Back to Journals » Journal of Inflammation Research » Volume 17
Role of Immune Inflammation in Recurrent Spontaneous Abortions
Authors Wen X, Dong P, Liu J, Wang SJ, Li J
Received 28 August 2024
Accepted for publication 6 November 2024
Published 22 November 2024 Volume 2024:17 Pages 9407—9422
DOI https://doi.org/10.2147/JIR.S488638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Xi Wen,1 Peng Dong,1 Jia Liu,1 Shi-Jun Wang,1 Jian Li1,2
1Department of Gynecology and Obstetrics, Xuanwu Hospital, Capital Medical University, Beijing, 100053, People’s Republic of China; 2Department of Family Planning, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, 100010, People’s Republic of China
Correspondence: Jian Li, Department of Family Planning, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, No. 17 Qihe Lou, Dongcheng District, Beijing, 100010, People’s Republic of China, Tel +86 13661343676, Email [email protected] Shi-Jun Wang, Department of Gynecology and Obstetrics, Xuanwu Hospital, Capital Medical University, Changchun Street, Xicheng District, Beijing, 100053, People’s Republic of China, Tel +86 13601222289, Email [email protected]
Objective: This study aimed to investigate the role of immune inflammation in recurrent spontaneous abortions (RSA).
Methods: In this study, decidua tissues from 12 patients were collected. These included six individuals with RSA in the RSA group and six in the control group. The differences in gene and metabolite expression in the decidua of the placenta between normal pregnancies and patients with RSA were compared using transcriptomic and metabolomic analyses. The differentially expressed genes and metabolites were further analyzed through functional enrichment analysis using high-throughput sequencing technology.
Results: There was a significant upregulation of genes associated with immunity and inflammation in the RSA group compared to the control group. The TNF signaling pathway was upregulated in the RSA group. Inflammatory mediators were expressed at higher levels in the RSA group, and arachidonic acid metabolism was the most significant differential metabolite set. The regulation of inflammatory mediators of transient receptor potential (TRP) channels were enriched in RSA cases. The integrated analysis of the data further suggests that the immune-inflammatory response might be an important factor in RSA. The expression levels of genes related to inflammation and hypoxia in tissues from patients with RSA were verified using quantitative reverse transcription polymerase chain reaction (qRT-PCR), and this revealed that the expression of MARK10 and TNFAIP3 genes was significantly upregulated in samples from RSA patients compared to normal tissues.
Conclusion: The findings suggest a strong association between immune-related inflammation and RSA. Addressing metabolic and inflammatory aspects in patients with RSA may potentially help enhance pregnancy outcomes.
Keywords: decidua tissue, immune inflammation, metabolomics, recurrent spontaneous abortion, transcriptomics
Introduction
Recurrent spontaneous abortion (RSA) is a relatively common condition affecting women of reproductive age.1,2 Approximately, less than 5% of women experience two consecutive miscarriages, and about 1% experience three or more pregnancy losses, leading to a significant burden on the patient’s physical and mental health, family harmony, and socioeconomic status.
The etiology of recurrent spontaneous abortion is complex, among which immune abnormalities are often considered a significant contributor to pregnancy failure, potentially accounting for approximately 50% of recurrent abortion cases.3,4 Immunological studies have shown that the main features of RSA are a predominance of TH1-type immunity and increased levels of reactive oxygen species (ROS).5 Increased expression of the inflammasome NLRP3 has been observed in the endometrium of women with unexplained RSA.6 The activation of various immune cell types can initiate systemic inflammation, which may result in compromised placental formation and subsequent abortion.7 Immune dendritic cells play a crucial role in the pathogenesis of RSA.8 An imbalance in the Th17/Treg cell ratio, dysfunction in the natural killer (NK) cell immunity, and impaired polarization of macrophages in the decidua in early pregnancy all has been found to potentially result in RSA.9–11 Interestingly, genes involved in immune response, metabolism, and angiogenesis, among others, have also been shown to be associated with RSA.12
RSA has also been linked to abnormal patterns of placental vascular bed formation.13 Inadequate neoangiogenesis during placental development is considered one of the main causes of recurrent pregnancy losses.14 Vascular endothelial growth factor (VEGF) plays a crucial role in follicular maturation and is essential for the development of an extensive vascular network through decidualization, which supports the growth of the embryo and placenta, sustaining early pregnancy.15 Additionally, placental vascular thrombosis may also result in RSA.16
The decidua refers to the posterior endometrium during pregnancy, and it consists primarily of decidual stromal cells, trophoblasts, and immune cells. The decidua provides an optimal microenvironment for embryo implantation, development, placenta formation, immune regulation, nutrient support, and the maintenance of pregnancy. Impaired differentiation of endometrial stromal cells (ESC) into specialized decidua cells may contribute to RSA.17,18 The etiology of 50% of RSA cases remains unknown and is thought to be related to abnormalities in the endometrial microenvironment.19
Unfortunately, existing research protocols for treating RSA have not fully addressed the underlying pathogenesis. While the diagnosis of RSA is established, uncertainty in pathogenesis and variable clinical manifestations continue to hinder progress in predicting and treating it.20 RSA remains a complex, multi-stage biological process influenced by factors related to changes at the genetic, protein, and metabolic levels.21 As high-throughput technologies continue to evolve, there has been a gradual increase in the number of omics studies of RSA. Through diverse analyses of differential genes and metabolites, these studies have provided opportunities to explore the pathogenesis of RSA.22
Therefore, this study aimed to utilize transcriptomics and metabolomics to conduct a combined analysis and comparatively analyze the differential genes and metabolites expressed in the placental decidua in normal pregnancies and patients with RSA. The study aimed to further analyze transcriptional pathways and metabolic pathways closely related to the development of RSA and to validate them using quantitative reverse transcription polymerase chain reaction (qRT-PCR). The ultimate goal was to speculate on the pathogenesis of RSA, identify potential targets, and explore therapeutic pathways for patients with RSA.
