Back to Journals » Journal of Inflammation Research » Volume 17
Role and Mechanism of Mechanical Load in the Homeostasis of the Subchondral Bone in Knee Osteoarthritis: A Comprehensive Review
Authors Chen L, Zhang Z, Liu X
Received 3 September 2024
Accepted for publication 15 November 2024
Published 21 November 2024 Volume 2024:17 Pages 9359—9378
DOI https://doi.org/10.2147/JIR.S492415
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Lin Chen,1,* Zhan Zhang,2,* Xueyong Liu1
1Department of Rehabilitation, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China; 2Department of Orthopedics, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xueyong Liu, Department of Rehabilitation, Shengjing Hospital of China Medical University, No. 16, Puhe Street, Shenyang, Liaoning, People’s Republic of China, Tel +86 18940112266, Email [email protected]
Abstract: Osteoarthritis (OA) is one of the most prevalent degenerative joint diseases, and the knee joint is particularly susceptible to it. It typically affects the entire joint and is marked by the erosion of cartilage integrity, chondrocytopenia, subchondral bone sclerosis and the mild synovial inflammation. Pathological changes in the subchondral bone often serve as initiating factors for joint degeneration. Various predisposing factors, including metabolic disorders, oxidative stress, and abnormal mechanical loading, regulate OA pathogenesis. Of them, mechanical loading is closely associated with the maintenance of the subchondral bone. Disrupted mechanical loading, leading to subchondral bone remodeling, can potentially trigger OA, whereas appropriate loading might ameliorate its progression. Therefore, this narrative review aimed to discuss existing knowledge and explore how mechanical loading mediates changes in the subchondral bone, influencing the development of knee osteoarthritis. Special emphasis is placed on its role and underlying mechanisms in maintaining joint homeostasis.
Keywords: mechanical load, bone remodeling, homeostasis, osteogenic differentiation
Introduction
Osteoarthritis (OA) is the most prevalent degenerative joint disease, affecting at least 300 million people worldwide. The leading cause of disability in the elderly is OA, responsible for global hip and knee replacement surgeries, and imposes a substantial economic burden on society and families. Of these joint diseases, knee osteoarthritis (KOA) has a high incidence.1 The loss of joint cartilage is a prominent feature of OA progression; however, it is now widely recognized that all joint structures are affected. Articular cartilage and subchondral bone interact with the osteochondral units in terms of biomechanical function. Compared with uninjured joints, more pronounced subchondral bone alterations are observed in joints with meniscal tears or ligament injuries.2 The widespread use of imaging techniques has also shown a close correlation between pathological changes in the subchondral bone and cartilage damage, which is an initiating factor in the degenerative changes of joints.3 Therefore, targeting the subchondral bone for treatment is important. Importantly, these measures need to be implemented before substantial structural and functional changes occur in the joint tissues.
Currently, the main treatment methods for OA include medication, surgery, and lifestyle intervention, however, these treatment methods have limitations. Although medication can relieve symptoms, patient compliance is low and there is a risk of drug dependence; surgical treatment is limited by the lifespan of the implant and is not suitable for all patients, with joint replacement often being the final destination. A study published in 20214 revealed that total knee replacements and total hip replacements affect patients in a similar way in the early postoperative period. In view of this, exploring an efficient and safe conservative treatment modality is essential for the management of KOA. In recent years, important advances have been made in the field of conservative treatment by Eleuterio A Sánchez Romero et al. In 2020,5 they published a study examining the effect of adding dry needling (DN) therapy to an exercise program on pain intensity and disability in patients with KOA, and although the results did not show that DN significantly reduced pain or improved disability in patients, they provided a good basis for conservative treatment in KOA management provides a new perspective to think about. Subsequently, in 2021,6 the team further investigated the efficacy of manipulative therapy (MT) in patients with KOA-related pain and found that MT may be a safe and effective treatment that significantly improves pain and shortens pain duration, a finding that further enriches the theory and practice of conservative treatment in the field of KOA.
In addition, the patient education (PE) strategy proposed by Pierluigi Sinatti in 20207 sheds new light on the treatment of KOA. The finding that PE has a positive effect on reducing OA-related pain and improving function in the hip and knee and positively affects the combination of conservative treatments with it has deepened our understanding of the role of conservative treatments in KOA from a new perspective.
Mechanical loading can be described as the physical forces acting on the human body or its movements, ranging from organs, tissues, and systems to the molecular and cellular levels, which are necessary for bodily function.8 The common forms of mechanical loading include tension, compression, fluid shear stress, and vibration. Excessive obesity or abnormal lower limb alignment leads to overloading of the internal load in the knee joint, which is one of the primary risk factors for KOA progression.9 As early as 2014, the use of magnetic resonance imaging to determine the response of knee joint cartilage to mechanical loading using T1rho and T2 relaxation times in patients with KOA was explored.10 However, hypertension has recently emerged as an independent risk factor for OA and is the most common vascular disease. This suggests that systemic hemodynamic mechanical stress plays a role in subchondral bone remodeling and OA pathogenesis.11 Additionally, a study conducted in 2023 indicated a vascular mechanical basis for OA.12 Tissues within the subchondral bone, including the trabeculae, cortical plates, and periosteal bone, undergo remodeling and adaptation in response to mechanical loading through changes in different bone cell types, alterations in the extracellular matrix, and interactions with bone marrow cells, blood vessels, and nerves. Therefore, appropriate modification and manipulation of the mechanical environment of the knee joint can prevent injuries, correct abnormalities, and accelerate healing and recovery. This review provides a comprehensive overview of the current knowledge regarding the influence of mechanical loading on the pathogenesis of KOA. We analyzed the potential underlying targets of mechanical loading on the subchondral bone and discussed the advantages and disadvantages of the mechanical interventions currently applied in clinical practice or research. This study aimed to provide new insights into preventing and treating KOA.
Methods
Perform a systematic bibliographic search of humans, animals, and cells using the PubMed search engine with no time limit using combinations of the following search terms: osteoarthritis, knee osteoarthritis, subchondral bone homeostasis, bone remodeling, bone resorption, bone sclerosis, bone marrow mesenchymal stem cells, bone cells, osteoblasts, osteoclasts, osteoclasts, neurovascular units, mechanical stress, mechanical loading, signaling pathways, therapeutics, clinical applications. The inclusion criteria focused on the exploration of the physiological and pathological state of subchondral bone in OA, specifically addressing the effects of mechanical stress and mechanical loading on subchondral bone homeostasis and how these external factors act on osteoblasts, osteoclasts, and other cells within the subchondral bone, as well as exploring the changes in the signaling pathways involved in the process of these cells in response to mechanical stress. In addition, the inclusion criteria covered clinical or basic studies on the effects of treatments related to mechanical loading on KOA and their potential mechanisms of action. The exclusion criteria, in turn, explicitly excluded the following categories of articles: any article that did not cover the content described in the above inclusion criteria; any article that contained duplicated data or information; and all literature published in languages other than English. A total of 2159 relevant articles were identified. According to the inclusion and exclusion criteria, a total of 1294 articles were deleted, and a total of 823 articles in English were retrieved after the initial screening, excluding those that were not highly relevant to this study and those that were outdated, and screened according to the reading titles and abstracts, and finally 111 articles were included in the review according to the criteria. PRISMA flow chart was available in Supplementary Figure 1.
Results
Characteristics of Subchondral Bone Homeostasis Imbalance in KOA
The articular cartilage, subchondral bone, and calcified cartilage form a bone-cartilage bio-composite material within the knee joint that uniquely adapts to transfer loads during weightbearing and joint movement. The differing capacities of cartilage and bone to adapt to local mechanical and injurious impacts may play a pivotal role in the natural history of OA joint pathology and have implications for therapeutic interventions. Subchondral bone transitions between soft and hard tissues. Products transferred from the subchondral bone provide essential nourishment to deep joint cartilage and offer mechanical support, serving as a cushion during shock absorption and somewhat preventing and repairing cartilage damage owing to excessive stress.13 At the cellular level, researchers have summarized the relationship between osteoclasts (OC) in subchondral bone and chondrocytes (CC) in articular cartilage, proposing the concept of “OC–CC” crosstalk. This concept suggests that alterations in the microstructure and mechanical characteristics of the subchondral bone may directly or indirectly affect cartilage metabolism and the pathogenesis and progression of OA.14 This imbalance in the homeostasis of the subchondral bone not only exacerbates the KOA, but may also further deteriorate the patient’s health by affecting systemic physiological mechanisms, such as intestinal flora balance.15 Of particular interest to us is OA, the most prevalent chronic form of temporomandibular joint (TMJ) disorders and the most severe type of temporomandibular disorders (TMD). The causes of TMD are complex and varied, and are generally recognized to be closely related to factors such as poor functioning habits, sleep bruxism, and sleep disorders. Insomnia and apnea are the most prevalent forms of sleep disorders in the TMD patient population. Some scholars have endeavored to explore the existing literature in an effort to discover whether scientific evidence exists to substantiate an association between patients with temporomandibular joint osteoarthritis (TMJ-OA) and increased sleep disturbances or decreased sleep quality.16 Unfortunately, however, the results of the study did not provide sufficient evidence to support the hypothesis that patients with TMJ-OA are more likely to suffer from sleep disorders or poor sleep quality. Nonetheless, this study opens up new perspectives to explore the pathomechanisms of OA and to develop more effective intervention strategies.
