Back to Journals » Journal of Inflammation Research » Volume 17
Sinulariolide Suppresses Inflammation of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis and Mitigates Collagen-Induced Arthritis Symptoms in Mice
Authors Tsai SW, Cheng YC, Chao YH, Yang DH
Received 5 May 2024
Accepted for publication 26 October 2024
Published 6 November 2024 Volume 2024:17 Pages 8299—8311
DOI https://doi.org/10.2147/JIR.S476847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Sen-Wei Tsai,1,2 Yu-Chieh Cheng,3 Ya-Hsuan Chao,4 Deng-Ho Yang4– 7
1Department of Physical Medicine and Rehabilitation, Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taichung, 402, Taiwan; 2School of Medicine, Tzu Chi University, Hualien, 970, Taiwan; 3Department of Orthopaedics, Tungs’ Taichung Metro Harbor Hospital, Taichung, 433, Taiwan; 4The iEGG and Animal Biotechnology Center, National Chung Hsing University, Taichung, 402, Taiwan; 5Department of Internal Medicine, Taichung Armed-Forces General Hospital, Taichung, 411, Taiwan; 6Division of Rheumatology/Immunology/Allergy, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, 114, Taiwan; 7Department of Medical Laboratory Science and Biotechnology, Central Taiwan University of Science and Technology, Taichung, 406, Taiwan
Correspondence: Deng-Ho Yang, Department of Internal Medicine, Taichung Armed Forces General Hospital, No. 348, Sec. 2, Chung-Shan Road, Taiping Dist, Taichung, 41152, Taiwan, Tel +886-4-23934191, Fax +886-4-23934192, Email [email protected]
Background: Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by active polyarthritis, which leads to functional loss and joint deformities. Natural compounds derived from marine organisms are considered valuable immune-modulating agents. This study aimed to assess the anti-inflammatory effect of sinulariolide, a soft coral-derived compound, on RA fibroblast-like synoviocytes and its therapeutic efficacy against collagen-induced arthritis (CIA).
Methods: To determine the effects of sinulariolide on tumor necrosis factor-alpha (TNF-α)-induced inflammation, MH7A cells pre-treated with 10 ng/mL TNF-α for 24 h were treated with sinulariolide. The effect of sinulariolide on proinflammatory cytokine expressions at both the mRNA and protein levels in the MH7A cells was assessed using real-time-polymerase chain reaction and enzyme-linked immunosorbent assay (ELISA). Further, we analyzed the effect of sinulariolide on the activation of mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways using Western blotting and the TransAM NF-κB p65 kit. To comprehensively evaluate the potential application of sinulariolide in the treatment of inflammatory diseases, we used a well-established collagen-induced arthritis (CIA) mouse model. We examined the tissue sections of the ankle joints of the mice, assessed synovial hyperplasia, inflammatory cell infiltration, and cartilage damage, and used ELISA to analyze changes in cytokine expression in the hind paw tissues.
Results: MH7A cells treated with sinulariolide showed a notable reduction in the expression of proinflammatory cytokines, which could be due to decreased activation of the MAPK and NF-kB pathways. Additionally, sinulariolide-treated mice showed significantly reduced joint swelling and lower clinical arthritis scores than those in the normal and control groups. Significant reductions in synovial hyperplasia, inflammatory cell infiltration, and cartilage damage were observed in the tissue sections of the ankle joints of the mice treated with sinulariolide. Furthermore, the expression of inflammatory cytokines in the hind paw tissue of the mice treated with sinulariolide was significantly decreased.
Conclusion: Sinulariolide inhibited the progression of inflammation in MH7A cells. Sinulariolide treatment significantly reduced clinical arthritis symptoms and histological inflammatory responses in mice with CIA. Sinulariolide may serve as a potential therapeutic agent for RA.
Keywords: fibroblast-like synoviocytes, soft coral, sinulariolide, inflammation, collagen-induced arthritis, rheumatoid arthritis
Background
Rheumatoid Arthritis (RA) is a systemic inflammatory disease primarily characterized by polyarthritis and synovitis.1 Uncontrolled progression of RA can lead to joint deformities and bone damage.2 Studies on RA-related inflammatory pathways have revealed enhanced activity of various inflammatory cells, including T cells, B cells, macrophages, osteoclasts, and neutrophils.3–5 These immune cells, when activated, release a multitude of proinflammatory cytokines and chemokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and IL-6, which play pivotal roles in the inflammatory process.6,7 A major hallmark of RA is hyperplasia and inflammatory response of synovial cells.8,9 Fibroblast-like synoviocytes (FLS) are commonly found in synovial tissues of patients with RA.10 The pathological role of FLS in RA-related chronic synovitis is primarily reflected in an imbalance between their growth and apoptosis.11 Studies have shown that FLS, when stimulated by proinflammatory cytokines, may exhibit tumor-like behaviors, such as aggressive proliferation and resistance to apoptosis.10,11 Excessive activity and infiltration of FLS in RA-induced inflamed joints contribute to the transformation of the normal synovium into aggressively proliferating tissues, forming synovial-like structures in the joints.12 Moreover, activated FLS plays a crucial regulatory role in synovitis by secreting large amounts of inflammatory factors.12 Therefore, the effective inhibition of FLS activity may be significant in the treatment of RA-related synovial inflammation.