Materials and Methods
Sample Collection and Clinical Characteristics
Decidual tissue was obtained from 12 patients who provided their informed consent for participating in the study, with the approval from Beijing Obstetrics and Gynecology Hospital’s Ethics Committee (Approval Number: linyanshen[2016]020). The experimental group consisted of decidua tissue from patients with RSA (n = 6), while the control group included decidua tissue from patients who had elected to undergo an abortion (n = 6). Patients with RSA underwent surgery within three days of the clinical indication of fetal heart cessation. Chromosomal abnormalities, autoimmune diseases, endocrine abnormalities, uterine anatomical malformations, infection and other such conditions were excluded. Patients who underwent abortions had previously had healthy children. RSA patients all did not have baby and the past number of spontaneous abortions is between 2 to 4.
Transcriptomics Sequencing
Firstly, RNA was extracted, and its integrity was accurately evaluated. Subsequently, the library was constructed using Illumina’s NEBNext® Ultra™ RNA Library Prep Kit. Using qRT-PCR, the effective concentration of the library was then precisely quantified, ensuring that it was higher than 2 nM, to guarantee the library’s quality. Following successful validation of the library, sequencing was performed with Illumina NovaSeq 6000. Use fastp v 0.19.3 to filter the original sequencing data. Use HISAT v2.1.0 to construct the index, and compare clean reads to the reference genome. Use featureCounts v1.6.2 to calculate the gene alignment. Use rMATS v3.1.0 to analyze variable splicing events. Use GATK v4.1.9.0 to analyze the variant sites, and use annovar to annotate the variant sites. STAR-Fusion v1.5.0 software was used to detect fusion transcripts using the fusion output of STAR v2.6.1d alignments.
Metabolomics Sequencing
Samples stored in a −80°C freezer were initially thawed on ice and subjected to three freeze-thaw cycles. The samples were rewarmed, and centrifuged, and 200 μL of the supernatant was transferred for liquid chromatography-mass spectrometry (LC-MS) analysis. The data acquisition instrument system mainly includes Ultra Performance Liquid Chromatography (UPLC) (ExionLC AD, https://sciex.com.cn/) and Tandem mass spectrometry (MS/MS) (QTRAP®6500, https://sciex.com.cn/). Metabolite qualitative and quantitative analysis uses the software Analyst 1.6.3 to process mass spectrometry data.
qRT-PCR
Tissues preserved at −80°C were processed by adding 1 mL of TRIzol after grinding, homogenization, incubation, centrifugation, and resuspension. Subsequently, 1 μL of the sample was extracted and analyzed using the Ultra-Micro Nucleic Acid Protein Assay Instrument (with a detection concentration of A260/A280 at 1.9–2.1). Total RNA was converted into cDNA using a reverse transcription kit. A quantity of 200 μL of DEPC-H2O (Generay, D1007-500 mL) was diluted and stored at −20°C. A 96-well plate (Applied Biosystems, 4346906) was spiked with cDNA per well, and 40 cycles of amplification were performed to obtain cycle threshold (Ct) values. The Ct values were analyzed with the 2-ΔΔCt method. The primer sequences utilized in this study are presented in Table 1.
 |
Table 1 Primers and Sequences Used in the Study |
Data Processing Methods
Transcriptomics Difference Analysis
Box plots were used to demonstrate the minimal dispersion in the distribution of gene expression levels across individual samples. Pearson’s correlation coefficient (R) served as an index to assess the relevance of biological replicates, and sample correlation plots were constructed. An R2 value approaching 1 indicates a strong correlation between the two replicate samples, affirming the reliability of the differentially expressed genes (DEGs).
The DEGs were analyzed using volcano plots. K-means clustering analysis was performed by normalizing the fragments per kilobase of transcript per million mapped reads (FPKM) values of all DEGs using the “scale()” function in R. This was followed by Z-score normalization of the DEGs to generate clustered heat maps of the grouped genes, aiming to comprehend the dynamics of DEGs under normal and RSA conditions.
Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Reactome enrichment analyses were performed on the DEGs to better understand their functions and potential pathways in the placental decidua tissues of patients with recurrent miscarriages and normal patients. The enrichment analysis is performed based on the hypergeometric test. For KEGG, the hypergeometric distribution test is performed with the unit of pathway; for GO, it is performed based on the GO term.
