Back to Journals » Journal of Multidisciplinary Healthcare » Volume 18
Sleep and Sugar: Deciphering the 2003–2023 Research Landscape on Sleep Disorders and Diabetes Mellitus via Bibliometric Study
Authors Jiang Z , Tan J, Zhou Z, Zheng H, Li Y, Wang H, Yang Q, Tian H, Chen H, Xie J, Li Z , Chen Y
Received 13 November 2024
Accepted for publication 10 June 2025
Published 7 July 2025 Volume 2025:18 Pages 3859—3875
DOI https://doi.org/10.2147/JMDH.S506219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jacqueline Dunbar-Jacob
Ziquan Jiang,* Jianhui Tan,* Zhongming Zhou, Huihui Zheng, Yanpo Li, Haiting Wang, Qiuping Yang, Huiting Tian, Haolin Chen, Jiayi Xie, Zhiyang Li, Yexi Chen
Department of Thyroid, Breast and Hernia Surgery, General Surgery, The Second Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, 515041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yexi Chen, Department of Thyroid, Breast and Hernia Surgery, General Surgery, The Second Affiliated Hospital of Shantou University Medical College, No. 69 North Dongxia Road, Shantou, Guangdong, 515041, People’s Republic of China, Tel +8613509880123, Email [email protected]
Objective: This study aims to provide a bibliometric analysis of the research landscape on sleep disorders and diabetes mellitus, exploring the volume and impact of scholarly publications, identifying leading contributors, and discerning emerging trends and hotspots within this field.
Methods: Utilizing the Web of Science™ Core Collection, we conducted a detailed search and analysis of publications related to sleep disorders and diabetes from January 2003 to December 2023. Tools like CiteSpace and VOSviewer were employed for data visualization, highlighting the contributions of authors, institutions, countries, and journals.
Results: We identified 816 publications, with a notable increase in research output over time. The analysis revealed significant contributions from the USA and prominent institutions like Johns Hopkins University. Critical research hotspots include the bidirectional relationship between sleep disorders and diabetes, pathophysiological mechanisms, and the impact of targeted interventions on disease management.
Conclusion: The growing body of literature underscores the complex interplay between sleep disorders and diabetes mellitus, pointing to a need for further investigation into their bidirectional relationships, underlying mechanisms, and the efficacy of interventions. This study offers a roadmap for future research, suggesting an integrated approach to understanding and managing these conditions.
Keywords: bibliometric analysis, sleep disorders, diabetes, sleep quality, metabolic diseases, insulin resistance
Introduction
Diabetes is an epidemic with an increasingly high incidence rate. The number of individuals diagnosed with diabetes—characterized by fasting plasma glucose levels of ≥7.0 mmol/L, HbA1c of ≥6.5% (48 mmol/mol), or a 2-hour plasma glucose level of ≥11.1 mmol/L during an oral glucose tolerance test (OGTT)—is projected to surge, posing significant threats globally.1–3 Type 2 diabetes mellitus (T2DM) accounts for the majority of diabetes cases, approximately 96.0% (95% UI 95.1–96.8).4 According to the Global Diabetes Report, factors contributing to the sharp rise in prevalence include ethnicity, family history of diabetes, lack of exercise, aging, overweight, or obesity.3 Evidence suggests that sleep disorders should also be considered a risk factor for T2DM, as, besides leading to some of the aforementioned risk factors, sleep disorders appear to be independently associated with diabetes.5–7
The International Classification of Sleep Disorders categorizes sleep disorders into six types: Insomnia Disorders, Sleep-Related Breathing Disorders, Central Disorders of Hypersomnolence, Circadian Rhythm Sleep-Wake Disorders, Parasomnias, Sleep-Related Movement Disorders.8 Research over the past decade indicates that sleep disorders not only affect cognitive function and daily performance9,10 but may also increase the risk of cardiovascular diseases and mortality.11,12 Moreover, there is a causal relationship between sleep issues, glucose regulation, and appetite control, with poor sleep quality and insufficient sleep considered significant contributors to the global increase in obesity rates and the incidence of T2DM.13–15 Research indicates that sleeping fewer than five hours per day or experiencing low-quality sleep is linked to a higher likelihood of developing T2DM, with a pooled RR of 1.48 (95% CI:1.25,1.76).16 Therefore, effective management and treatment of sleep disorders are crucial for the prevention of T2DM.