Key approaches for managing RA include the administration of non-steroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, corticosteroids, and biological therapies.13,14 These medications reduce pain, control inflammatory processes, and regulate the immune system. Notably, biological therapies are geared towards blocking TNF-α and IL-6 and managing T and B cell functions.15,16 However, these conventional treatments may lead to adverse effects or be ineffective in many patients.13,17,18 Consequently, natural compounds derived from marine life have emerged as valuable tools for developing novel immunomodulatory medications.19,20 Sinulariolide, a cyclic diterpene found in the soft coral Sinularia flexibilis, is known for its diverse biological functions, including immunomodulatory and anticancer effects.21–23 This substance impedes the activation and development of dendritic cells when stimulated with endotoxins. Previous studies have indicated the effectiveness of sinulariolide against various types of cancer, such as liver cancer,24,25 bladder cancer,26,27 lung adenocarcinoma,23 stomach cancer,28 and melanoma.29 Additionally, 11-epi-sinulariolide acetate, sourced from Sinularia querciformis, reportedly reduces inflammation and bone destruction in a rat model of adjuvant-induced arthritis.30 The specific effects of sinulariolide and its mechanism of action in combatting arthritis and inflammation are subjects of ongoing research. Our study aimed to assess the healing effects of sinulariolide on human RA MH7A synovial cells and its influence on a DBA/1 mouse model of collagen-induced arthritis.
Material
Aim, Design, and Setting
Our study aimed to assess the healing effects of sinulariolide on human RA MH7A synovial cells and its influence on a DBA/1 mouse model of collagen-induced arthritis.
Cell Culture
MH7A cells of the RA-FLS variety were sourced from Chih-Hsin Tang at the China Medicine University (Taichung, Taiwan) and were originally obtained from the Riken Cell Bank (RCB1512, Ibaraki, Japan). The cells were cultured in a medium containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA) and antibiotics, specifically 100 U/mL penicillin/streptomycin (Sigma-Aldrich, St Louis, MO, USA). The culture medium was enhanced using Dulbecco’s modified Eagle’s medium (HyClone, Logan, Utah, USA). The cells were kept at a steady temperature of 37 °C in an environment with controlled humidity, comprising 95% air and 5% CO2. The MH7A cells were treated with different concentrations of sinulariolide (provided by Dr. Jui-Hsin Su, National Museum of Marine Biology and Aquarium, Pingtung, Taiwan) for 24, 48, and 72 h. To induce inflammatory conditions, the cells were treated with 10 ng/mL TNF-α (Peprotech, Rocky Hill, NJ) for 24 h.
Cell Counting Kit‐8 (CCK-8) Assay
To determine the viability of MH7A cells at different sinulariolide concentrations, a CCK-8 assay was performed (AAT Bioquest, Inc., CA, USA). We began by distributing 2×104 MH7A cells into each segment of a 96-well plate. Subsequently, the cells were treated with varying concentrations of sinulariolide (0–100 μM) for 24–72 h. Post-treatment, we introduced 10 μL of the CCK-8 solution into every well and maintained the plates at 37 °C for an additional 2 h. The final step involved quantifying the viability of the MH7A cells by measuring optical density at 450 nm using a microplate reader (Tecan Sunrise, Grödig, Austria).