Changes in the subchondral bone are part of the coupled process between osteoclast-mediated old bone resorption and osteoblast-mediated new bone formation, whether at the cellular or histological level. An imbalance in metabolism between these two cell types alters the bone mass and causes microstructural changes.17 The characteristics of subchondral bone lesions differ in various stages of OA. In the early stages of OA, a significant increase is observed in osteoclast activity beneath the subchondral plate, which results in a notable increase in the porosity of the cortical plate and gradual thinning of the trabeculae. In later stages, osteoblast activity in the subchondral trabecular bone becomes more pronounced, leading to the thickening of the subchondral plate and trabeculae, accompanied by subchondral bone sclerosis. Notably, despite the increase in bone volume in the later stages, local bone turnover rates rise and calcium-collagen ratios decrease, resulting in inadequate bone tissue mineralization and reduced elastic modulus. As OA progresses, in addition to the formation of osteophytes and subchondral bone cysts at the joint edges, the subchondral cortical plate becomes flattened and deformed, a process referred to as “bone attrition”. Moreover, aberrant remodeling of the subchondral bone can lead to abnormal vascular neogenesis, with these vascular components accompanied by the invasion of sensory and sympathetic nerves into the subchondral bone and cartilage tissue. Meanwhile, the infiltration of peripheral inflammatory factors exacerbates this condition by disrupting the homeostasis of the cartilage and subchondral bone.18 (Figure 1).
Impact of Mechanical Load on the Onset and Progression of KOA
A previous study investigated the influence of mechanical loads during walking on changes in the subchondral bone plate and cartilage in rats with early-stage KOA. By comparing the location of subchondral bone plate lesions and mechanical loads, the research showed that biomechanical signals not only affect cartilage metabolism but also impact the remodeling of the subchondral bone. Furthermore, uncoupled remodeling of the subchondral bone is a key factor leading to an imbalanced mechanical force distribution within the knee joint, further damaging the subchondral bone and surface cartilage.19 Therefore, the capacity of the subchondral bone to adapt to local mechanical and injurious impacts may be significant in the natural history of OA joint pathology and may have implications for therapeutic interventions.
Individuals engaged in regular physical activity experience beneficial mechanical stress that contributes to strengthening their weight-bearing bones. However, pilots’ weight-bearing bones can lose 1–1.5% of their bone density per month because they spend extended periods in a microgravity environment, leading to increased bone resorption and reduced bone formation.20 Furthermore, prolonged bed rest and paralysis significantly reduce the mechanical stress applied to the bones, resulting in disuse osteoporosis. Overall, excessive, inadequate, or unstable mechanical loading can lead to changes in the subchondral bone, subsequently contributing to OA.21,22
In animal experiments, inducing abnormal mechanical loads on joints is a common method for establishing OA models. For instance, overloading the tibia can simulate anterior cruciate ligament injury,23 or subjecting mouse knee joints to repeated cyclic loading or single non-traumatic loads,24 and prolonged axial loading,25 can lead to morphological and cellular changes resembling those seen in bone-related arthritis. Notably, in rat and mouse models of traumatic bone-related arthritis, avoiding excessive knee joint load effectively ameliorates cartilage degradation and subchondral bone alterations offering robust theoretical support for non-weight-bearing rest in patients with OA.26–29 However, appropriate joint loading may not necessarily have a negative effect on disease progression. Several studies have demonstrated that mechanical interventions, such as walking and extracorporeal shockwave therapy (ESWT), can effectively improve OA symptoms and slow its progression. The intensity, duration, and frequency of these interventions require special attention.30–32 Therefore, mechanical loading is a double-edged sword, and its contribution to the onset and progression of OA should be taken into account. (Table 1).
 |
Table 1 Researches on Animal Models Pertaining to Mechanical Loading in Osteoarthritis |
Regulation of Factors Affecting Subchondral Bone Homeostasis by Mechanical Load
The intrinsic and continuous remodeling process of the bone is designed to regulate its microstructure and overall geometry in response to ever-changing mechanical environments. Various cells control the conversion of physical forces they experience into biochemical signals, thereby regulating bone adaptation and regeneration. This was confirmed by the presence of numerous mechanically sensitive structures within the cells. The mechanosensitive ion channel Piezo1 is essential for changes in gene expression induced by fluid flow shear stress (FFSS) in bone cells.36 Calcium ion channels have long been associated with the mechanical transduction of bone cells. With the expanding research, abnormal proliferation and differentiation of bone marrow mesenchymal stem cells, osteoblasts, and osteoclasts, and angiogenesis, are considered important factors contributing to the imbalance in subchondral bone homeostasis. Mechanical loading plays a critical regulatory role in these processes. (Table 2).
 |
Table 2 Cellular Responses to Various Mechanical Stresses in Osteoarthritis |
Regulatory Role of Mechanical Load on Bone Mesenchymal Stem Cells
Bone mesenchymal stem cells (BMSCs) are a cluster of cells with self-renewal capabilities that can differentiate into osteoblasts, adipocytes, chondrocytes, and myocytes in an appropriate microenvironment. Mechanical loading leads to changes in intra-bone marrow pressure and generates shear stress. BMSCs respond to mechanical stimulation by sensing subtle deformations of the extracellular matrix (ECM). They promote bone healing through cell-cell interactions and the secretion of growth factors, including bone morphogenetic protein (BMPs) and vascular endothelial growth factor (VEGF).44
Chen et al exposed human bone marrow-derived MSCs to mechanical cyclic stretching at amplitudes of 2.5, 5, and 10% for 10 h daily. After 3 days, appropriate stretching increased MSC proliferation and promoted osteogenic differentiation. Mechanical stretching significantly increases the transcription of osteoblast-specific markers.37 Another study found that application of low-magnitude vibration in the early stages of postmenopausal osteoporosis could inhibit its progression. Mechanistic investigations indicated that vibration activated the canonical wingless-like (Wnt)/β-catenin pathway in BMSCs through estrogen receptor α, promoting MSC osteogenic differentiation.38 Similarly, low-amplitude, high-frequency vibrations increase miRNA-335-5p expression in BMSCs through the same pathway to enhance osteogenic differentiation.35 However, excessive mechanical stress inducing BMSC osteogenic differentiation can lead to temporomandibular joint OA involving osteoclasts in the process.17 Additionally, mechanical loading has received significant attention for regulating the chondrogenic differentiation of BMSCs. Applying dynamic compressive loads with inherent frequency and intensity to 3D-cultured BMSC-collagen scaffold constructs revealed that compression facilitated BMSC adhesion, proliferation, uniform distribution, and ECM secretion. This process facilitated the chondrogenic differentiation of BMSCs, resulting in increased gene expression levels of COL2α1, AGG, and SOX9, thereby preventing lower levels of the hypertrophic marker COL10α1. This approach significantly enhanced the mechanical strength of the BMSC-collagen scaffold structure, thereby better mimicking the structure and function of natural cartilage.39
Stem cells are more sensitive to mechanical stimuli than somatic cells, and biomechanical signals play a crucial role in regulating the phenotypic differentiation of stem cells. MSCs possess immunomodulatory properties and can suppress inflammatory responses. Under mechanical loading, MSCs may influence arthritis development by modulating the production of inflammatory factors. Therefore, the significance of BMSCs in mechanical loading research on OA is reflected in their cellular biological responses and regulatory roles under mechanical loading conditions and their potential in joint tissue repair and protection.
Regulatory Role of Mechanical Load on Osteocytes
Bone cells exist within the mineralized bone matrix and comprise over 95% of the total cell count in bone tissue.45 They form numerous dendritic processes embedded in the bone matrix, constituting the lacunar-canalicular system. Fluid movement within this system serves as the primary means for bone cells to sense changes in their mechanical environments. Upon stimulation, the bone cells initiate repair by recruiting osteoblasts and osteoclasts. For subchondral bone, bone formation only increases under loading stimuli; however, excessive mechanical loads can lead to sclerosis. Sclerostin (SOST), which is secreted by bone cells, is a key protein in the mechanostress response that inhibits bone formation. Wild-type and SOST knockout mice with OA exhibit a more active bone-forming state than wild-type mice with increased bone formation and decreased bone resorption rates.46 The absence of SOST may exacerbate OA in mice by promoting subchondral bone sclerosis, indicating that SOST plays a stress-dependent protective role during the early stages of OA.
