 nodes, P = 0.948), there was no significant difference in metastasis rates (1.1% vs 6.5%, P = 0.053), and the two groups had similar risk profiles in terms of tumor size, LVSI, and histologic grade (all P > 0.05). During the early phase of KD-RAS adoption, surgeons preferentially selected low-risk cases (eg, grade 1 tumors <2 cm), where lymphadenectomy could be omitted per Chinese guidelines.21 This pattern contrasts with claims that technical precision alone drives dissection rates—enhanced instrumentation should theoretically increase nodal harvest quantity, not just dissection frequency.22
The marginally prolonged time to first flatus (3.0 vs 2.9 hr, P < 0.001) and hospital stay (5.0 vs 4.4 days, P = 0.001) in the KD-RAS group were primarily due to institutional protocols rather than clinical necessity. Specifically, Chinese guidelines recommend 72-hour postoperative observation for new surgical technologies,23 whereas Western centers often prioritize same-day discharge under ERAS protocols.24 In addition, persistent cultural preferences for inpatient recovery in China further contributed to extended stays, despite evidence supporting outpatient robotic procedures.25 Importantly, complication rates remained comparable between the two groups (2.4% vs 3.5% Clavien-Dindo grade II), confirming safety equivalence.
From a cost-effectiveness perspective, the KD-RAS group demonstrated a 24% reduction in total hospitalization expenses (4.1 vs 4.7 million CNY, P < 0.001), which can be attributed to several strategic advantages. The use of reusable instruments allowed for 20 sterilization cycles compared to only 10 for DV-RAS, reducing per-case costs by 38%.26 Local manufacturing eliminated 15% import tariffs and 30% maintenance fees, while simplified domestic supply chains enabled faster consumable replenishment (2 vs 14 days for imported systems).27 These savings persisted even when excluding hospitalization costs, with surgical expenses alone being significantly lower in the KD-RAS group (1.6 vs 2.1 million CNY, P < 0.001).
The KD-RAS system offers several distinctive advantages. Its open multi-screen console design enhances the comfort of the surgeon, reduces intraoperative fatigue, and facilitates real-time surgical observation and teaching. Additionally, it also has remote surgical capabilities to overcome geographical barriers, thereby improving accessibility to advanced surgical treatments, as demonstrated by successful case implementations. While this study has demonstrated the efficacy and safety profile of the KD-RAS system through comparisons of the preoperative, intraoperative, and postoperative conditions, it still has some limitations. The single-center design and relatively small sample size may affect the generalizability of the findings. Furthermore, the lack of long - term follow - up data prevents evaluating sustained clinical outcomes.
Conclusion
This study establishes KD-RAS as a viable, cost-effective alternative to DV-RAS in endometrial cancer surgery. Technical disparities in operative time diminish with experience, while inherent economic advantages persist. By contextualizing findings within regional healthcare norms, we provide a roadmap for global adoption of domestic robotic platforms.
Data Sharing Statement
The data which was used and/or analyzed during this study is available from the corresponding author on reasonable request.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Harbin Medical University Cancer Hospital (Approval No. KY2021-46). Written informed consent was obtained from all participants.
Funding
This work was supported by the grants from Beijing Cancer Prevention & Treatment Society (No. IZXUEYANZI 2019-1003), and the grants from Beijing Li Ze Chanrity Foundation (No. KY0005), and National Cancer Center Climbing Fund (No. NCC201908B07).