RNA Isolation and Real-Time Real Time-Polymerase Chain Reaction (RT-PCR)
After being treated for 24 h with sinulariolide at concentrations of either 12.5 or 25 μM, combined with 10 ng/mL of human TNF-α, the MH7A cells underwent RNA extraction. This extraction process involved the use of TRIzol reagent (EBL Biotechnology, Taipei, Taiwan) according to the manufacturer’s guidelines. The extracted RNA was converted to cDNA using the Superscript III Reverse Transcriptase System (Invitrogen). Next, quantitative RT-PCR was conducted using an ABI 7500 Fast RT-PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc., Singapore). The PCR process involved the use of 2X Universal SYBR Green Fast qPCR Mix (ABclonal, Xinbei, Taiwan) to facilitate the detection and quantification of cDNA. The following primer sequences were used: mouse IL-1beta, forward 5′-TCGTGCTGTCGGACCCATA-3′ and reverse 5′-TTGTTGGTTGATATTCTGTCCATTG-3′; mouse IL-6, forward 5′-TCGGAGGCTTAATTACACATGTTC-3′ and reverse 5′-TGCCATTGCACAACTCTTTTCT-3′ and reverse 5′-GGAGTGGGTGTCGCTGTTG-3′.
Enzyme‐Linked Immunosorbent Assay (ELISA)
MH7A cells were treated with sinulariolide (12.5 or 25 μM) and 10 ng/mL human TNF‐α for 24 h and centrifuged. The resulting supernatants were collected, and the levels of prostaglandin E2 (PGE2), IL‐6, IL‐1β, and matrix metalloproteinase‐1 were measured using the corresponding commercially available ELISA kits (R&D Systems Co, Ltd, Minneapolis, MN).
Determination of Nitric Oxide (NO) Level
Further, a NO detection kit (Jiancheng Bioengineering, Nanjing, China) was used to determine the concentration of NO in the supernatant.
Western Blot Analysis
The MH7A cells, after being subjected to a preliminary 2 h treatment with sinulariolide, were subsequently stimulated with recombinant human TNF-α, and their suspensions were gathered at the 90-min mark. This procedure was performed to assess the phosphorylation events in the mitogen-activated protein kinase (MAPK) signaling pathway. For lysate preparation, the cells were lysed in a lysis buffer containing protease inhibitors on ice for 30 min. The protein content of the lysates was quantified using the bicinchoninic acid technique. In the subsequent stage of protein examination, 40 μg of the lysates was heated and applied to 8–10% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels for electrophoretic separation. Thereafter, the proteins were electro-transferred onto nitrocellulose membranes. These membranes underwent an overnight incubation at 4 °C with specific primary antibodies that recognize phospho-p38 (Thr180/Tyr182), p38, phospho-ERK (Thr202/Tyr204), total ERK, phosphor-JNK, JNK, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Following this, a mouse anti-rabbit IgG secondary antibody tagged with horse-radish peroxidase in a 1:2000 dilution was applied, and the membranes were incubated once more overnight at 4 °C. Proteins were detected using enhanced chemiluminescence solution (Visual Protein, Taipei, Taiwan) and visualized using the Hansor Luminescence Image System (Taichung, Taiwan). Densitometric assessment of the specific bands was conducted using ImageJ software (National Institutes of Health, Bethesda, MD, USA), and this analysis was normalized to the GAPDH content in each sample.
Preparation of Nuclear Extracts and Measurement of Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB) Activity
NE-PER reagent (Thermo Fisher Scientific, Waltham, MA, USA), designed for nuclear and cytoplasmic extraction, was used to obtain nuclear fractions from the MH7A cells. The cells had been pre-treated with sinulariolide for 2 h and stimulated with recombinant human TNF-α for 90 min. Following the extraction, the activation of the NF-κB p65 subunit was measured using the TransAM NF-κB p65 kit (Active Motif, Carlsbad, CA, USA). This ELISA-based kit was used in accordance with the manufacturer’s instructions.
Animals
Female DBA/1 mice aged approximately 6–8 weeks and of a similar weight range were acquired from the Jackson Laboratory (Bar Harbor, ME, USA). The mice were housed and cared for under specific pathogen-free conditions, regulated temperature and humidity, and a consistent light/dark cycle. The animals had unrestricted access to a standard rodent diet and water. All procedures involving these animals were conducted in compliance with the regulations set by the Institutional Animal Care and Use Committee (IACUC) of the National Chung Hsing University. The animal study protocols were approved by the committee, and an identification number (NCHU-IACUC No, 110–047) was assigned before the study began.
Arthritis Induction and Assessment
To establish collagen-induced arthritis (CIA), bovine type II collagen (CII) provided by Chondrex, Inc. (WA, USA) was first dissolved and diluted to a concentration of 2 mg/mL in 10 nM acetic acid. The collagen solution was equally mixed with complete Freund’s adjuvant, containing 250 μg of heat-inactivated Mycobacterium tuberculosis H37Ra (Sigma-Aldrich, St. Louis, MO, USA). The resulting emulsion was then administered intradermally to the DBA/1 mice at the base of their tails in doses of 200 μL per mouse. A follow-up booster shot consisting of CII in incomplete Freund’s adjuvant was administered intradermally to the same group of mice 21 days after the first injection. The severity of arthritis in the mice was evaluated using a double-blind scoring system. Each limb was individually rated on a scale of 0 (no symptoms) to 4 (severe symptoms) based on the degree of redness and swelling.