Focal adhesions, comprising focal adhesion kinase (FAK), vinculin, and integrins, provide a platform for transmitting mechanical signals from the extracellular matrix to the cell cytoskeleton, promoting cell adhesion, spreading, and migration. FAK is involved in sensing intracellular and extracellular mechanical forces. Research has found that FAK in bone cells mediates the phosphorylation of histone deacetylase 5 tyrosine, drives its nuclear translocation, and increases SOST expression. However, FFSS can induce the dephosphorylation of FAK, inhibiting SOST function and promoting bone formation.41 Another study demonstrated that FFSS elevates intracellular cyclic adenosine monophosphate levels and activates protein kinase A by acting on the EP2 and EP4 receptors on the cell surface, effectively preventing dexamethasone-induced apoptosis of osteocyte-like cells.40
However, osteocyte activity is relatively stable, and their involvement in bone remodeling occurs slowly. They serve as primary mechanical sensors within the subchondral bone and are crucial to overall skeletal health. Consequently, investigating the mechanistic role of osteocytes in subchondral bone remodeling in mechanical environments is important.
Regulatory Role of Mechanical Load on Osteoblasts and Osteoclasts
Osteoclasts are multinucleated cells present in the bone marrow of hematopoietic lineage progenitors. Mature osteoclasts attach to aging bone regions and achieve bone resorption by releasing hydrogen ions and catalytic enzymes to dissolve the bone. Osteoblasts primarily produce collagen substances (osteoids), which subsequently mineralize to form mature bone, counteracting bone resorption.47 Studies have demonstrated that the duration and magnitude of mechanical loading play a pivotal role in hematopoietic progenitor differentiation into osteoclasts, implying that osteoclasts may possess mechanosensing abilities. In co-culture systems, mesenchymal stem cells or bone cells exposed to oscillating fluid flow can inhibit osteoclast generation.42 Therefore, osteoclasts are not only mechanosensitive cells but also effectors of other mechanosensitive cells. Consequently, osteoclasts may regulate bone quality through direct mechanosensing or by receiving signals from osteoblastic lineage cells.
Receptor Activator of Nuclear Factor-κB Ligand (RANKL) is one of the most critical molecules in bone remodeling and has been identified as a key signaling molecule for the differentiation of bone macrophages into mature osteoclasts.43 Abnormal mechanical loading can stimulate osteoblasts to produce RANKL which then binds to the RANK receptor on the surface of osteoclasts, activating the NF-κB signaling pathway.48 Subsequently, the activated NF-κB transcription factor enhances the expression of several osteoclastogenic factors, including IL-1β, IL-6, and PGE2, leading to bone resorption. Activation of the RANK/RANKL pathway is regulated by osteoprotegerin, a protein produced by osteoblasts that binds to RANKL, thereby preventing bone resorption and promoting bone formation.49
Microgravity environments can simulate physiological conditions experienced by patients with OA on Earth, such as reduced bone density and decreased joint loading, offering a unique opportunity for OA research. Studies have found that microgravity environments can lead to the disassembly of the cellular cytoskeleton and changes in the arrangement and orientation of microfilaments and microtubules.50 During spaceflight, increased bone resorption can result in the loss of calcium and minerals from bones, altering the endocrine regulation of calcium metabolism. Microgravity can also induce the disruption of actin microfilaments in osteoblasts, reducing RUNX2 expression and migration and consequently inhibiting the expression of osteogenic-related genes.51 Additionally, in response to mechanical stimulation in osteoblasts, PIEZO1 promotes the expression of COL2α1 and COL9α2 through YAP1 expression, and in turn, these collagen isoforms control the differentiation of osteoclasts.34
Osteoblasts and osteoclasts are closely interconnected, and the RANK/RANKL/OPG signaling pathway is a key regulator of their balance. Understanding how mechanical loading affects their interactions and equilibrium can improve our understanding of OA pathogenesis and guide the development of new therapeutic approaches. Osteoclasts are highly active during the early stages of OA. Therefore, targeting osteoclasts using mechanical loading interventions may be a promising alternative strategy for treating OA.
Potential Impact of Mechanical Load on Subchondral Bone Angiogenesis
Angiogenesis plays a crucial role in bone remodeling during the treatment of bone repair disorders, including fractures and osteonecrosis. H-type vessels were identified as distinct subtypes of blood vessels that highly express CD31 and endomucin (EMCN).52 They can also induce angiogenesis and bone formation.
Recent studies have revealed that weight gain may influence the mechanosensory pathway by activating the mechanosensitive receptor, PIEZO1. This subsequently leads to an increase in the kinase FAM20C within osteoblasts, which is primarily involved in the secretion of phosphorylated proteins. FAM20C contributes to bone mineralization by promoting the accumulation of phosphorylated dentin matrix protein 1 (DMP1), a key factor in bone mineralization. However, DMP1 serves to inhibit the phosphorylation of vascular endothelial growth factor receptor 2, thereby suppressing the signaling of VEGF. Secreted DMP1 transforms H-type vessels into quiescent L-type vessels, limiting skeletal growth activity and enhancing skeletal mineralization. This suggests that mechanical forces tightly link bone matrix mineralization to the inhibition of vascular formation, thereby restricting bone growth in adolescents.53
Furthermore, moderate mechanical loads can accelerate angiogenesis through reciprocal interactions between osteoblasts, osteoclasts, and H-type vessels,54 playing a role in remodeling the subchondral bone and promoting bone healing through multiple pathways. In early-stage OA, activated osteoclasts lead to an increase in H-type vessels and oxygen concentration, thereby reducing the expression of hypoxia-inducible factor (HIF) 1α in chondrocytes, resulting in cartilage degradation.55 Under abnormal stress, MSCs exhibit enhanced angiogenic potential, probably because of the downregulation of exosomal miR-214-3p expression by mechanical stimulation, which in turn stimulates the formation of H-type vessels and the secretion of VEGF.56
Therefore, abnormal mechanical loads may be initial factors contributing to subchondral bone remodeling and H-type vessel formation during OA onset. This effect can be achieved through various pathways, including blood supply modulation, influence on bone metabolism, regulation of growth factor release, and transmission of mechanical signals. A thorough understanding of these mechanisms aids in unveiling the physiological regulatory processes within bone tissue and their potential roles in bone health and disease. However, further experimental research is needed to elucidate the upstream signaling pathways that regulate the formation of H-type vessels in the subchondral bone during OA.
Regulation of Mechanical Load Signaling Pathways and Their Effects on Factors Influencing Subchondral Bone Homeostasis Mechanisms
Recent research has revealed a close association between an imbalance in subchondral bone homeostasis and aberrant signaling pathway regulation. According to relevant experimental studies, multiple signaling pathways are involved in regulating subchondral bone remodeling under mechanical loads. Common signaling pathways include Wnt/β-catenin and TGF-β/BMPs, while other pathways, such as SDF-1/CXCR4, have been reported. These signaling pathways balance bone formation and resorption, inhibit the expression of inflammatory factors, restrain vascular generation, and ultimately protect the subchondral bone.
Wnt/β-Catenin
The Wnt glycoproteins are a family of over 19 secreted proteins. Owing to their lipid side chains, Wnt molecules are insoluble, and their reduced solubility significantly limits their capacity for physical signal transduction in various tissues. The Wnt pathway is divided into two categories based on its dependency on β-catenin: the canonical pathway dependent on β-catenin and the non-canonical pathway independent of β-catenin. This signaling cascade exhibits paracrine and autocrine effects, thus participating in various cellular activities.57
The Wnt/β-catenin signaling plays a pivotal role in skeletal development as an essential anabolic pathway.58 Processes including mesenchymal stem cell aggregation, chondrogenic differentiation, chondrocyte hypertrophy, growth plate chondrocyte and osteoblast differentiation, and maturation are mediated by this signaling pathway. Moderate activation of the canonical Wnt pathway is necessary to maintain healthy articular cartilage and subchondral bone. Mice with a heterozygous deletion of β-catenin in bone cells lose the ability to form new bone even under mechanical loading stimuli.59 Studies have shown that mechanical pressure can reverse the decreased expression of Wnt-related proteins following the onset of OA.60 Rapid activation of Wnt signaling increases the number of osteoblasts and enhances the expression of osteogenic differentiation-related genes, including ALP, OCN, RUNX2, and Osterix, while significantly reducing the expression of osteoclast markers, such as CTSK and RANKL.61 This leads to improved subchondral bone density reduction and the inhibition of abnormal bone remodeling. However, despite the rapid activation of β-catenin signaling in bone cells by mechanical loading, excessive loading can have negative effects.62 In addition to bone cells, mechanical loading can transmit signals to chondrocytes via the Wnt pathway. For instance, excessive mechanical stretching increases the levels of inflammatory cytokines in the conditioned media of osteoblasts, which induces subchondral bone loss and leads to catabolic metabolism and apoptosis of chondrocytes.63
In summary, the Wnt/β-catenin signaling significantly influences bone tissue growth, repair, adaptive changes, and metabolic balance. Mechanical loading can activate or inhibit this pathway, thereby regulating the behavior of bone cells and affecting overall skeletal health. Nonetheless, additional research is required to gain a deeper understanding of the regulatory mechanisms of the Wnt/β-catenin signaling in subchondral bone and its significance in bone health and disease. (Figure 2).
 |
Figure 2 Mechanosensory Regulation of the Classical Wnt/β-catenin Signaling Pathway’s Impact on Subchondral Bone Homeostasis Mechanism Diagram. |
TGF-β/Smad
The transforming growth factor-β (TGFβ) superfamily comprises BMPs, TGFβs, growth and differentiation factors (GDFs), and other factors.64 In the canonical pathway, TGFβ/BMP signaling relies on intracellular Smads, wherein transmembrane receptors are activated by specific ligand binding, leading to the direct phosphorylation of Smad2/3 proteins. This facilitates the translocation of signaling molecules into the cell nucleus, where they interact with other transcription factors and play a significant role in the development of OA.