DESeq2 v1.22.1 /edgeR v3.24.3 was used to analyze the differential expression, and the P value was corrected using the Benjamini & Hochberg method. The corrected P value and |log2foldchange| are used as the threshold for significant difference expression. The protein interaction analysis of differentially expressed genes is based on the STRING database of known and predicted protein-protein interactions, building networks based on known interactions of species. Use gsea-3.0.jar for gene set enrichment analysis. Use WGCNA v1.69 for weighted gene co-expression network analysis.
Differential Analysis of Metabolomics in RSA
Metabolomics can reflect changes in biological pathways that occur during specific pathological processes, thus providing crucial insights into disease mechanisms.
The total ion current (TIC) was utilized to analyze the reproducibility and reliability of metabolite extraction and detection. Additionally, quality control (QC) sample correlation analysis and coefficient of variation (CV) value distribution plots were employed to assess the stability of the process data.
The samples were subjected to principal component analysis (PCA) for a comprehensive analysis of the overall metabolism and intergroup metabolite differences. To further refine the differentiation between groups, the metabolite group data were analyzed using the orthogonal projections to latent structures discriminant analysis (OPLS-DA) model. S-plot graphs were generated from the OPLS-DA data to compare the normal and the RSA groups. To demonstrate the differential status of metabolites, volcano plots were generated using a combination of variable importance in projection (VIP). Fold change (FC) and P value/false discovery rate (FDR) as triple screening criteria.
Various graphical representations were utilized to identify specific differential metabolites associated with normal and RSA conditions and to characterize potential biomarkers of RSA more clearly. Bar plots were used to depict the variance in metabolite abundance. Differences in metabolite content were illustrated with heat maps, while violin plots were used to visualize the distribution of differential metabolites among the subgroups based on marker intensity levels between the two groups.
To further assess the level of metabolic similarity and mutual regulation among the identified differential metabolites, correlation analysis of the significantly different metabolites using Pearson’s correlation coefficient was conducted. Chord diagrams, correlation heat maps, and correlation network diagrams were also generated to show the relationships among the differential metabolites. A KEGG enrichment analysis for differential metabolites was also undertaken to further illustrate the potential functional significance of the correlations between differentially abundant metabolites (DAMs) in the normal and RSA groups.
Combined Transcriptomics and Metabolomics Analysis
The integration of transcriptomics and metabolomics analyses provides a comprehensive view of how genetic changes at the transcriptional level determine phenotypic changes. PCA analysis was conducted independently for both the transcriptome and metabolome datasets. Subsequently, a correlation clustering heat map was generated, incorporating all correlation calculations for DEGs and DAMs.
All identified DEGs and DAMs were selected to construct orthogonal projections to latent structures (O2PLS) models for integrating the two datasets. DEGs and DAMs in the normal and RSA placental decidua tissues were enriched simultaneously and visualized using an enrichment bubble chart.
To further analyze the relationship between DEGs and DAMs, the correlation results of DEGs and DAMs with Pearson’s correlation coefficients > 0.80 and P values < 0.05 were selected for each pathway. These results were used to generate correlation network diagrams. Additionally, canonical correlation analysis (CCA) was performed to illustrate the overall correlation between the transcriptomic and metabolomic indicators across the two groups.
Results
Clinical Characteristics
There were no statistical differences in age, BMI, or weeks of gestation between patients with RSA and women who underwent normal abortions (Table 2).
 |
Table 2 Baseline Characteristics of Patients |
Analysis of Transcriptomic Differences
To explore the differential gene expression in the decidua of patients with normal pregnancy and those with RSA, six normal and five RSA samples were selected for transcriptomic analysis (out of the original total of 12 samples, one sample was excluded due to test failure). Quality control assessments that were initially conducted on the sequencing data revealed that the sequencing quality was satisfactory (Supplementary Figure 1).
Box plots (Figure 1A) indicated consistent gene expression levels across all samples. The sample correlation plots that were generated indicated a strong correlation between replicate samples and confirmed the reliability of the identified differentially expressed genes (Figure 1B). Principal component analysis (PCA) of the DEGs (Figure 1C) showed significant differences between the normal and RSA samples, with the first two principal components accounting for 25.15% and 13.72% of the variance, respectively.
Differential gene expression analysis (Figure 1D) identified a total of 1431 differentially expressed genes, of which 617 genes were downregulated and 814 genes were upregulated (|log2Fold Change| ≥ 1 and P < 0.05) (Supplementary Table 1). These DEGs were classified into five main clusters (Figure 1E), with genes within the same cluster exhibiting similar trends of change under various experimental conditions, implying potential functional similarities. The clustering heat map results highlighted distinct differences in gene expression between the normal and RSA groups, further confirming the high variability in gene expression profiles between the two groups (Figure 1F).