Treatment
The mice were divided into four distinct groups: healthy, normal DBA/1 mice that served as the normal control group; mice with CIA-induced arthritis treated with a vehicle control containing 10% dimethyl sulfoxide and 90% glyceryl trioctanoate; and two groups of mice receiving sinulariolide treatment at doses of either 10 mg/kg or 20 mg/kg. Sinulariolide-treated mice with CIA were intraperitoneally injected with sinulariolide daily for 21 days, starting 3 weeks after the initial CII injection.
Histological Analysis
The ankle joints of the mice were subjected to histological examination after sectioning in paraffin. Each joint tissue section, with a thickness of 5 μm, was stained using hematoxylin and eosin to highlight the tissue structure. To assess the severity of arthritis, histopathological features were scored using a scoring system.31 This evaluation was conducted by two independent investigators in a double-blind setting to ensure an unbiased assessment of severity.
To measure the cytokine levels in the hind paw tissues, the paws of the DBA/1 mice from each group were homogenized and lysed with tissue lysis buffer and UltraCruz Protease Inhibitor Cocktail (Santa Cruz Biotechnology Inc., Dallas, TX, USA) and then centrifuged twice at 840 × g for 10 min at 4 °C for supernatant collection. The concentrations of TNF-α, IL-6, IL-1β, IL-8, and IL-17A in the supernatant were determined using the corresponding mouse ELISA kit (BioLegend).
Statistical Analysis
Data are presented as means ± standard deviation, and these values were obtained from triplicate measurements unless specified otherwise. A two-tailed Student’s t-test was used to compare two individual datasets. When comparing multiple experimental groups, either a one-way or two-way analysis of variance, followed by a posthoc Tukey HSD test, was used. All statistical analyses were performed using GraphPad Prism v5.0 software. Statistical significance was set at p < 0.05. 3.
Results
Effect of Sinulariolide on MH7A Cell Viability
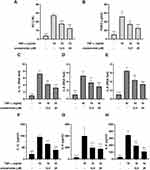
To assess the cytotoxic effect of sinulariolide (Figure 1A) on MH7A cells, we conducted CCK-8 assays after treating the cells with different concentrations of sinulariolide for 24, 48, and 72 h. According to the results shown in Figure 1B–D, MH7A cell viability was suppressed when the concentration of sinulariolide exceeded 50 μM within 72 h, as well as when it exceeded 100 μM at 48 and 72 h. To ensure that the cytotoxicity of sinulariolide does not interfere with the evaluation of inflammatory cell hormone expression, we proceeded with the experiments using sinulariolide at concentrations of 12.5 and 25 μM.
Sinulariolide Inhibited the Secretion of Proinflammatory Cytokines by MH7A Cells Treated with TNF-α
To assess the effect of sinulariolide on TNF-α-induced inflammation in MH7A cells, MH7A cells were treated with 12.5 and 25 μM sinulariolide with 10 ng/mL TNF-α for 24 h. The outcomes pertaining to the secretion of NO and PGE2 are depicted in Figure 2A and B. Exposure to only TNF-α resulted in a significant increase in NO and PGE2 levels, whereas these TNF-α-induced effects were ameliorated following sinulariolide treatment. Meanwhile, RNA and protein expressions of IL‐1β, IL‐6, and IL-8 in MH7A cells were induced by TNF‐α, which were suppressed following treatment with 12.5 and 25 μM sinulariolide (Figure 2C–H). Summarily, these results indicate that sinulariolide can mitigate TNF-α-induced inflammation in MH7A cells.
Sinulariolide Downregulated the MAPKs and NF-κB Pathways in MH7A Cells Treated with TNF-α
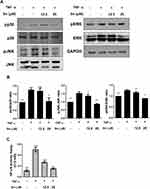
In addition to investigating the anti-inflammatory effects of sinulariolide on FLS in RA, we explored the potential molecular mechanisms underlying the effects of sinulariolide on MH7A cells. The activation of MAPK and NF-κB is crucial for the inflammation of FLS, leading to the release of proinflammatory cytokines.32 We used Western blot analysis to examine the phosphorylation levels of ERK, JNK, and p38 MAPKs and assessed the activation of MAPK and NF-κB in MH7A cells with or without sinulariolide treatment under TNF-α stimulation through p65 binding experiments. The results revealed that within 90 minutes, sinulariolide inhibited TNF-α-induced phosphorylation of p38 and JNK, whereas the expression of p-ERK appeared to remain unchanged after sinulariolide treatment (Figure 3A and B). Conversely, the nuclear translocation of NF-κB p65 increased over time under TNF-α stimulation, but it significantly decreased after sinulariolide treatment (Figure 3C). These findings suggest that sinulariolide’s effect on MH7A cells may be associated with the deactivation of MAPK and NF-κB.