Studies have shown that changes in the subchondral bone structure alter the distribution of mechanical stress on articular cartilage, with concentrated TGF-β activity in areas of high mechanical stress.65 Increased TGFβ1 activity in the subchondral bone of individuals with KOA is spatially associated with bone mass and the severity of disease damage.66 Carson-Burns et al conducted studies using mice with TGFβ type II receptor (TβRII) deletion in bone cells and assessed defects in cartilage degeneration, subchondral bone plate thickness, and subchondral bone plate sclerostin expression.67 They demonstrated that TGFβ signaling in bone cells is essential for the mechanical sensitivity response to injury, which regulates MSCs’ osteogenic and osteoclastic activity. This signaling pathway is positively correlated with late-stage subchondral bone remodeling in OA, and its inhibition can prevent pathological changes in the subchondral bone and improve bone quality.68 Another study revealed that aberrant activation of TGF-β in subchondral bone marrow increases osteoprogenitors and uncoupled bone resorption and formation in a rheumatoid arthritis animal model. Systemic or local inhibition of TGF-β activity in the subchondral bone can attenuate rheumatoid arthritis related cartilage degeneration.69
The role of TGFβ signaling in subchondral bone remodeling has been extensively studied, and the mechanisms involved are relatively understood. Therefore, in recent years, many researchers have explored how biological molecules inhibit aberrant subchondral bone remodeling through TGF-β signaling mediated by Smad2/3. Examples include 15-lipoxygenase-1(15-LOX-1)70 and nangibotide (LR12).71 Currently, various interventions are used for mechanical loading. Combining these biological molecules with mechanical forces may produce more significant protective effects on subchondral bone, potentially yielding more pronounced results. (Figure 3).
 |
Figure 3 Mechanosensory Regulation of TGF-β/Smad Signaling Pathway’s Impact on Subchondral Bone Homeostasis Mechanism Diagram. |
Yap/Taz
The Hippo signaling pathway is characterized by a cascade of protein kinases and transcription factors, that are highly sensitive to various external mechanical stimuli. Among these, YAP/TAZ serves as the core molecule in the Hippo signaling pathway. Upon activation of the Hippo pathway, YAP/TAZ undergo phosphorylation, leading to their sequestration in the cytoplasm and subsequent degradation via the proteasomal system. When the Hippo signaling pathway is inhibited, unphosphorylated YAP/TAZ enters the nucleus and binds to the TEAD family of transcription factors to regulate downstream gene expression, influencing skeletal development and homeostasis.72 Aberrant Hippo signaling can lead to skeletal defects and diseases, including osteogenesis imperfecta73 and disuse osteoporosis74.
However, mechanical cues can independently modulate the activity of YAP, circumventing the Hippo signaling pathway in addition to the canonical Hippo signaling pathway. For instance, vibration enhances the nuclear translocation of YAP, subsequently regulating downstream BMP2 expression to promote MSC osteogenic differentiation.75 Zarka et al76 developed 2D stretching and 3D compression cell culture models using MLO-Y4 osteocyte-like cell lines embedded in concentrated collagen hydrogels. This study showed that mechanical loading induces the nuclear translocation of YAP/TAZ, upregulates their target genes and proteins, and increases the expression of a subset of mechanically sensitive genes and chemokines in bone cells, including CXCL1, M-CSF, CXCL2, CXCL3, CXCL9, and CXCL10. Knockdown of YAP/TAZ weakened the increase in M-CSF and CXCL3 levels in bone cells after mechanical unloading. Moreover, RNA-seq analysis demonstrated that YAP/TAZ knockdown mediates the regulation of several genes, including those involved in dendritic cell formation in bone cells, indicating that YAP/TAZ signaling is essential for osteocyte-like cell mechanotransduction, governing gene expression profiles, and controlling chemokine expression. Mechanical stimulation is a crucial pathogenic factor in the ossification of the posterior longitudinal ligament (OPLL). After extracting primary cells from the posterior longitudinal ligament tissues of patients with and without OPLL and subjecting them to sinusoidal uniaxial cyclic stretching, the mechanical stretching load resulted in significant nuclear translocation of YAP after 3 days, along with increased expression of genes and proteins associated with OPLL-induced bone formation compared with the control group without significant changes.77
As early as 2013, researchers have proposed that mechanical forces serve as master regulators of YAP/TAZ in a multicellular environment.78 This process is interconnected with various signaling pathways, such as Hippo, WNT, and GPCR, and can directly mediate YAP nuclear translocation. Mechanical and Hippo signals represent two parallel inputs in regulating YAP/TAZ, which provide insights into the signal transduction of YAP/TAZ in response to mechanical loading. (Figure 4).
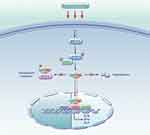 |
Figure 4 Mechanosensory Regulation of YAP/TAZ Signaling Pathway’s Impact on Subchondral Bone Homeostasis Mechanism Diagram. |
Sdf-1/Cxcr4
Matrix-derived factor (SDF)-1 belongs to the CXC chemokine subfamily (chemokine 2 [CXCL12]) and is involved in regulating the extracellular matrix, inflammatory factors, and subchondral bone homeostasis. CXCR4 is a receptor that is homologous to CXC. Previous studies have shown that CXCR4 promotes angiogenesis in vitro and in vivo. SDF-1 can activate CXCR4 and regulate various biological processes, such as cell proliferation, differentiation, chemotaxis, survival, and apoptosis. The SDF-1/CXCR4 axis plays a significant role in OA.79
SDF-1 levels were significantly elevated in rat knee joints after ACLT combined with DMM surgery was performed in human OA subchondral bone samples. Continuous administration of AMD3100 (an SDF-1 receptor inhibitor) to OA rats resulted in a substantial reduction in cartilage damage extent. Moreover, the number of osteoblasts showing positivity for osteoblastic markers such as Osterix in the subchondral bone significantly decreased.80 Yang et al81 found that in mechanically induced temporomandibular joint OA (TMJOA), several SDF-1-positive cells in the subchondral bone mainly originate from osteoblasts. When the SDF-1 signaling pathway was inhibited using AMD3100, Col II expression was upregulated in the cartilage, whereas the expression of CXCR4, SDF-1, and MMP13 was downregulated. These findings suggest that the increased expression of SDF-1 derived from osteoblasts not only promotes osteoblastic differentiation but may also contribute to cartilage degradation by engaging SDF-1 with CXCR4.
Furthermore, knee joint loading regulates the migration of adipose-derived stem cells to OA sites through the SDF-1/CXCR4 axis.82 The SDF-1/CXCR4 axis can recruit BMSCs and human umbilical vein endothelial cells by upregulating the phosphorylation of extracellular signal-regulated kinase (ERK) and enhancing osteogenic differentiation and vascular formation. After treatment with inhibitors of this pathway, the number of TRAP-stained positive osteoclasts in the subchondral bone of the ACLT group returned to normal, and the CD31+/EMCN+ blood vessels returned to normal levels, showing reduced cartilage destruction and bone resorption. Another study reported that low-magnitude high-frequency vibration promotes the healing of osteoporotic fractures by enhancing SDF-1/CXCR4 signaling in MSCs, which enhances their proliferation and migration.83
Therefore, inhibiting the SDF-1/CXCR4 axis can alleviate abnormal bone formation and angiogenesis, making it a potential therapeutic target for improving the subchondral bone microenvironment in OA.