Disclosure
Tianbo Liu and Li Ma are co-first authors for this study. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–1428. doi:10.1016/S0140-6736(22)00323-3
2. Galant N, Krawczyk P, Monist M, et al. Molecular classification of endometrial cancer and its impact on therapy selection. Int J Mol Sci. 2024;25(11):5893. doi:10.3390/ijms25115893
3. Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. doi:10.1016/S0020-7292(06)60031-3
4. Chung S, Shen J, Kohn N, et al. Characteristics of early-stage endometrial cancer and factors that influence disease recurrence. J Clin Oncol. 2021;39(15_suppl):e17567–e17567. doi:10.1200/jco.2021.39.15_suppl.e17567
5. Metindir J, Dilek GB. The role of omentectomy during the surgical staging in patients with clinical stage I endometrioid adenocarcinoma. J Cancer Res Clin Oncol. 2008;134(10):1067–1070. doi:10.1007/s00432-008-0389-z
6. Stewart KI, Fader AN. New developments in minimally invasive gynecologic oncology surgery. Clin Obstet Gynecol. 2017;60(2):330–348. doi:10.1097/GRF.0000000000000286
7. Reyes DA, Tang B, Cuschieri A. Minimal access surgery (MAS)-related surgeon morbidity syndromes. Surg Endosc. 2006;20(1):1–13. doi:10.1007/s00464-005-0315-2
8. Zanagnolo V, Garbi A, Achilarre MT, Minig L. Robot-assisted surgery in gynecologic cancers. J Minim Invasive Gynecol. 2017;24(3):379–396. doi:10.1016/j.jmig.2017.01.006
9. Wen Z, Yang YX, Yu S, et al. KangDuo surgical robot versus da Vinci robotic system in urologic surgery: a systematic review and meta-analysis. J Robot Surg. 2024;19(1):6. doi:10.1007/s11701-024-02165-5
10. Xiong S, Fan S, Chen S, et al. Robotic urologic surgery using the KangDuo-Surgical Robot-01 system: a single-center prospective analysis. Chin Med J. 2023;136(24):2960–2966. doi:10.1097/CM9.0000000000002920
11. Fan S, Zhang Z, Wang J, et al. Robot-assisted radical prostatectomy using the KangDuo Surgical Robot-01 system: a prospective, single-center, single-arm clinical study [published correction appears in J Urol. 2022;208(3):744. doi: 10.1097/JU.0000000000002838]. J Urol. 2022;208(1):119–127. doi:10.1097/JU.0000000000002498
12. Fan S, Hao H, Chen S, et al. Robot-assisted laparoscopic radical prostatectomy using the KangDuo Surgical Robot System vs the da Vinci Si Robotic System. J Endourol. 2023;37(5):568–574. doi:10.1089/end.2022.0739
13. Li X, Xu W, Fan S, et al. Robot-assisted partial nephrectomy with the newly developed KangDuo Surgical Robot Versus the da Vinci Si Surgical System: a double-center prospective randomized controlled noninferiority trial. Eur Urol Focus. 2023;9(1):133–140. doi:10.1016/j.euf.2022.07.008
14. Zhang Z, Li Z, Xu W, et al. Robot-assisted radical nephroureterectomy using the KangDuo Surgical Robot-01 System versus the da Vinci System: a multicenter prospective randomized controlled trial. Int Braz J Urol. 2024;50(6):727–736. doi:10.1590/S1677-5538.IBJU.2024.0230
15. Berek JS, Matias-Guiu X, Creutzberg C, et al. FIGO staging of endometrial cancer: 2023 [published correction appears in Int J Gynaecol Obstet. 2024;166(3):1374. doi: 10.1002/ijgo.15193]. Int J Gynaecol Obstet. 2023;162(2):383–394. doi:10.1002/ijgo.14923
16. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
17. Feng Q, Yuan W, Li T, et al. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7(11):991–1004. doi:10.1016/S2468-1253(22)00248-5
18. Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19(8):1051–1060. doi:10.1016/S1470-2045(18)30357-7
19. Liu Y, Wang Y, Wang C, et al. Comparison of short-term outcomes of robotic-assisted radical colon cancer surgery using the Kangduo Surgical Robotic System and the Da Vinci Si Robotic System: a prospective cohort study. Int J Surg. 2024;110(3):1511–1518. doi:10.1097/JS9.0000000000000976