Bibliometrics, an area within information science, concentrates on the quantitative and qualitative analysis of the literature and its metric features. This method uncovers the structural distribution, relationships, and clustering within research fields, establishing itself as a primary approach for assessing the credibility, quality, and impact of scholarly works.17 In detail, it involves evaluating the contributions and influence of authors, countries/regions, institutions, subjects, and journals, alongside examining the research’s current state, trends, and emerging topics. Tools such as VOSviewer and CiteSpace, extensively utilized for bibliometric analysis and visualization, effectively display thematic content evolution, facilitating an intuitive comprehension of advancements in pertinent areas.18,19
Notably, bibliometric analysis is increasingly valuable in medicine and public health to understand research trends. Several articles have used it to reveal the relationship between diabetes and its risk factors such as Biological Clock, gut flora, Physical Activity, etc.20–22 However, the realm of research combining sleep disorders with diabetes has yet to be scrutinized through bibliometric methods.23–25 This investigation aims to close this lacuna by harnessing data from the Web of Science™ Core Collection (WoSCC) for a bibliometric study on sleep disorders and diabetes, accompanied by descriptive analysis. By examining the current status, trending topics, and visionary research directions in sleep disorders and diabetes, this study contributes to a deep understanding of the theme and furnishes direction for future research pathways.
Method
Search Strategy
To ensure a comprehensive and focused retrieval of relevant literature, we conducted searches within the Web of Science Core Collection (WoSCC). The search strategy was meticulously designed to capture publications addressing both sleep disorders and diabetes mellitus. We employed the following topic search query across all databases within WoSCC: 1: (TS = (“Dyssomnia$”)) OR TS = (“Sleep Disorder$”); 2: TS = (“diabete$”); 3: #2 AND #1, with the time frame set from January 1, 2003, to December 31, 2023, without any restrictions on language, document type, or data type.
Data Collection
The literature search was conducted to retrieve publications within a defined timeframe from 2003 to 2023. This 20-year period was chosen to provide a contemporary overview of the evolving research landscape in sleep disorders and diabetes. No restrictions were applied regarding language or geographical location to ensure a global perspective. Following the initial retrieval, we conducted a review of titles and abstracts to screen for relevance to the topic and to remove any duplicate records. All retrieved records were downloaded from WoSCC in “plain text” format, selecting the option for “Full Record and Cited References”. This export format is specifically required for compatibility and optimal analysis using VOSviewer and CiteSpace software, which were employed for subsequent bibliometric analysis and visualization.
Data Analysis
We employed CiteSpace and VOSviewer to analyze the articles’ years, authors, co-cited authors, countries, keywords, institutions, and journals. Data were compiled and analyzed in Microsoft Excel 2021, and relevant charts were created using Origin 2023 software. Additionally, in the analysis of countries/regions, literature from Taiwan, Hong Kong, and Macau was categorized under China, while the UK encompassed England, Wales, Scotland, and Northern Ireland. Synonyms within the keywords were also consolidated.
We applied social network analysis to map out the intricate web of relationships among various research elements.26 The methodology allowed for the visualization of research intensity and connectivity, with larger nodes indicating higher occurrence rates and thicker connecting lines denoting stronger relationships. This bibliometric technique sheds light on the prevailing hotspots and evolving trends within the research on sleep disorders and diabetes, providing a clear depiction of the research landscape over time and across geographies. VOSviewer was specifically used to dissect keyword co-occurrence, authors, and co-cited authors.
Lastly, CiteSpace was employed for its proficiency in bibliometric and visualization analysis, aimed at pinpointing latent trends and movements within the field.19 Through CiteSpace, we visualized the surge of keywords, effectively mapping the trajectory of the research domain. The methodological approach of our study, including the steps and tools used, is encapsulated in the schematic representation provided in Figure 1.