RANTES-CCR-Akt2
Research has shown that in vivo biomechanical loading is a decisive factor in the transition from systemic autoimmunity to joint inflammation.84 T cell activation-regulated secretion of the chemokine RANTES (regulated upon activation, normal T cell expressed and secreted) is a chemotactic factor that recruits immune cells by binding to chemokine receptors (CCRs).85 RANTES is highly expressed in the tissues, synovial fluid, and peripheral blood of patients with rheumatoid arthritis and OA. The Akt pathway mediates macrophage recruitment and osteoclast regulation.86 Based on this, Feng et al87 proposed the hypothesis of the “RANTES-CCRs-Akt2 axis mediating TMJOA subchondral bone loss.” Their findings showed that RANTES is a potential biomarker of early TMJOA in humans. An increase in the number of macrophages beneath the articular cartilage and increased RANTES expression in the subchondral bone were associated with early TMJOA. The RANTES-CCRs-Akt2 axis is activated due to increased joint loading, promoting osteoclast formation and resulting in bone loss. (Figure 5).
 |
Figure 5 Mechanosensory Regulation of SDF-1/CXCR4 and RANTES-CCR-Akt2 Signaling Pathway’s Impact on Subchondral Bone Homeostasis Mechanism Diagram. |
Some signaling pathways have been specifically discussed in temporomandibular joint arthritis; however, the knee and temporomandibular joints are mobile joints, and a shared mechanism of pathogenesis occurs between them. The roles of the aforementioned signaling pathways are interconnected, and specific effects may be influenced by factors, including different cell types, types of mechanical stimulation, stimulus intensity, and duration. A comprehensive understanding of the impact of mechanical loading on subchondral bone requires further research.
Therapeutic Research on Improving KOA Through Mechanical Loading
Organs of the musculoskeletal system endure continuous mechanical loads, including bone compression, muscle stretching, and fluid shear stress on the blood vessels. Different forms of mechanical forces exhibit varying magnitudes, patterns, and durations and play a crucial role in cell growth and differentiation.
Exercise Therapy
Exercise plays a positive role in mitigating age-related disturbances and repairing osteoporosis and OA tissues. It can improve osteochondral crosstalk and enhance the subchondral bone microenvironment by regulating it.88 Human studies have shown that physical activity can enhance the efficacy of bone and cartilage regeneration therapies and promote skeletal health.89 One study indicated that participants who engaged in walking exercises had a reduced likelihood of experiencing frequent new knee joint pain compared with patients with KOA who did not engage in walking exercises, and the progression of medial joint space narrowing was less common.90 In another study, running was considered a form of mechanical load, and micro-computed tomography analysis revealed that running mice exhibited enhanced subchondral bone formation, increased thickness of the subchondral bone plate, and enlarged trabecular volume, thereby strengthening subchondral bone remodeling.91 However, excessive exercise-induced load overload may lead to diffuse microdamage and microcracks in the subchondral bone.92 This early microdamage contributes to OA occurrence and progression. Therefore, this issue should be addressed. Despite studies having shown generally positive effects of exercise on the cartilage, its impact depends on the type and intensity of the activity.
Overall, exercise therapy helps maintain joint flexibility and range of motion, enhances blood circulation around the joints, aids nutrient delivery, strengthens muscles, and alleviates joint burden. However, severely damaged joints can cause pain and further damage. Some patients may find it challenging to engage in effective exercise because of pain or the severity of their condition. Thus, personalized exercise plans are necessary for rehabilitation treatments because different individuals may require different types and intensities of exercise.
ESWT
ESWT is a physical therapy approach widely used for musculoskeletal disorders in recent years. Many studies have suggested that ESWT can convert mechanical force into biological signaling, with possible mechanisms, including pain relief,93 nerve terminal blockade, inflammation reduction, vasodilation, regeneration, and osteogenesis promotion. This leads to the alleviation of local symptoms, promotion of tissue repair, and functional improvement. A systematic review of randomized clinical trials indicated that ESWT effectively improved pain and function in patients with KOA.94 Another study evaluated the effectiveness and safety of ESWT in patients with KOA.95 Recent research on the effect of ESWT on KOA suggests that the subchondral bone is the main target, contributing to its density improvement and yielding superior results, compared with treatments targeting only the cartilage region.96
However, the molecular mechanisms underlying ESWT effect on KOA involving the subchondral bone remain unclear. However, the optimal treatment intensity, frequency, and timing require further investigation. This intervention offers a new treatment option for patients with limited mobility.
Low-Intensity Pulsed Ultrasound (LIPUS)
LIPUS is a mechanical wave that generates minor mechanical stress and effectively promotes the healing of bone fractures. Approved by the United States Food and Drug Administration, LIPUS has been widely used to treat bone diseases, such as traumatic fractures and OA.97 Research has indicated that LIPUS stimulation decreases the expression of bone resorption markers, including tissue inhibitor of metalloproteinase K, matrix metalloproteinase 9, and tartrate-resistant acid phosphatase (TRAP), demonstrating that LIPUS treatment can stimulate bone regeneration and reduce bone resorption.98 Yi et al’s study found that LIPUS significantly inhibits the activation of the TGF-β1/Smad3 signaling pathway in the subchondral bone of TMJOA rabbits, leading to reduced IL-6 production. This reveals some of its anti-inflammatory mechanisms and offers a potential therapeutic strategy for inflammatory diseases, such as OA.99
A growing body of research indicates that LIPUS has significant effects on early OA treatment. It reduces pain, facilitates functional recovery, promotes cartilage repair, enhances the morphology and histological characteristics of the subchondral bone, and boosts subchondral bone regeneration in patients with OA. LIPUS represents an advanced technology that blends physics with medicine, which is becoming a new option between conservative treatment and open surgery and a potential hotspot for future research.
Whole-Body Vibration (WBV)
WBV comprises synchronous mechanical vibrations generated by a vibrating platform that propagate along the human body. WBV training is a novel exercise therapy aimed at optimizing joint function, primarily by improving muscle coordination. A systematic review and meta-analysis conducted in 2022 revealed that combining WBV with exercise outperforms exercise alone in enhancing pain relief, physical function, and knee extensor strength.100 Both high and low-frequency WBV have demonstrated positive effects on pain intensity, physical function, and knee extensor strength. WBV can be incorporated into conservative treatment plans for patients with knee OA; however, further larger-scale studies are needed to validate the optimal approach for implementing WBV.
Ye et al investigated the efficacy of whole-body vibration therapy on KOA cartilage and subchondral bone in an experimental study. Their experiments, which included micro-CT and histology, demonstrated that WBV could increase the bone volume fraction, trabecular thickness, and trabecular number, while decreasing the bone surface area to bone volume ratio. Furthermore, WBV promoted the expression of aggrecan and inhibited the expression of MMP13, ADAMTS4, and IL-1β. Based on these findings, it was concluded that WBV could slow cartilage degeneration and preserve the subchondral bone microstructure.101 In the same year, Wang et al demonstrated in their experiment that WBV could alleviate the degradation of early-stage OA in rat knee joints, with lower frequencies showing better effects.102 However, another study found that continuous low-frequency and low-amplitude vibration increased the production of TNF-α in the mouse knee joint synovial fluid, suggesting a novel TNF-α-regulated mechanism for WBV-induced cartilage degeneration.103 Therefore, based on the current clinical and animal experimental results, the impact of whole-body vibration with different amplitudes and frequencies on knee OA remains controversial, and the potential targets and mechanisms require further investigation.
In summary, WBV is a relatively straightforward treatment that can be performed at home. However, it may result in dizziness and nausea in some patients, and treatment effects vary. Therefore, selecting an appropriate vibration frequency and intensity based on the patient’s health conditions and capabilities is required.
In conclusion, each patient’s situation is unique, and an appropriate treatment approach should be selected based on medical advice, the patient’s condition, and their health status. Different treatment methods can be used to improve therapeutic outcomes.
Discussion
Limitations
This review only summarizes and analyzes the regulation of mechanical stress on the factors affecting subchondral bone homeostasis in English, and there is a lack of research data in other languages and a lack of relevant experimental data. At present, there is a lack of clinical studies on the direct regulation of subchondral bone mechanical stress, and its mechanism of action on subchondral bone homeostasis is not completely clear, which still needs to be explored more deeply in the future.
Future Directions and Clinical Implications
In order to more accurately assess and measure loads on joint surfaces and to delve deeper into the underlying mechanisms of KOA, there is a need to develop more accurate and reliable animal models and joint load measurement systems. This will help to mimic the pathogenesis of KOA in humans and provide more accurate data to support research on the effects of mechanical loading on KOA. Since current studies have mainly focused on articular cartilage and relatively little attention has been paid to the subchondral bone, future studies should strengthen the investigation of the role of the subchondral bone in the formation and development of KOA and its relationship with mechanical loading. Through in-depth study of the biological properties of the subchondral bone, its metabolic mechanism and its interaction with articular cartilage, a new mechanism for the pathogenesis of KOA can be revealed, providing new targets for treatment. More reliable evidence is needed to support clinical application when using mechanical interventions for the treatment of KOA. Future studies should further optimize parameters such as intensity, dose, and time of mechanical intervention, and explore the efficacy and safety of different intervention strategies for KOA patients. This will help to develop a more personalized and precise treatment plan to improve the outcome of KOA.
An in-depth understanding of the role of the osteochondral unit in the formation and development of OA and the effect of mechanical loading on KOA is of great clinical significance. First, it helps us to better understand the pathogenesis of KOA and provide new ideas and methods for prevention and treatment. Second, by optimizing the mechanical intervention treatment strategy, we can provide more effective and safer treatment options for KOA patients, reduce their pain and dysfunction, and improve their quality of life. Finally, these research results can also provide a scientific basis for the development of related medical devices and drugs, and promote the progress and development of the KOA treatment field.