The top 50 Gene Ontology (GO) terms identified in the GO enrichment analysis of the DEGs (Figure 2A) were classified into three domains: biological processes, cellular components, and molecular functions. In the domain of biological processes, these genes were primarily involved in extracellular structural organization, extracellular matrix organization, and cellular response to organic circulating compounds. They were also significantly involved in maternal processes such as female pregnancy, decidualization, maternal placenta development, endothelial cell migration, response to oxygen levels, response to decreased oxygen levels, response to hypoxia, and smooth muscle cell proliferation. In terms of cellular composition, these genes were predominantly enriched in the collagen-containing extracellular matrix. In terms of molecular functions, these genes were mainly enriched for receptor-ligand activity, cytokine activity, cytokine receptor binding, and platelet-derived growth factor binding (P value < 0.05, Supplementary Table 2).
GO analysis indicated that the aforementioned gene functions may be associated with the mechanism of RSA occurrence. Enrichment analysis of KEGG pathways (Figure 2B) revealed significant enrichment in pathways such as the TNF signaling pathway, cytokine-cytokine receptor interactions, and the platelet-activated signaling pathway. Conventional enrichment analysis, which typically relies on hypergeometric distribution, may overlook genes that are biologically important but not significantly differentially expressed. Therefore, a gene set enrichment analysis (GSEA) was conducted on the differentially expressed genes between the decidua of normal and RSA patients. The results revealed an upregulation in the TNF signaling pathway in the RSA group compared to the normal group (Figure 2Ci and Cii).
Collectively, the results of the transcriptomics analysis suggest that immune-inflammatory responses in RSA placental decidua tissues may have contributed to RSA.
Metabolomic Analysis of RSA Differences
The considerable overlap of the curves of total ion flow for metabolite detection indicated a high level of reproducibility and reliability of metabolite extraction and detection (Supplementary Figure 2A), as well as good data stability and quality throughout the detection process (Supplementary Figure 2B and C).
PCA was conducted on both sets of samples (Figure 3A). The PCA patterns of the first two principal components (PCs) in both groups of samples were found to be similar. In the Normal-RSA group, PC1 accounted for 25.27% of the variance, while PC2 explained 22.64%. The samples within the same group clustered together but were distinct from the samples in the other group. The PCA results indicated a clear tendency for metabolite segregation between the two groups, showing significant overall metabolic differences and distinct metabolite profiles between the groups (Figure 3B). The volcano plot analysis identified a total of 853 differentially expressed metabolites, with 19 downregulated and 91 upregulated metabolites (Figure 3C).
Notably, the 166 DAMs identified in the KEGG annotation table of differentially significant metabolites (Supplementary Table 3) were classified into 11 subclasses corresponding to the major seven categories shown in the heat map of grouped differential metabolite content (Figure 3D). These categories included nucleotides and their metabolites, amino acids and their metabolites, fatty acyls, alcohols and amines, carbohydrates and their metabolites, organic acids and their derivatives, and hormones and hormone-related substances.
Among the alcohols and amines, the expression of the inflammatory mediator histamine was higher in the RSA group compared to the normal group. Additionally, within the category of organic acids and their compounds, the levels of the anaerobic oxidation products DL-3-phenyllactic acid and hydroxyphenyllactic acid were similarly elevated in the RSA group compared to the normal group. In the differential metabolite abundance bar plot (Figure 3E), the top 10 most highly expressed metabolites were 2-methylglutaric acid, L-arginine-L-glycine, L-glycine-L-arginine, 3-methylglutaric acid, kojibiose, N-acetyllactosamine, 2′-deoxycytidine-5′-monophosphoric acid, 12-ketoacyl-5Z,8Z,10E,14Z arachidonicacid, LPC (12:0/0:0), and (±)5,6-epoxy-8Z,11Z,14Z-eicosatrienoic acid. The 10 metabolites that were downregulated in expression were cytosine, isocytosine, glycine-lysine, N-methyl-4-aminobutyric acid, glutamate-proline, N6-acetyl-L-lysine, 6-aminonicotinamide, L-hydroxyproline-L-valine, N-adrenaline, and dextrinic acid.
The corresponding subgroup VIP TOP 50 differential metabolite violin plots (Figure 3F) showed that inflammatory mediators such as arachidonic acid metabolites (5,6-EET, 12-OxoETE, etc). and prostaglandins (Prostaglandin E2) were expressed at higher levels in the RSA group than in the normal group.
Collectively, these results suggest that the placental decidua tissue of patients with RSA is metabolically active and could elicit an inflammatory response.
Analysis of the correlations among the significantly different metabolites revealed a pattern of very strong correlation, characterized by predominantly positive correlations and a few negative correlations (Figure 4A). KEGG enrichment analysis of differential metabolites (Figure 4B) revealed that the differential metabolites in the normal and RSA groups were significantly enriched in the arachidonic acid metabolism pathway, which is regulated by inflammatory mediators and transient receptor potential (TRP) channels.
The KEGG classification map of differential metabolites (Figure 4C) categorized metabolic pathways into five subcategories: organismal systems, metabolism, human diseases, gene information processing, and environmental information processing. In the category of organismal systems, metabolites were enriched in pathways regulating inflammatory mediators via TRP channels. In the category of metabolic pathways, metabolites were enriched in arachidonic acid metabolism. In the human disease category, there was an enrichment in antioxidant properties, while in that of environmental information processing, enrichment was noted in the HIF-1 signaling pathway, the FoxO signaling pathway, and the PI3K-Akt signaling pathway. These findings suggest that alterations in these pathways may be related to the pathogenesis of RSA.