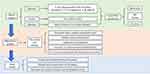 |
Figure 1 Selection of Papers and Flow Diagram for Research Framework. |
Result
Bibliometric Analysis of Publication Output
Our extensive search yielded 816 publications, classified into eight distinct categories: Article, Review Article, Meeting Abstract, Proceeding Paper, Editorial Material, Early Access, Letter, Book Chapter, and Retracted Publication. Among these, Articles emerged as the predominant category, comprising 71.81% of the total, followed by Review Articles at 22.92% (Figure 2a). The predominance of Articles and Review Articles, encompassing a total of 773 papers, indicates their comprehensive coverage of the field’s research directions and focal points. Consequently, our subsequent analysis will concentrate on these two document types to elucidate the prevailing trends and topics of interest.
 |
Figure 2 (a) Percentage representation of report types. (b) Yearly distribution of research categories and paper counts for Sleep disorder and diabetes. |
Figure 2b delineates the annual publication output within the literature, as depicted through a line graph that portrays the total number of publications per year alongside a stacked bar graph demonstrating the evolution of the distribution of the top six research categories. The period from 2000 to 2023 witnessed a pronounced escalation in publication numbers, with a noteworthy dip observed in 2014 followed by a significant resurgence in 2015, indicating a volatile yet overall upward trajectory in research interest and output. By 2023, publications reached a peak, with 98 documents published within the year, underscoring an intensified focus on diabetes and sleep disorders research.
Moreover, the stacked bar graph portion of Figure 2b accentuates the significant growth over time in the ‘Endocrinology Metabolism’, ‘Medicine General Internal’, and ‘Clinical Neurology’ categories, with the latter being the predominant category, comprising 19.24% of the total paper output. Along with these fields, ‘Public Environmental Occupational Health’, ‘Neurosciences’, and ‘Respiratory System’ are substantial contributors to the literature, collectively accounting for 49.27% of the publications.
Journals, Co-Cited Journals and Publications
Using VOSviewer, we analyzed journals and co-cited journals to identify the most active and influential journals in the field. The analysis revealed that 470 journals published papers relevant to this domain, with 19 journals publishing more than five papers each. The top ten active journals collectively published 143 papers, accounting for 18.5% of the total publications in the WoS core database. The highest-ranking journal was Sleep Medicine, which published 26 papers with an impact factor (IF) of 4.5. It was followed by PLOS ONE, Journal of Clinical Sleep Medicine, Sleep, and Sleep and Breathing. Among the top ten journals, two had an impact factor (IF) greater than 5.0. Additionally, the average number of citations per paper for each journal was calculated by dividing the total number of citations by the number of papers published. The journal Sleep published only 16 papers on sleep disorders and cancer but was cited 1777 times. Therefore, it had the highest average citation rate of 111.1 per paper, ranking first (Table 1).
Out of 7081 co-cited journals, 11 were cited more than 500 times. As shown in Table 2, Sleep had the highest number of co-citations (2081 times), followed by Sleep Medicine, Diabetes Care, and the American Journal of Respiratory and Critical Care Medicine. Among the top ten journals with the most co-citations, five had an IF exceeding 10.0 (Table 2).
 |
Table 1 The Top 10 Journals for Sleep Disorder and Diabetes Research |
 |
Table 2 The Top 10 Co-Cited Journals for Sleep Disorder and Diabetes Research |
 |
Table 3 Top 10 Cited References of Publications in Sleep Disorder and Diabetes Research |
 |
Table 4 The Top 10 Countries/Regions for Sleep Disorder and Diabetes Research |
 |
Table 5 The Top 10 Institutions for Sleep Disorder and Diabetes Research |
 |
Table 6 The Top 12 Authors for Sleep Disorder and Diabetes Research |
Table 3 summarizes the top 10 publications ranked according to total citations. Four of these publications have been cited more than 300 times, namely articles by Koren, D, Manna, P, Briancon-Marjollet, A, and Samanta, S, among others. The paper published by Koren, D et al in 2016, “Role of sleep quality in the metabolic syndrome”, has the highest number of citations, totaling 448.
Countries/Regions and Institutions
The analysis encompasses 773 papers from 79 countries and 1510 institutions. Figure 3a and Table 4 depict the quantity of literature from each country and the collaboration intensity, where larger nodes represent a greater volume of publications, and thicker connecting lines indicate stronger international collaborations. The USA leads in publication volume with 277 papers (35.83%), followed by China with 120 (15.52%), the UK with 54 (6.99%), and Italy with 50 (6.47%). Apart from African nations, connections between countries are remarkably close, with the USA maintaining collaborative ties with 51 countries/regions, notably with China (n=18), the UK (n=12), and Spain (n=11).
 |
Figure 3 (a) Volume of literature and intensity of cooperation in each country. (b) Intensity of cooperation between institutions. |
In terms of publication output, the leading institutions include Johns Hopkins University (19, 2.46%), the University of Chicago (18, 2.33%), the University of Pittsburgh (17, 2.20%), China Medical University (15, 1.94%), and the Affiliated Hospital of China Medical University (14, 1.81%). The top ten list features only two Chinese institutions, underscoring the predominance of American entities in this research domain (Figure 3b and Table 5).