Conclusions
The osteochondral unit, comprising cartilage and subchondral bone, is pivotal in OA formation and progression. Moderate mechanical loading positively influences the metabolism of both components. However, studies directly evaluating joint surface loads and elucidating KOA mechanisms are limited due to the absence of suitable animal models and load measurement systems. Current research focuses mostly on articular cartilage, neglecting the subchondral bone due to sampling challenges. Reliable evidence supporting the clinical application of mechanical interventions for KOA, including intensity, dosage, and timing, is lacking. In conclusion, mechanical loading plays a key role in subchondral bone changes and will be a future research focus for KOA prevention and treatment, potentially offering new alternatives for mobility-limited OA patients.
Acknowledgments
We want to acknowledge Shengjing Hospital and all of the participants who contributed to this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Basic Research Program of Liaoning Province (No.2022JH2/101300030) and Shenyang Science and Technology Plan, Public Health Research and Development Special Project (Medicine-Industrial Collaborative Innovation Research Project) (No.21-172-9-07).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sharma L. Osteoarthritis of the Knee. N Engl J Med. 2021;384(1):51–59. doi:10.1056/NEJMcp1903768
2. Holzer LA, Kraiger M, Talakic E, et al. Microstructural analysis of subchondral bone in knee osteoarthritis. Osteoporos Int. 2020;31(10):2037–2045. doi:10.1007/s00198-020-05461-6
3. Xing W, Cai M, Li C, Nie Z, Tang W. Computed tomography image analysis of neuromuscular electrical stimulation in the treatment of knee osteoarthritis with different radiologic characteristics based on iterative reconstruction algorithm. World Neurosurg. 2021;149:372–379. doi:10.1016/j.wneu.2020.10.032
4. Sánchez-Romero EA, Battaglino A, Campanella W, Turroni S, Bishop MD, Villafañe JH. Impact on blood tests of lower limb joint replacement for the treatment of osteoarthritis. Top Geriatr Rehabil. 2021;37(4):227–229. doi:10.1097/TGR.0000000000000337
5. Sánchez Romero EA, Fernández-Carnero J, Calvo-Lobo C, Ochoa Sáez V, Burgos Caballero V, Pecos-Martín D. Is a combination of exercise and dry needling effective for knee OA? Pain Med Malden Mass. 2020;21(2):349–363. doi:10.1093/pm/pnz036
6. Sánchez-Romero EA, González-Zamorano Y, Arribas-Romano A, et al. Efficacy of manual therapy on facilitatory nociception and endogenous pain modulation in older adults with knee osteoarthritis: a case series. Appl Sci. 2021;11(4):1895. doi:10.3390/app11041895
7. Sinatti P, Sánchez Romero EA, Martínez-Pozas O, Villafañe JH. Effects of patient education on pain and function and its impact on conservative treatment in elderly patients with pain related to hip and knee osteoarthritis: a systematic review. Int J Environ Res Public Health. 2022;19(10):6194. doi:10.3390/ijerph19106194
8. Lu TW, Chang CF. Biomechanics of human movement and its clinical applications. Kaohsiung J Med Sci. 2012;28(2 Suppl):S13–25. doi:10.1016/j.kjms.2011.08.004
9. Han X, Cui J, Chu L, et al. Abnormal subchondral trabecular bone remodeling in knee osteoarthritis under the influence of knee alignment. Osteoarthritis Cartilage. 2022;30(1):100–109. doi:10.1016/j.joca.2021.10.005
10. Souza RB, Kumar D, Calixto N, et al. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(10):1367–1376. doi:10.1016/j.joca.2014.04.017
11. Ni R, Guo XE, Yan C, Wen C. Hemodynamic stress shapes subchondral bone in osteoarthritis: an emerging hypothesis. J Orthop Translat. 2021;32:85–90. doi:10.1016/j.jot.2021.11.007
12. Beverly M, Murray DW. Walking on water: subchondral vascular physiology explains how joints work and why they become osteoarthritic. EFORT Open Rev. 2023;8(6):436–442. doi:10.1530/EOR-23-0002
13. Fell NLA, Lawless BM, Cox SC, et al. The role of subchondral bone, and its histomorphology, on the dynamic viscoelasticity of cartilage, bone and osteochondral cores. Osteoarthritis Cartilage. 2019;27(3):535–543. doi:10.1016/j.joca.2018.12.006
14. Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. 2021;80(4):413–422. doi:10.1136/annrheumdis-2020-218089
15. Sánchez Romero EA, Meléndez Oliva E, Alonso Pérez JL, et al. Relationship between the Gut Microbiome and Osteoarthritis Pain: review of the Literature. Nutrients. 2021;13(3):716. doi:10.3390/nu13030716
16. Sánchez Romero EA, Martínez-Pozas O, García-González M, et al. Association between sleep disorders and sleep quality in patients with temporomandibular joint osteoarthritis: a systematic review. Biomedicines. 2022;10(9):2143. doi:10.3390/biomedicines10092143
17. Wang L, Tian Y, Liu M, et al. Excessive mechanical stress induced temporomandibular joint osteoarthritis via osteoclasts-mediated osteogenic differentiation of BMSCs. J Oral Rehabil. 2022;49(10):1020–1029. doi:10.1111/joor.13360
18. Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9(1):20. doi:10.1038/s41413-021-00147-z
19. Ziemian SN, Ayobami OO, Rooney AM, et al. Low bone mass resulting from impaired estrogen signaling in bone increases severity of load-induced osteoarthritis in female mice. Bone. 2021;152:116071. doi:10.1016/j.bone.2021.116071
20. Vico L, Hargens A. Skeletal changes during and after spaceflight. Nat Rev Rheumatol. 2018;14(4):229–245. doi:10.1038/nrrheum.2018.37
21. Zhu J, Zhu Y, Xiao W, Hu Y, Li Y. Instability and excessive mechanical loading mediate subchondral bone changes to induce osteoarthritis. Ann Transl Med. 2020;8(6):350. doi:10.21037/atm.2020.02.103
22. Ootake T, Ishii T, Sueishi K, et al. Effects of mechanical stress and deficiency of dihydrotestosterone or 17β-estradiol on Temporomandibular Joint Osteoarthritis in mice. Osteoarthritis Cartilage. 2021;29(11):1575–1589. doi:10.1016/j.joca.2021.08.005
23. Hislop BD, Devine C, June RK, Heveran CM. Subchondral bone structure and synovial fluid metabolism are altered in injured and contralateral limbs 7 days after non-invasive joint injury in skeletally-mature C57BL/6 mice. Osteoarthritis Cartilage. 2022;30(12):1593–1605. doi:10.1016/j.joca.2022.09.002
24. Ko FC, Dragomir CL, Plumb DA, et al. Progressive cell-mediated changes in articular cartilage and bone in mice are initiated by a single session of controlled cyclic compressive loading. J Orthop Res off Publ Orthop Res Soc. 2016;34(11):1941–1949. doi:10.1002/jor.23204
25. Li M, Xie WQ, He M, et al. Characterization of the subchondral bone and pain behavior changes in a novel bipedal standing mouse model of facet joint osteoarthritis. Biomed Res Int. 2020;2020:8861347. doi:10.1155/2020/8861347
26. Takahashi I, Takeda K, Matsuzaki T, Kuroki H, Hoso M. Reduction of knee joint load suppresses cartilage degeneration, osteophyte formation, and synovitis in early-stage osteoarthritis using a post-traumatic rat model. PLoS One. 2021;16(7):e0254383. doi:10.1371/journal.pone.0254383
27. He Z, Nie P, Lu J, et al. Less mechanical loading attenuates osteoarthritis by reducing cartilage degeneration, subchondral bone remodelling, secondary inflammation, and activation of NLRP3 inflammasome. Bone Jt Res. 2020;9(10):731–741. doi:10.1302/2046-3758.910.BJR-2019-0368.R2
28. Ziemian SN, Witkowski AM, Wright TM, Otero M, van der Meulen MCH. Early inhibition of subchondral bone remodeling slows load-induced posttraumatic osteoarthritis development in mice. J Bone Miner Res. 2021;36(10):2027–2038. doi:10.1002/jbmr.4397
29. Hsia AW, Jbeily EH, Mendez ME, et al. Post-traumatic osteoarthritis progression is diminished by early mechanical unloading and anti-inflammatory treatment in mice. Osteoarthritis Cartilage. 2021;29(12):1709–1719. doi:10.1016/j.joca.2021.09.014