To identify biologically significant differential metabolites, the top 50 metabolite sets ranked by P value were selected for Metabolite Set Enrichment Analysis (MSEA) (Figure 4D). The analysis revealed that arachidonic acid metabolism was the most significant differential metabolite set, indicating that the inflammatory metabolic response in RSA may be of biological importance.
In summary, the results of the metabolomics analysis suggest that placental decidua tissue in RSA pregnancies may be associated with an immune-inflammatory response.
Combined Analysis of Transcriptomics and Metabolomics
The results of the PCA analysis for the transcriptome and metabolome (Figure 5Ai and Aii) showed significant differences between the normal and RSA groups. In the transcriptomic analysis, PC1 accounted for 25.15% of the variance, and PC2 accounted for 13.72%. Similarly, in the metabolomic analysis, PC1 explained 29.51% of the variance, while PC2 explained 21.21%.
The results of all correlation calculations of differential genes and differential metabolites were used to generate a correlation clustering heat map (Figure 5B). The heat map was categorized into 11 subclasses: alcohols and amines, amino acids and their metabolites, benzene and alternative derivatives, bile acids, carbohydrates and their metabolites, fatty acids, glycoproteins, hormones and hormone-related compounds, nucleotides and their metabolites, organic acids and their derivatives, and others. The results showed that most genes were either positively or negatively correlated with the corresponding metabolites.
Integration analysis between the transcriptomic and metabolomic data sets (Figure 5Ci and Cii) revealed the top ten metabolites with the most significant impact on transcriptomics and the five genes with the most significant impact on metabolomics. This analysis showed a strong correlation between the two datasets, providing reliable evidence of their interrelationship.
Enrichment analysis indicated that the DEGs and DAMs were involved in similar pathways, including the inflammatory mediator-regulated TRP channels, the HIF-1 signaling pathway, and the FoxO signaling pathway (Figure 6A). Correlative network maps and canonical correlation analysis (CCA) maps further confirmed the enrichment in inflammation-related pathways, highlighting the role of inflammatory mediator-regulated TRP channels (Figure 6Bi and Bii). These channels are known to produce a variety of inflammatory mediators, such as prostaglandin E2 (PGE2) and pro-inflammatory cytokines, during tissue injury or inflammation. These mediators, in turn, can indirectly regulate the activity of TRP channels.
In summary, the integrated analysis of transcriptome and metabolome data suggests that the underlying disease mechanism of RSA may be associated with the immune-inflammatory response.
Validation of Representative Genes by qPCR
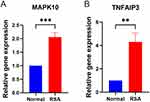
qRT-PCR analysis, performed to validate the expression levels of inflammation-related genes in individual tissues from patients with RSA and normal tissues, revealed significantly elevated expression of genes associated with immune inflammation in the tissues of patients with RSA compared to those of normal individuals (Figure 7).
Discussion
This study is the first integrated analysis of metabolomics and transcriptomics in the context of RSA. The differential expression of genes and variations in metabolic pathways identified through this integrated analysis of decidual samples from patients with RSA compared to samples from the control group may be intricately linked to the pathogenesis of RSA.
The transcriptomic analysis revealed a significantly higher expression of immune-inflammation-related genes in the RSA group compared to the normal group. Concurrently, the metabolomic analysis revealed the enrichment of metabolites involved in the regulation of inflammatory mediators via TRP channels. By combining transcriptomic and metabolomic analyses, we hypothesized that immune inflammation could be an important factor contributing to the development of the disease in patients with unexplained recurrent abortions.
Immune factors contribute to approximately 50% of the pathogenesis of RSA.2 A variety of immune cells, such as NK cells, macrophages, and DC cells, are present at the maternal-fetal interface.23 NK cells are the most abundant immune cells in both maternal and fetal tissues,24 and some immunotherapies targeting NK cells have yielded promising outcomes.25 In their study, Daher et al26 noted increased NK cell activity and elevated levels of Th1-type cytokines, such as IFN-r and TNF-a, in patients with RSA, linking these findings to immune inflammation.