Sinulariolide Attenuated CIA in DBA/1 Mice
To comprehensively assess the potential effect of sinulariolide in treating inflammatory diseases, we used a well-established CIA mouse model. The DBA/1 mice were administered CII to induce CIA. The DBA/1 mice with CII-induced CIA exhibited pathological features and tissue damage similar to those of human RA. As described in the Methods section, following the initial collagen injection, mice in the vehicle control and sinulariolide experimental groups were treated daily with either the vehicle or sinulariolide (10 or 20 mg/kg), and the clinical arthritis scores of the hind paws were evaluated. Mice receiving sinulariolide treatment showed significantly reduced clinical arthritis scores (Figure 4A) and maintained a stable body weight (Figure 4B) compared with those in the normal and vehicle control groups. Additionally, histological examination of the ankle joint tissues revealed a notable decrease in synovial hyperplasia, inflammatory cell infiltration, and cartilage damage in mice treated with sinulariolide, with corresponding tissue scores lower than those of the vehicle control mice (Figure 4C and D). Furthermore, Safranin-O staining also indicated that the sinulariolide-treated CIA mice exhibited less cartilage damage (Supplementary Figure 1). These results suggested that sinulariolide treatment alleviated arthritis symptoms and joint tissue degeneration in mice with CIA.
Sinulariolide Suppressed the Production of Inflammatory Cytokines in the Hind Limbs of Mice with CIA
To further investigate the effect of sinulariolide on the immune response in mice with CIA, we analyzed the cytokine profiles of sinulariolide-treated and untreated mice. On day 42, after the conclusion of the experiment, we uniformly processed the hind paw samples from the mice to obtain supernatants for ELISA. Compared with mice treated with the vehicle, mice with CIA treated with sinulariolide (Figure 5) exhibited significantly reduced levels of proinflammatory cytokines and chemokines, such as TNF-α, IL-6, IL-1β, and IL-8, as well as Th17-associated cytokine (IL-17A) and Th1-associated cytokine (interferon-gamma (IFN-γ)).
Discussion
FLS plays a crucial role in the development of RA-related synovial inflammation and can contribute to cartilage damage and non-osseous support structures. Our study demonstrates that sinulariolide can effectively suppress proinflammatory responses in FLS without causing significant cytotoxicity. We observed a remarkable anti-inflammatory effect on active RA-FLS, as evidenced by reduced levels of IL-6, IL-1β, and IL-8 in cell cultures. Sinulariolide’s ability to inhibit the inflammatory response of RA-FLS was dose-dependent in vitro, and this immune modulation occurred through the MAPK pathway by inhibiting p38, JNK, and NF-κB pathway.
Active arthritis and synovitis with progressive bone destruction are usually observed during chronic RA progression. Synovial inflammation-related invasive hyperplastic tissue that forms a pannus develops in the joints. FLS play dual roles as passive immune responders to inflammatory signals and immunoreactive response after activation.33 Increased infiltration of FLS with high expression of inflammatory cytokines and chemokines has been observed in synovial joints with RA.34,35 Adequate immunomodulatory function and activity of RA-FLS can control joint inflammation or destruction.36 A reduction in FLS in RA can develop following anti-inflammatory therapy.37,38 Our study showed that sinulariolide can reduce the activity of TNF-α induced active FLS. Significant reductions in NO and PGE2 levels were observed after sinulariolide therapy (Figure 3A and B). Moreover, proinflammatory cytokine levels decreased in RA-FLS. In our two mouse models of arthritis, significant improvements in arthritis and histological findings were observed after treatment with sinulariolide. Abnormally high expression of MAPK in the synovial tissue of joints with RA is associated with synovial proliferation and joint destruction.36,39 Active proliferation and migration of RA-FLS may progress via the MAPK pathway.40 MAPK inhibitors induce apoptosis by downregulating cyclooxygenase-2 expression and activity in human RA-FLS.41 Significant inhibition of MAPK due to reduced phosphorylation of p-JNK and p38 was observed in our study (Figure 4A). In a previous study, sinulariolide was shown to inhibit gastric cancer cell migration and invasion by downregulating the MAPK signaling pathway.28 Sinulariolide significantly inhibits hepatocellular carcinoma cell migration and invasion in cell culture, and this reduction in metastasis was mediated by the suppression of MAPK.25 From the epigenomic analysis of RA-FLS, NF-κB activation serves as one of TNF-α induced inflammatory genes.42 In our study, the significant inhibition of NF-κB activity was dose-dependent (Figure 4B). The regulation of the MAPK signaling pathway plays a major role in the immunomodulatory effects of sinulariolide. From different studies on the pathogenesis of RA, apoptosis of RA-FLS with reduced proinflammatory factors can progress through the MAPK signaling pathways.39,43,44
Network pharmacology analysis further supports our findings, as it identified 438 common genes between the RA-related and Sinulariolide-related gene sets (Supplementary Figure 2). This suggests that Sinulariolide may target molecular pathways involved in the pathogenesis of RA. The analysis revealed several key signaling pathways related to inflammation and immune response, including the T cell receptor signaling pathway, TNF signaling pathway, MAPK signaling pathway, IL-17 signaling pathway, and NF-κB signaling pathway. These pathways are critical in the development and progression of RA, indicating that Sinulariolide could potentially modulate these signaling mechanisms to exert its anti-inflammatory effects.