30. Zhong Z, Liu B, Liu G, et al. A randomized controlled trial on the effects of low-dose extracorporeal shockwave therapy in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2019;100(9):1695–1702. doi:10.1016/j.apmr.2019.04.020
31. Uysal A, Yildizgoren MT, Guler H, Turhanoglu AD. Effects of radial extracorporeal shock wave therapy on clinical variables and isokinetic performance in patients with knee osteoarthritis: a prospective, randomized, single-blind and controlled trial. Int Orthop. 2020;44(7):1311–1319. doi:10.1007/s00264-020-04541-w
32. Ho KD, Yang CL, Lo HY, Yeh HJ. Extracorporeal shockwave therapy with a modified technique on tendon and ligament for knee osteoarthritis: a randomized controlled trial. Am J Phys Med Rehabil. 2022;101(1):11–17. doi:10.1097/PHM.0000000000001730
33. Jia H, Ma X, Wei Y, et al. Loading-induced reduction in sclerostin as a mechanism of subchondral bone plate sclerosis in mouse knee joints during late-stage osteoarthritis. Arthritis Rheumatol Hoboken NJ. 2018;70(2):230–241. doi:10.1002/art.40351
34. Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. 2020;11(1):282. doi:10.1038/s41467-019-14146-6
35. Zhao W, Tang Y, Yang Y, Wang M, Low-Magnitude YH. High-frequency vibration promotes osteogenic differentiation via intensifying miRNA-335-5p expression. J Environ Pathol Toxicol Oncol off Organ Int Soc Environ Toxicol Cancer. 2019;38(3):271–283. doi:10.1615/JEnvironPatholToxicolOncol.2019030625
36. Li X, Han L, Nookaew I, et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife. 2019;8:e49631. doi:10.7554/eLife.49631
37. Chen X, Yan J, He F, et al. Mechanical stretch induces antioxidant responses and osteogenic differentiation in human mesenchymal stem cells through activation of the AMPK-SIRT1 signaling pathway. Free Radic Biol Med. 2018;126:187–201. doi:10.1016/j.freeradbiomed.2018.08.001
38. Li H, Wu W, He X, et al. Applying vibration in early postmenopausal osteoporosis promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells and suppresses postmenopausal osteoporosis progression. Biosci Rep. 2019;39(9):BSR20191011. doi:10.1042/BSR20191011
39. Cao W, Lin W, Cai H, et al. Dynamic mechanical loading facilitated chondrogenic differentiation of rabbit BMSCs in collagen scaffolds. Regen Biomater. 2019;6(2):99–106. doi:10.1093/rb/rbz005
40. Kitase Y, Barragan L, Qing H, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the β-catenin and PKA pathways. J Bone Miner Res off J Am Soc Bone Miner Res. 2010;25(12):2657–2668. doi:10.1002/jbmr.168
41. Sato T, Verma S, Andrade CDC, et al. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat Commun. 2020;11(1):3282. doi:10.1038/s41467-020-17099-3
42. Liao C, Cheng T, Wang S, Zhang C, Jin L, Yang Y. Shear stress inhibits IL-17A-mediated induction of osteoclastogenesis via osteocyte pathways. Bone. 2017;101:10–20. doi:10.1016/j.bone.2017.04.003
43. Kodrič K, Zupan J, Kranjc T, et al. Sex-determining region Y (SRY) attributes to gender differences in RANKL expression and incidence of osteoporosis. Exp Mol Med. 2019;51(8):1–16. doi:10.1038/s12276-019-0294-3
44. Schreivogel S, Kuchibhotla V, Knaus P, Duda GN, Petersen A. Load-induced osteogenic differentiation of mesenchymal stromal cells is caused by mechano-regulated autocrine signaling. J Tissue Eng Regen Med. 2019;13(11):1992–2008. doi:10.1002/term.2948
45. Yan Y, Wang L, Ge L, Pathak JL. Osteocyte-mediated translation of mechanical stimuli to cellular signaling and its role in bone and non-bone-related clinical complications. Curr Osteoporos Rep. 2020;18(1):67–80. doi:10.1007/s11914-020-00564-9
46. Li J, Xue J, Jing Y, et al. SOST deficiency aggravates osteoarthritis in mice by promoting sclerosis of subchondral bone. BioMed Res Int. 2019;2019:7623562. doi:10.1155/2019/7623562
47. Ikebuchi Y, Aoki S, Honma M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561(7722):195–200. doi:10.1038/s41586-018-0482-7
48. Fang C, Guo JW, Wang YJ, et al. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacol Sin. 2022;43(5):1299–1310. doi:10.1038/s41401-021-00747-9
49. Sun Q, Zhang Y, Ding Y, et al. Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis. Cells. 2022;11(17):2760. doi:10.3390/cells11172760
50. Vorselen D, Roos WH, MacKintosh FC, Wuite GJL, van Loon JJWA. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J off Publ Fed Am Soc Exp Biol. 2014;28(2):536–547. doi:10.1096/fj.13-236356
51. Xu H, Wu F, Zhang H, et al. Actin cytoskeleton mediates BMP2-Smad signaling via calponin 1 in preosteoblast under simulated microgravity. Biochimie. 2017;138:184–193. doi:10.1016/j.biochi.2017.04.015
52. Li H, Liao L, Hu Y, et al. Identification of type H vessels in mice mandibular condyle. J Dent Res. 2021;100(9):983–992. doi:10.1177/00220345211002120
53. Dzamukova M, Brunner TM, Miotla-Zarebska J, et al. Mechanical forces couple bone matrix mineralization with inhibition of angiogenesis to limit adolescent bone growth. Nat Commun. 2022;13(1):3059. doi:10.1038/s41467-022-30618-8
54. Lu J, Zhang H, Cai D, et al. Positive-feedback regulation of subchondral H-type vessel formation by chondrocyte promotes osteoarthritis development in mice. J Bone Miner Res off J Am Soc Bone Miner Res. 2018;33(5):909–920. doi:10.1002/jbmr.3388
55. Zhang H, Wang L, Cui J, et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci Adv. 2023;9(14):eabo7868. doi:10.1126/sciadv.abo7868
56. Wang X, Li X, Li J, et al. Mechanical loading stimulates bone angiogenesis through enhancing type H vessel formation and downregulating exosomal miR-214-3p from bone marrow-derived mesenchymal stem cells. FASEB J off Publ Fed Am Soc Exp Biol. 2021;35(1):e21150. doi:10.1096/fj.202001080RR
57. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi:10.1016/j.cell.2017.05.016
58. Li X, Liu D, Li J, et al. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J. 2019;33(8):8913–8924. doi:10.1096/fj.201802711R
59. Javaheri B, Stern AR, Lara N, et al. Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res off J Am Soc Bone Miner Res. 2014;29(3):705–715. doi:10.1002/jbmr.2064
60. Zheng W, Ding B, Li X, Liu D, Yokota H, Zhang P. Knee loading repairs osteoporotic osteoarthritis by relieving abnormal remodeling of subchondral bone via Wnt/β-catenin signaling. FASEB J off Publ Fed Am Soc Exp Biol. 2020;34(2):3399–3412. doi:10.1096/fj.201902117R
61. Yang J, Xu Y, Xue X, Zhang M, Wang S, Qi K. MicroRNA-26b regulates BMSC osteogenic differentiation of TMJ subchondral bone through β-catenin in osteoarthritis. Bone. 2022;162:116448. doi:10.1016/j.bone.2022.116448
62. Chen X, Guo J, Yuan Y, et al. Cyclic compression stimulates osteoblast differentiation via activation of the Wnt/β-catenin signaling pathway. Mol Med Rep. 2017;15(5):2890–2896. doi:10.3892/mmr.2017.6327
63. He Z, Liu M, Zhang Q, et al. Wnt/β-catenin signaling pathway is activated in the progress of mandibular condylar cartilage degeneration and subchondral bone loss induced by overloaded functional orthopedic force (OFOF). Heliyon. 2022;8(10):e10847. doi:10.1016/j.heliyon.2022.e10847
64. Zhang Y, Que J. BMP signaling in development, stem cells, and diseases of the gastrointestinal tract. Annu Rev Physiol. 2020;82(1):251–273. doi:10.1146/annurev-physiol-021119-034500
65. Zhen G, Guo Q, Li Y, et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat Commun. 2021;12(1):1706. doi:10.1038/s41467-021-21948-0
66. Muratovic D, Findlay DM, Quarrington RD, et al. Elevated levels of active Transforming Growth Factor β1 in the subchondral bone relate spatially to cartilage loss and impaired bone quality in human knee osteoarthritis. Osteoarthritis Cartilage. 2022;30(6):896–907. doi:10.1016/j.joca.2022.03.004
67. Bailey KN, Nguyen J, Yee CS, Dole NS, Dang A, Alliston T. Mechanosensitive control of articular cartilage and subchondral bone homeostasis in mice requires osteocytic transforming growth factor β signaling. Arthritis Rheumatol Hoboken NJ. 2021;73(3):414–425. doi:10.1002/art.41548