Similarly, Azizi et al proposed that metabolic dysregulation might induce immune inflammation and oxidative stress, potentially contributing to the occurrence of RSA.27 In another study, Wang et al found that the cytotoxic properties of CD8+ effector T cells and NK cells were significantly enhanced in the peripheral blood of patients with RSA, indicating an enhanced systemic pro-inflammatory state. Additionally, they found that, in patients with RSA, the proportion of decidual natural killer (dNK) subpopulations supporting embryonic growth was reduced, while the proportion of dNK subpopulations with cytotoxic and immunoreactive features was significantly increased.28
In pregnant women with unexplained recurrent spontaneous abortion (URSA), elevated levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and Th17/Treg ratios may result in an immune imbalance at the maternal-fetal interface, potentially leading to miscarriages.29 An analysis of microRNA (miRNA) data revealed that differentially expressed miRNAs were highly enriched in the TNF signaling pathway. NF-κB1, a regulator of innate and adaptive immunity, was found to induce the expression of pro-inflammatory genes, particularly IL-6, and interleukin-8 (IL-8), by activating NF-κB1 signaling.30 In another study, it was found that mesenchymal stem cells (MSCs) of the decidua play a role in promoting fetal-maternal tolerance through their immunomodulatory effects.31 Another research study revealed significantly upregulated levels of apoptosis-related genes and pro-inflammatory cytokine genes (TNF-α, IL-6, and IL-8) in the placental tissues of patients with RSA.32
An analysis involving data from the GEO database highlighted the role of NK cells in the maintenance of pregnancy and how they may influence the development of RSA by regulating genes such as CASP3 and PARP1.33 It was also found that women with recurrent pregnancy loss (RPL) may have heightened inflammatory immune responses and decreased immunoregulation.34 The metabolite coenzyme Q10 (CoQ10), known to maintain redox homeostasis in vivo, was found to reduce oxidative stress (OS), regulate gene expression, and suppress inflammatory responses.35
In our study, TNFAIP3 was significantly upregulated in the decidua tissue of patients with RSA compared to patients with normal abortions. This upregulation suggests that TNFAIP3 may be involved in regulating the inflammatory environment of the decidua, potentially contributing to the pathogenesis of RSA.36 Previous studies have also emphasized the role of TNFAIP3 in multiple inflammatory diseases, further supporting its relevance in RSA.37 Therefore, targeting TNFAIP3-mediated pathways may provide new therapeutic avenues for managing RSA by modulating the inflammatory milieu of the decidua.
MAPK10, another gene involved in the regulation of cellular processes such as apoptosis, differentiation, and stress response, is mainly expressed in neuronal tissues but has also been found to be associated with other tissues under stress conditions.38 In our study, MAPK10 was identified as one of the genes differentially expressed in the decidua of RSA patients, with significant upregulation compared to patients with normal abortions. This finding points to the potential role of MAPK10 in apoptosis and stress response pathways within the decidua, which may contribute to the poor pregnancy outcomes observed in RSA. Upregulation of MAPK10 may lead to increased decidua apoptosis, which could compromise the integrity of the maternal-fetal interface. Given the involvement of MAPK10 in the JNK signaling pathway, which has been demonstrated to play a role in various reproductive processes such as embryo attachment and placental development,39 the dysregulation of MAPK10 in patients with RSA emphasizes its potential as a biomarker and therapeutic target for improving pregnancy outcomes.
In this study, using transcriptomic and metabolomic analyses, a comprehensive analysis of the molecular variances between patients with RSA and those with normal abortions was conducted. We found a significant enrichment of DEGs and DAMs in several key biological pathways, including the TNF signaling pathway, cytokine-cytokine receptor interactions, platelet-activated signaling pathway, arachidonic acid metabolism, and regulation of TRP channels by inflammatory mediators.
The TNF signaling pathway is known to play a key role in inflammatory and immune responses, which are critical processes in maintaining pregnancy and addressing complications. Dysregulation of this pathway has been associated with a variety of reproductive disorders, including recurrent abortions and preeclampsia, among other conditions. Similarly, the cytokine-cytokine receptor interaction pathway is critical for cell signaling in the immune response. Alterations in this pathway may be involved in the pathophysiology of RSA by affecting immune tolerance at the maternal-fetal interface.40
The modulation of TRP channels by inflammatory mediators suggests a link between ion channel activity and inflammatory responses in RSA. TRP channels are involved in a variety of physiological processes, including sensory perception and vascular tone. Dysregulation of these channels may impact placental blood flow and fetal development.41
The qRT-PCR validation of key genes such as TNFAIP3 and MAPK10 in this study further underscores the involvement of immune and inflammatory responses in RSA. TNFAIP3 acts as a negative regulator of the NF-κB signaling pathway and plays a crucial role in controlling inflammation, while MAPK10 is involved in inflammatory and apoptotic signaling pathways.
There are several limitations to this study. First, the relatively small sample size of 12 patients (6 with RSA and 6 with normal abortion) may limit the generalizability of the results. Furthermore, the absence of thorough clinical validation necessitates the confirmation of these molecular variances in larger, independent cohorts. Finally, the use of multiple datasets and analytical techniques may introduce batch effects, potentially impacting the reliability of the results. Future studies should aim to address these limitations by expanding the sample size and employing robust methodologies to minimize batch effects.
Conclusion
Our transcriptomic and metabolomic studies have highlighted significant associations between RSA, immunity, and inflammation, providing valuable insights into the molecular differences between patients with RSA and those with normal abortions. These findings suggest potential clinical implications, suggesting that enhancing metabolic pathways and addressing inflammation, both systemically and locally, could potentially improve pregnancy outcomes in this patient population. However, due to the complex and heterogenous etiology of unexplained recurrent miscarriage, the relationship between inflammatory pathways and RSA merits further investigation.