Our study showed that sinulariolide may inhibit RA-FLS activity via the MAPK signaling pathway. Treatment with 11-epi-sinulariolide acetate exerts anti-inflammatory effects and suppresses the progression of bone destruction in rats with adjuvant-induced arthritis.30 This finding of arthritis control was observed in the two mouse models of arthritis in our study. Significant improvements in joint-related histological inflammation, including synovial hyperplasia, inflammatory cell infiltration, and cartilage damage, were observed in vitro after sinulariolide therapy. From the peripheral serum of mice, expressions of proinflammatory mediators, including TNF-α, IL-6, IL-1β, IL-8, IL-17A, and IFN-γ decreased after sinulariolide therapy. Our study supports the anti-inflammatory effect of sinulariolide by inhibiting RA-FLS in vivo.
Conclusions
Sinulariolide, a natural compound, demonstrated the ability to effectively manage active synovitis and arthritis in two mouse models of RA. Our study revealed a significant reduction in RA-FLS activity. The anti-inflammatory effects of sinulariolide were achieved by inhibiting the MAPK and NF-κB pathways in RA-FLS. Sinulariolide, a marine-life-derived compound, holds promise as a potential therapeutic agent for the treatment of RA.
Abbreviations
RA, rheumatoid arthritis; RA-FLS, rheumatoid arthritis fibroblast-like synoviocytes; TNF-α, tumor necrosis factor-alpha; IL, interleukin; FLS, fibroblast-like synoviocytes; RT-PCR, real time-polymerase chain reaction; ELISA, enzyme‐linked immunosorbent assay; PGE2, prostaglandin E2; NO, nitric oxide; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; CII, bovine type II collagen; CCK-8, cell counting kit-8.
Data Sharing Statement
The data of this study were collected from Taichung Armed forces General Hospital, National Chung Hsing University and Tri-Service General Hospital in Taiwan.
Ethical Approval and Consent to Participate
The study was approved by the National Chung Hsing University Animal Ethics Committee (NCHU-IACUC No. 110-047). All animal studies were conducted in accordance with the guidelines and regulations of the Laboratory Animal Care Center of National Chung Hsing University.
Consent for Publication
The authors agree with the publication of this paper.
Acknowledgments
This study received assistance of the Department of Medical Education and Research, Taichung Armed Forces General Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Taichung Armed Forces General Hospital (TCAFGH-E-112048 and TCAFGH-D-112015), the Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (Grant Number: TCMF-A-108-1(113)), and partially by the National Science and Technology Council, R.O.C. (Grant Number: 111-2314-B-30).
Disclosure
The authors declare that they have no competing interests.
References
1. Chauhan K, Jandu JS, Brent LH, Al-Dhahir MA. Rheumatoid arthritis. Treasure Island (FL): StatPearls; 2024.
2. Perricone C, Shoenfeld Y. Mosaic of autoimmunity- The novel factors of autoimmune diseases revisited. Harefuah. 2020;159:57–60.