68. Dai G, Xiao H, Liao J, et al. Osteocyte TGFβ1‑Smad2/3 is positively associated with bone turnover parameters in subchondral bone of advanced osteoarthritis. Int J Mol Med. 2020;46(1):167–178. doi:10.3892/ijmm.2020.4576
69. Xu X, Zheng L, Bian Q, et al. Aberrant activation of TGF-β in subchondral bone at the onset of rheumatoid arthritis joint destruction. J Bone Miner Res off J Am Soc Bone Miner Res. 2015;30(11):2033–2043. doi:10.1002/jbmr.2550
70. Wan Y, Lv Y, Li L, Yin Z. 15-Lipoxygenase-1 in osteoblasts promotes TGF-β1 expression via inhibiting autophagy in human osteoarthritis. Biomed Pharmacother Biomedecine Pharmacother. 2020;121:109548. doi:10.1016/j.biopha.2019.109548
71. Zhong Y, Xu Y, Xue S, et al. Nangibotide attenuates osteoarthritis by inhibiting osteoblast apoptosis and TGF-β activity in subchondral bone. Inflammopharmacology. 2022;30(3):1107–1117. doi:10.1007/s10787-022-00984-2
72. Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19(7):480–494. doi:10.1038/s41573-020-0070-z
73. Liu Y, Wang Z, Ju M, et al. Modification of COL1A1 in autologous adipose tissue-derived progenitor cells rescues the bone phenotype in a mouse model of osteogenesis imperfecta. J Bone Miner Res off J Am Soc Bone Miner Res. 2021;36(8):1521–1534. doi:10.1002/jbmr.4326
74. Yang BC, Kuang MJ, Kang JY, Zhao J, Ma JX, Ma XL. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem Biophys Res Commun. 2020;524(4):883–889. doi:10.1016/j.bbrc.2020.02.001
75. Dong L, Song Y, Zhang Y, et al. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol. 2021;236(9):6376–6390. doi:10.1002/jcp.30312
76. Zarka M, Etienne F, Bourmaud M, et al. Mechanical loading activates the YAP/TAZ pathway and chemokine expression in the MLO-Y4 osteocyte-like cell line. Lab Investig J Tech Methods Pathol. 2021;101(12):1597–1604. doi:10.1038/s41374-021-00668-5
77. Zhu Z, Tang T, He Z, et al. Uniaxial cyclic stretch enhances osteogenic differentiation of OPLL-derived primary cells via YAP-Wnt/β-catenin axis. Eur Cell Mater. 2023;45:31–45. doi:10.22203/eCM.v045a03
78. Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–1059. doi:10.1016/j.cell.2013.07.042
79. Liu H, Liu H, Deng X, et al. CXCR4 antagonist delivery on decellularized skin scaffold facilitates impaired wound healing in diabetic mice by increasing expression of SDF-1 and enhancing migration of CXCR4-positive cells. Wound Repair Regen. 2017;25(4):652–664. doi:10.1111/wrr.12552
80. Chen Y, Lin S, Sun Y, et al. Attenuation of subchondral bone abnormal changes in osteoarthritis by inhibition of SDF-1 signaling. Osteoarthritis Cartilage. 2017;25(6):986–994. doi:10.1016/j.joca.2017.01.008
81. Yang J, Li Y, Liu Y, et al. Role of the SDF-1/CXCR4 signaling pathway in cartilage and subchondral bone in temporomandibular joint osteoarthritis induced by overloaded functional orthopedics in rats. J Orthop Surg. 2020;15(1):330. doi:10.1186/s13018-020-01860-x
82. Zhang Y, Li X, Li J, et al. Knee loading enhances the migration of adipose-derived stem cells to the osteoarthritic sites through the SDF-1/CXCR4 regulatory axis. Calcif Tissue Int. 2022;111(2):171–184. doi:10.1007/s00223-022-00976-y
83. Wei FY, Chow SK, Leung KS, et al. Low-magnitude high-frequency vibration enhanced mesenchymal stem cell recruitment in osteoporotic fracture healing through the SDF-1/CXCR4 pathway. Eur Cell Mater. 2016;31:341–354. doi:10.22203/ecm.v031a22
84. Cambré I, Gaublomme D, Burssens A, et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat Commun. 2018;9(1):4613. doi:10.1038/s41467-018-06933-4
85. Feng SY, Lei J, Chen HM, Wang YX, Yap AUJ, Fu KY. Increased chemokine RANTES in synovial fluid and its role in early-stage degenerative temporomandibular joint disease. J Oral Rehabil. 2020;47(9):1150–1160. doi:10.1111/joor.13041
86. Lee JW, Hoshino A, Inoue K, et al. The HIV co-receptor CCR5 regulates osteoclast function. Nat Commun. 2017;8(1):2226. doi:10.1038/s41467-017-02368-5
87. Feng SY, Lei J, Li YX, et al. Increased joint loading induces subchondral bone loss of the temporomandibular joint via the RANTES-CCRs-Akt2 axis. JCI Insight. 2022;7(21):e158874. doi:10.1172/jci.insight.158874
88. Zhang S, Li T, Feng Y, et al. Exercise improves subchondral bone microenvironment through regulating bone-cartilage crosstalk. Front Endocrinol. 2023;14:1159393. doi:10.3389/fendo.2023.1159393
89. Smith JK. Exercise as an adjuvant to cartilage regeneration therapy. Int J Mol Sci. 2020;21(24):9471. doi:10.3390/ijms21249471
90. Lo GH, Vinod S, Richard MJ, et al. Association between walking for exercise and symptomatic and structural progression in individuals with knee osteoarthritis: data from the osteoarthritis initiative cohort. Arthritis Rheumatol Hoboken NJ. 2022;74(10):1660–1667. doi:10.1002/art.42241
91. Yao Z, Chen P, Wang S, et al. Reduced PDGF-AA in subchondral bone leads to articular cartilage degeneration after strenuous running. J Cell Physiol. 2019;234(10):17946–17958. doi:10.1002/jcp.28427
92. Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone. 2010;47(4):766–772. doi:10.1016/j.bone.2010.07.006
93. Liao CD, Huang YY, Chen HC, Liou TH, Lin CL, Huang SW. Relative effect of extracorporeal shockwave therapy alone or in combination with noninjective treatments on pain and physical function in knee osteoarthritis: a network meta-analysis of randomized controlled trials. Biomedicines. 2022;10(2):306. doi:10.3390/biomedicines10020306
94. Avendaño-Coy J, Comino-Suárez N, Grande-Muñoz J, Avendaño-López C, Gómez-Soriano J. Extracorporeal shockwave therapy improves pain and function in subjects with knee osteoarthritis: a systematic review and meta-analysis of randomized clinical trials. Int J Surg Lond Engl. 2020;82:64–75. doi:10.1016/j.ijsu.2020.07.055
95. Wang YC, Huang HT, Huang PJ, Liu ZM, Shih CL. Efficacy and safety of extracorporeal shockwave therapy for treatment of knee osteoarthritis: a systematic review and meta-analysis. Pain Med Malden Mass. 2020;21(4):822–835. doi:10.1093/pm/pnz262
96. Chou WY, Cheng JH, Wang CJ, Hsu SL, Chen JH, Huang CY. Shockwave targeting on subchondral bone is more suitable than articular cartilage for knee osteoarthritis. Int J Med Sci. 2019;16(1):156–166. doi:10.7150/ijms.26659
97. Wu Y, Zhu S, Lv Z, et al. Effects of therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil. 2019;33(12):1863–1875. doi:10.1177/0269215519866494
98. Carina V, Costa V, Pagani S, et al. Inhibitory effects of low intensity pulsed ultrasound on osteoclastogenesis induced in vitro by breast cancer cells. J Exp Clin Cancer Res CR. 2018;37(1):197. doi:10.1186/s13046-018-0868-2
99. Yi X, Liu J, Cheng MS, Zhou Q. Low-intensity pulsed ultrasound inhibits IL-6 in subchondral bone of temporomandibular joint osteoarthritis by suppressing the TGF-β1/Smad3 pathway. Arch Oral Biol. 2021;125:105110. doi:10.1016/j.archoralbio.2021.105110
100. Qiu CG, Chui CS, Chow SKH, Cheung WH, Wong RMY. Effects of whole-body vibration therapy on knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med. 2022;
101. Ye W, Guo H, Yang X, Yang L, He C. Pulsed electromagnetic field versus whole body vibration on cartilage and subchondral trabecular bone in mice with knee osteoarthritis. Bioelectromagnetics. 2020;41(4):298–307. doi:10.1002/bem.22263
102. Wang L, Wang Z, Liu Q, Su J, Wang T, Li T. Effect of whole body vibration on HIF-2α expression in SD rats with early knee osteoarthritis. J Bone Miner Metab. 2020;38(4):491–500. doi:10.1007/s00774-020-01092-3
103. Yu PM, Lin Y, Zhang C, et al. Low-frequency vibration promotes tumor necrosis factor-α production to increase cartilage degeneration in knee osteoarthritis. Cartilage. 2021;13(2_suppl):1398S–1406S. doi:10.1177/1947603520931178
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