Abbreviations
RSA, Recurrent Spontaneous Abortion; DM, Decidual macrophage; ESC, endometrial stromal cells; ESHRE, European Society of Human Reproduction and Embryology; TRP, Transient receptor potential; KEGG, Kyoto Encyclopedia of Genes and Genomes; TNF, Tumor necrosis factor; DEGs, Differentially Expressed Genes; FPKM, Fragments Per Kilobase of exon model per Million mapped fragments; TIC, Total ions current; qRT-PCR, quantitative reverse transcription PCR.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Beijing Obstetrics and Gynecology Hospital of Capital Medical University (Approval Number: linyanshen[2016]020). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number: 81270733) and the Beijing Natural Science Foundation (Grant Number: 7132097). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors declare that they have no conflict of interests.
References
1. Tomkiewicz J, Darmochwał-Kolarz D. The Diagnostics and Treatment of Recurrent Pregnancy Loss. J Clin Med. 2023;12(14):4768. doi:10.3390/jcm12144768
2. Liu H, Lin XX, Huang XB, et al. Systemic Characterization of Novel Immune Cell Phenotypes in Recurrent Pregnancy Loss. Front Immunol. 2021;12:657552. doi:10.3389/fimmu.2021.657552. Erratum in: Front Immunol. 2021;12:722805. doi: 10.3389/fimmu.2021.722805.
3. Li D, Zheng L, Zhao D, Xu Y, Wang Y. The Role of Immune Cells in Recurrent Spontaneous Abortion. Reprod Sci. 2021;28(12):3303–3315. doi:10.1007/s43032-021-00599-y
4. Girardi G. Role of tissue factor in the maternal immunological attack of the embryo in the antiphospholipid syndrome. Clin Rev Allergy Immunol. 2010;39(3):160–165. doi:10.1007/s12016-009-8187-1
5. Talukdar A, Sharma KA, Rai R, Deka D, Rao DN. Effect of Coenzyme Q10 on Th1/Th2 Paradigm in Females with Idiopathic Recurrent Pregnancy Loss. Am J Reprod Immunol. 2015;74(2):169–180. doi:10.1111/aji.12376
6. Tersigni C, Vatish M, D’Ippolito S, Scambia G, Di Simone N. Abnormal uterine inflammation in obstetric syndromes: molecular insights into the role of chemokine decoy receptor D6 and inflammasome NLRP3. Mol Hum Reprod. 2020;26(2):111–121. doi:10.1093/molehr/gaz067
7. Hosseini MS, Ali-Hassanzadeh M, Nadimi E, Karbalay-Doust S, Noorafshan A, Gharesi-Fard B. Stereological study of the placental structure in abortion-prone mice model (CBA/J×DBA/2J). Ann Anat. 2020;230:151508. doi:10.1016/j.aanat.2020.151508
8. Lai N, Fu X, Hei G, et al. The Role of Dendritic Cell Subsets in Recurrent Spontaneous Abortion and the Regulatory Effect of Baicalin on It. J Immunol Res. 2022;2022:9693064. doi:10.1155/2022/9693064
9. Muyayalo KP, Li ZH, Mor G, Liao AH. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2018;80(4):e13018. doi:10.1111/aji.13018
10. Tao Y, Li YH, Zhang D, et al. Decidual CXCR4+ CD56bright NK cells as a novel NK subset in maternal-foetal immune tolerance to alleviate early pregnancy failure. Clin Transl Med. 2021;11(10):e540. doi:10.1002/ctm2.540
11. Yang L, Zhang X, Gu Y, et al. SEC5 is involved in M2 polarization of macrophages via the STAT6 pathway, and its dysfunction in decidual macrophages is associated with recurrent spontaneous abortion. Front Cell Dev Biol. 2022;10:891748. doi:10.3389/fcell.2022.891748
12. Pereza N, Ostojić S, Kapović M, Peterlin B. Systematic review and meta-analysis of genetic association studies in idiopathic recurrent spontaneous abortion. Fertil Steril. 2017;107(1):150–159.e2. doi:10.1016/j.fertnstert.2016.10.007
13. Wang N, Ge H, Zhou S. Cyclosporine A to Treat Unexplained Recurrent Spontaneous Abortions: a Prospective, Randomized, Double-Blind, Placebo-Controlled, Single-Center Trial. Int J Womens Health. 2021;13:1243–1250. doi:10.2147/IJWH.S330921
14. Kanki K, Ii M, Terai Y, Ohmichi M, Asahi M. Bone Marrow-Derived Endothelial Progenitor Cells Reduce Recurrent Miscarriage in Gestation. Cell Transplant. 2016;25(12):2187–2197. doi:10.3727/096368916X692753
15. Rizov M, Andreeva P, Dimova I. Molecular regulation and role of angiogenesis in reproduction. Taiwan J Obstet Gynecol. 2017;56(2):127–132. doi:10.1016/j.tjog.2016.06.019
16. Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–1111. doi:10.1016/j.fertnstert.2012.06.048.