20. Liu S, Zhang S, Li Z, et al. Insufficient post-operative energy intake is associated with failure of enhanced recovery programs after laparoscopic colorectal cancer surgery: a prospective cohort study. Front Nutr. 2021;8:768067. doi:10.3389/fnut.2021.768067
21. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–791. doi:10.1097/CM9.0000000000001474
22. Rogers T, Moschovas MC, Saikali S, et al. Triple-console robotic telesurgery: first impressions and future impact. J Robot Surg. 2024;18(1):381. doi:10.1007/s11701-024-02141-z
23. Togami S, Furuzono N, Fukuda M, Mizuno M, Yanazume S, Kobayashi H. Comparative analysis of surgical outcomes between the hinotori™ surgical robot system and da Vinci® Xi for simple hysterectomy with sentinel lymph node biopsy in low-risk endometrial cancer. Jpn J Clin Oncol. 2025;55(4):349–354. PMID: 39657986. doi:10.1093/jjco/hyae170
24. Togami S, Furuzono N, Kobayashi Y, et al. Comparative analysis of da Vinci® Xi and hinotori™ SRS robot-assisted surgery systems for gynecologic disorders: a retrospective study. Medicina. 2024;60(12):2014. PMID: 39768894; PMCID: PMC11727689. doi:10.3390/medicina60122014
25. Matsuura M, Nagao S, Kurokawa S, Tamate M, Akimoto T, Saito T. Early outcomes of three new robotic surgical systems in patients undergoing hysterectomy. Updates Surg. 2024;76(5):2051–2057. PMID: 38787495. doi:10.1007/s13304-024-01891-7
26. Kohjimoto Y, Yamashita S, Iwagami S, Muraoka S, Wakamiya T, Hara I. hinotoriTM vs. da Vinci®: propensity score-matched analysis of surgical outcomes of robot-assisted radical prostatectomy. J Robot Surg. 2024;18(1):130. PMID: 38498237. doi:10.1007/s11701-024-01877-y
27. Giannini A, Malacarne E, Sergiampietri C, et al. Comparison of perioperative outcomes and technical features using da Vinci Si and Xi robotic platforms for early stages of endometrial cancer. J Robot Surg. 2021;15(2):195–201. PMID: 32447594. doi:10.1007/s11701-020-01091-6
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Efficacy and Safety of Anlotinib in Extensive-Stage Small Cell Lung Cancer: A Multicenter Real-World Study
Zheng HR, Jiang AM, Gao H, Liu N, Zheng XQ, Fu X, Zhang R, Ruan ZP, Tian T, Liang X, Yao Y
Cancer Management and Research 2022, 14:2273-2287
Published Date: 2 August 2022
Relative Safety and Efficacy of Two Doses of Tandospirone Citrate for Generalized Anxiety Disorder: A Multicenter Randomized Controlled Trial
Li Q, Zhang H, Lin G, Shi S, Zhang Y, Ji J, Yang L, Yao J, Wu W
Neuropsychiatric Disease and Treatment 2022, 18:1653-1664
Published Date: 8 August 2022
A Phase IV Study on Safety, Tolerability and Efficacy of Dolutegravir, Lamivudine, and Tenofovir Disoproxil Fumarate in Treatment Naïve Adult Indian Patients Living with HIV-1
Dravid A, Morkar D, Prasad D, Ramapuram JT, Patel KV, Naik KS, Bhrusundi M, Kulkarni M, Hegde S, Anuradha S, Nageswaramma S, Madan S, Jayaprakash T, Kulkarni V
Pragmatic and Observational Research 2022, 13:75-84
Published Date: 10 August 2022
Biosimilars: Science, Implications, and Potential Outlooks in the Middle East and Africa
Batran RA, Elmoshneb M, Hussein AS, Hussien OM, Adel F, Elgarhy R, Morsi MI
Biologics: Targets and Therapy 2022, 16:161-171
Published Date: 6 October 2022
Clinical Evaluation of Risankizumab in the Treatment of Adults with Moderately to Severely Active Crohn’s Disease: Patient Selection and Reported Outcomes
Horst S, Cross RK
Drug Design, Development and Therapy 2023, 17:273-282
Published Date: 31 January 2023