3. Salehi S, Mahmoudinezhad Dezfouli SM, Azadeh H, Khosravi S. Immune dysregulation and pathogenic pathways mediated by common infections in rheumatoid arthritis. Folia Microbiol. 2023;68:325–335. doi:10.1007/s12223-023-01036-0
4. Jang S, Kwon EJ, Lee JJ. Rheumatoid arthritis: pathogenic roles of diverse immune cells. Int J Mol Sci. 2022;23:905. doi:10.3390/ijms23020905
5. Wu F, Gao J, Kang J, et al. B cells in rheumatoid arthritis: pathogenic mechanisms and treatment prospects. Front Immunol. 2021;12:750753. doi:10.3389/fimmu.2021.750753
6. Mateen S, Zafar A, Moin S, Khan AQ, Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta. 2016;455:161–171. doi:10.1016/j.cca.2016.02.010
7. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi:10.1038/nri2094
8. Mousavi MJ, Karami J, Aslani S, et al. Transformation of fibroblast-like synoviocytes in rheumatoid arthritis; from a friend to foe. Auto Immun Highlights. 2021;12:3. doi:10.1186/s13317-020-00145-x
9. Tsuchiya H, Ota M, Fujio K. Multiomics landscape of synovial fibroblasts in rheumatoid arthritis. Inflamm Regen. 2021;41:7. doi:10.1186/s41232-021-00157-8
10. Mahmoud DE, Kaabachi W, Sassi N, et al. The synovial fluid fibroblast-like synoviocyte: a long-neglected piece in the puzzle of rheumatoid arthritis pathogenesis. Front Immunol. 2022;13:942417. doi:10.3389/fimmu.2022.942417
11. Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi:10.1111/j.0105-2896.2009.00859.x
12. Cheng L, Wang Y, Wu R, et al. New insights from single-cell sequencing data: synovial fibroblasts and synovial macrophages in rheumatoid arthritis. Front Immunol. 2021;12:709178. doi:10.3389/fimmu.2021.709178
13. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021;73:924–939. doi:10.1002/acr.24596
14. Mian A, Ibrahim F, Scott DL. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol. 2019;3:42. doi:10.1186/s41927-019-0090-7
15. Sharma R, Sharma CL, Mahajan A. Biological agents targeting beyond TNF-alpha. Ind J Crit Care Med. 2008;12:181–189. doi:10.4103/0972-5229.45079
16. Skartsis N, Peng Y, Ferreira LMR, et al. IL-6 and TNFalpha drive extensive proliferation of human Tregs without compromising their lineage stability or function. Front Immunol. 2021;12:783282. doi:10.3389/fimmu.2021.783282
17. Mease PJ, Stryker S, Liu M, et al. Treatment patterns in rheumatoid arthritis patients newly initiated on biologic and conventional synthetic disease-modifying antirheumatic drug therapy and enrolled in a North American clinical registry. Arthritis Res Ther. 2021;23:236. doi:10.1186/s13075-021-02599-4
18. Singh JA. Treatment guidelines in rheumatoid arthritis. Rheum Dis Clin North Am. 2022;48:679–689. doi:10.1016/j.rdc.2022.03.005
19. Ghosh S, Sarkar T, Pati S, Kari ZA, Edinur HA, Chakraborty R. Novel bioactive compounds from marine sources as a tool for functional food development. Front Mar Sci. 2022;9. doi:10.3389/fmars.2022.832957
20. Kang HK, Lee HH, Seo CH, Park Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar Drugs. 2019;17. doi:10.3390/md17060350
21. Tursch B, Braekman JC, Daloze D, Hérin M, Karlsson R, Losman D. Chemical studies of marine invertebrates—XI. Tetrahedron. 1975;31:129–133. doi:10.1016/0040-4020(75)85006-X
22. Wu -Y-Y, Hsieh -C-C, Li C-Y, et al. Natural cembrane diterpenoids from the soft coral Sinularia querciformis. Nat Prod Commun. 2021;16:1934578X211059299. doi:10.1177/1934578X211059299
23. Hsiao KY, Wu YJ, Liu ZN, Chuang CW, Huang HH, Kuo SM. Anticancer effects of sinulariolide-conjugated hyaluronan nanoparticles on lung adenocarcinoma cells. Molecules. 2016;21:297. doi:10.3390/molecules21030297
24. Chen YJ, Su JH, Tsao CY, et al. Sinulariolide induced hepatocellular carcinoma apoptosis through activation of mitochondrial-related apoptotic and PERK/eIF2alpha/ATF4/CHOP pathway. Molecules. 2013;18:10146–10161. doi:10.3390/molecules180910146
25. Wu YJ, Neoh CA, Tsao CY, Su JH, Li HH. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/−9 through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci. 2015;16:16469–16482. doi:10.3390/ijms160716469
26. Cheng TC, Din ZH, Su JH, Wu YJ, Liu CI. Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/−9 and urokinase through the PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar Drugs. 2017;15. doi:10.3390/md15080238
27. Neoh CA, Wang RY, Din ZH, et al. Induction of apoptosis by sinulariolide from soft coral through mitochondrial-related and p38MAPK pathways on human bladder carcinoma cells. Mar Drugs. 2012;10:2893–2911. doi:10.3390/md10122893
28. Wu YJ, Lin SH, Din ZH, Su JH, Liu CI. Sinulariolide inhibits gastric cancer cell migration and invasion through downregulation of the EMT process and suppression of FAK/PI3K/AKT/mTOR and MAPKs signaling pathways. Mar Drugs. 2019;17. doi:10.3390/md17120668
29. Li HH, Su JH, Chiu CC, et al. Proteomic investigation of the sinulariolide-treated melanoma cells A375: effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade. Mar Drugs. 2013;11:2625–2642. doi:10.3390/md11072625
30. Lin YY, Jean YH, Lee HP, et al. A soft coral-derived compound, 11-epi-sinulariolide acetate suppresses inflammatory response and bone destruction in adjuvant-induced arthritis. PLoS One. 2013;8:e62926. doi:10.1371/journal.pone.0062926.