17. Teklenburg G, Salker M, Heijnen C, Macklon NS, Brosens JJ. The molecular basis of recurrent pregnancy loss: impaired natural embryo selection. Mol Hum Reprod. 2010;16(12):886–895. doi:10.1093/molehr/gaq079
18. Zeng Z, Liu F, Li S. Metabolic Adaptations in Pregnancy: a Review. Ann Nutr Metab. 2017;70(1):59–65. doi:10.1159/000459633
19. Luo J, Wang Y, Chang HM, Zhu H, Yang J, Leung PCK. ID3 mediates BMP2-induced downregulation of ICAM1 expression in human endometiral stromal cells and decidual cells. Front Cell Dev Biol. 2023;11:1090593. doi:10.3389/fcell.2023.1090593
20. Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020;6(1):98. doi:10.1038/s41572-020-00228-z
21. Yu N, Kwak-Kim J, Bao S. Unexplained recurrent pregnancy loss: novel causes and advanced treatment. J Reprod Immunol. 2023;155:103785. doi:10.1016/j.jri.2022.103785
22. Li J, Wang L, Ding J, et al. Multiomics Studies Investigating Recurrent Pregnancy Loss: an Effective Tool for Mechanism Exploration. Front Immunol. 2022;13:826198. doi:10.3389/fimmu.2022.826198
23. Yang Y, Song S, Gu S, et al. Kisspeptin prevents pregnancy loss by modulating the immune microenvironment at the maternal-fetal interface in recurrent spontaneous abortion. Am J Reprod Immunol. 2024;91(2):e13818. doi:10.1111/aji.13818
24. Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol. 2016;38(6):635–649. doi:10.1007/s00281-016-0574-0
25. Moffett A, Shreeve N. First do no harm: uterine natural killer (NK) cells in assisted reproduction. Hum Reprod. 2015;30(7):1519–1525. doi:10.1093/humrep/dev098
26. Daher S, de Arruda Geraldes Denardi K, Blotta MH, et al. Cytokines in recurrent pregnancy loss. J Reprod Immunol. 2004;62(1–2):151–157. doi:10.1016/j.jri.2003.10.004
27. Azizi R, Soltani-Zangbar MS, Sheikhansari G, et al. Metabolic syndrome mediates inflammatory and oxidative stress responses in patients with recurrent pregnancy loss. J Reprod Immunol. 2019;133:18–26. doi:10.1016/j.jri.2019.05.001
28. Wang F, Jia W, Fan M, et al. Single-cell Immune Landscape of Human Recurrent Miscarriage. Genomics Proteomics Bioinf. 2021;19(2):208–222. doi:10.1016/j.gpb.2020.11.002
29. Qian J, Zhang N, Lin J, et al. Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion. Biosci Trends. 2018;12(2):157–167. doi:10.5582/bst.2018.01012
30. Bahia W, Soltani I, Abidi A, et al. Identification of genes and miRNA associated with idiopathic recurrent pregnancy loss: an exploratory data mining study. BMC Med Genomics. 2020;13(1):75. doi:10.1186/s12920-020-00730-z
31. Büyükbayrak EE, Gündoğdu NEÖ, Gürkan N, Kahraman FR, Akalın M, Akkoç T. Immunological effects of human decidual mesenchymal stem cells in spontaneous and recurrent abortions. J Reprod Immunol. 2024;162:104193. doi:10.1016/j.jri.2024.104193
32. Al-Sheikh YA, Ghneim HK, Alharbi AF, Alshebly MM, Aljaser FS, Aboul-Soud MAM. Molecular and biochemical investigations of key antioxidant/oxidant molecules in Saudi patients with recurrent miscarriage. Exp Ther Med. 2019;18(6):4450–4460. doi:10.3892/etm.2019.8082
33. Liu Y, Chen P, Fei H, Li M, Li X, Li T. Natural killer cells contributed to recurrent miscarriage by SP1-CASP3-PARP1. Int Immunopharmacol. 2021;93:107424. doi:10.1016/j.intimp.2021.107424
34. Wang WJ, Salazar Garcia MD, Deutsch G, et al. PD-1 and PD-L1 expression on T-cell subsets in women with unexplained recurrent pregnancy losses. Am J Reprod Immunol. 2020;83(5):e13230. doi:10.1111/aji.13230
35. Nie X, Dong X, Hu Y, Xu F, Hu C, Shu C. Coenzyme Q10 Stimulate Reproductive Vatality. Drug Des Devel Ther. 2023;17:2623–2637. doi:10.2147/DDDT.S386974
36. Wertz IE, Dixit VM. Regulation of death receptor signaling by the ubiquitin system. Cell Death Differ. 2010;17(1):14–24. doi:10.1038/cdd.2009.168
37. Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12(11):774–785. doi:10.1038/nri3313
38. Coffey ET. Nuclear and cytosolic JNK signalling in neurons. Nat Rev Neurosci. 2014;15(5):285–299. doi:10.1038/nrn3729
39. Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4(5):E131–6. doi:10.1038/ncb0502-e131
40. Wang P, Pan J, Tian X, et al. Transcriptomics-determined chemokine-cytokine pathway presents a common pathogenic mechanism in pregnancy loss and spontaneous preterm birth. Am J Reprod Immunol. 2021;86(1):e13398. doi:10.1111/aji.13398
41. Persoons E, Hennes A, De Clercq K, et al. Functional Expression of TRP Ion Channels in Endometrial Stromal Cells of Endometriosis Patients. Int J Mol Sci. 2018;19(9):2467. doi:10.3390/ijms19092467
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.