31. Seeuws S, Jacques P, Van Praet J, et al. A multiparameter approach to monitor disease activity in collagen-induced arthritis. Arthritis Res Ther. 2010;12:R160. doi:10.1186/ar3119.
32. Nejatbakhsh Samimi L, Farhadi E, Tahmasebi MN, Jamshidi A, Sharafat Vaziri A, Mahmoudi M. NF-κB signaling in rheumatoid arthritis with focus on fibroblast-like synoviocytes. Autoimmun Highlights. 2020;11. doi:10.1186/s13317-020-00135-z
33. Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi:10.1038/nrrheum.2012.190
34. Murayama MA, Shimizu J, Miyabe C, Yudo K, Miyabe Y. Chemokines and chemokine receptors as promising targets in rheumatoid arthritis. Front Immunol. 2023;14:1100869. doi:10.3389/fimmu.2023.1100869
35. Alunno A, Carubbi F, Giacomelli R, Gerli R. Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets. BMC Rheumatol. 2017;1:3. doi:10.1186/s41927-017-0001-8
36. Tong Y, Li X, Deng Q, Shi J, Feng Y, Bai L. Advances of the small molecule drugs regulating fibroblast-like synovial proliferation for rheumatoid arthritis. Front Pharmacol. 2023;14:1230293. doi:10.3389/fphar.2023.1230293
37. Liu Y, Rao P, Qian H, et al. Regulatory fibroblast-like synoviocytes cell membrane coated nanoparticles: a novel targeted therapy for rheumatoid arthritis. Adv Sci. 2023; 10:e2204998. doi:10.1002/advs.202204998.
38. Tu J, Hong W, Zhang P, Wang X, Körner H, Wei W. Ontology and function of fibroblast-like and macrophage-like synoviocytes: how do they talk to each other and can they be targeted for rheumatoid arthritis therapy? Front Immunol. 2018;9:1467. doi:10.3389/fimmu.2018.01467
39. Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–916. doi:10.1136/ard.2007.074278
40. Liu F, Feng XX, Zhu SL, et al. Sonic hedgehog signaling pathway mediates proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via MAPK/ERK signaling pathway. Front Immunol. 2018;9:2847. doi:10.3389/fimmu.2018.02847
41. Liagre B, Leger DY, Vergne-Salle P, Beneytout JL. MAP kinase subtypes and Akt regulate diosgenin-induced apoptosis of rheumatoid synovial cells in association with COX-2 expression and prostanoid production. Int J Mol Med. 2007;19:113–122. doi:10.3892/ijmm.19.1.113
42. Loh C, Park SH, Lee A, Yuan R, Ivashkiv LB, Kalliolias GD. TNF-induced inflammatory genes escape repression in fibroblast-like synoviocytes: transcriptomic and epigenomic analysis. Ann Rheum Dis. 2019;78:1205–1214. doi:10.1136/annrheumdis-2018-214783
43. Zhang Q, Liu J, Zhang M, et al. Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules. 2019;9. doi:10.3390/biom9120795
44. Han Y, Wang J, Jin M, Jia L, Yan C, Wang Y. Shentong Zhuyu decoction inhibits inflammatory response, migration, and invasion and promotes apoptosis of rheumatoid arthritis fibroblast-like synoviocytes via the MAPK p38/PPARγ/CTGF pathway. BioMed Res Int. 2021;2021:6187695. doi:10.1155/2021/6187695
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.