Authors and Co-Cited Authors
A total of 4018 authors have contributed to research in this field. Regarding the number of published articles, 12 authors have published more than five articles each (Table 6). Kao, Chia-Hung leads with the highest number of publications (n = 10), followed by Fietze, Ingo (n = 7), Lin, Cheng-Li (n = 6), and Redline, Susan (n = 6). The table also presents the authors’ H-index, a mixed quantitative measure of a researcher’s academic achievements introduced in 2005 by Jorge E. Hirsch, a physicist at the University of California, San Diego.26 The H-index is utilized to evaluate both the quantity and impact level of a researcher’s scholarly output. In terms of the H-index, the top-ranked is Gozal, David (H-index=140), followed by Redline, Susan (H-index=129), Zee, Phyllis C. (H-index=94), and Bixler, Edward O. (H-index=81).
Using VOSviewer, authors cited at least 30 times (n = 67) were selected to construct a co-citation network map. Co-citation occurs when two or more authors are cited together by one or subsequent papers, forming a co-citation relationship. This knowledge map clearly displays the most frequently co-cited authors, with more prominent points indicating a higher frequency. Figure 4 reveals that the nodes representing Vgontzas, An, Spiegel, K, Grandner, Ma, and Knutson, Kl are the largest.
 |
Figure 4 The network map of Co-cited authors. |
Keyword Co-Occurrence, Clusters, and Evolution
Utilizing VOSviewer, we conducted a term co-occurrence and clustering analysis. Keywords provided by paper authors that appeared more than six times in the WoS core database were included in the final analysis. Among 3,399 keywords, 214 met the threshold. Figure 5a presents a visualized network of nodes and connecting lines, where node size correlates with keyword occurrence frequency, and line thickness indicates the strength of the relationship between keywords. The color of a node represents the cluster it belongs to, with different clusters indicated by different colors; there are four clusters in total. Red represents Cluster 1, yellow represents Cluster 2, green represents Cluster 3, and blue represents Cluster 4.
 |
Figure 5 Presents (a) a network visualization map and (b) an overlay highlighting the co-occurrence of keywords in sleep disorder and diabetes research. |
Cluster 1 focuses on the relationship between sleep disorders and various health outcomes, including diabetes, emphasizing metabolic diseases such as inflammation, cardiovascular diseases, and diabetes. It explores the pathophysiology mechanisms by which poor sleep quality can exacerbate or contribute to diabetes. Moreover, the frequent use of terms like “risk” “sleep” and “healthy” indicates a strong research interest in understanding how sleep impacts overall health and disease risk.
Cluster 2 delves into the connection between obstructive sleep apnea (OSA) and diabetes, particularly T2DM, highlighting OSA’s critical role in affecting glucose metabolism and oxidative stress. This cluster also examines how interventions such as Continuous Positive Airway Pressure (CPAP) can mitigate these effects, underscoring the interconnectedness of sleep disorders, diabetes management, and cardiovascular health.
Cluster 3 highlights the prevalence and association between insomnia, depression, and T2DM, stressing the importance of sleep duration and quality in diabetes management. It also emphasizes the impact of sleep disorders like Restless Legs Syndrome and sleep-disordered breathing on glycemic control, illustrating the complex interplay between sleep disorders and diabetes across different populations, including adults, women, and children.
Cluster 4 delves into the intricate connections between sleep disorders, obesity, and diabetes, highlighting insulin resistance, cardiovascular diseases, and metabolic syndrome as essential mediators. This cluster reflects the impact of sleep duration, circadian rhythms, physical activity, and dietary lifestyle factors on the development and management of diabetes and its related conditions, emphasizing the critical role of sleep quality on metabolic health and recommending targeted interventions to mitigate these risks.
Figure 5b presents an overlay visualization map displaying keywords from 2003 to 2023. The closer a node’s color is to red, the more recent the keyword’s emergence. Keywords with a color closer to blue represent those introduced before 2015, indicating that most keywords emerged in the last ten years. Among the four clusters, Clusters 2 and 4 represent relatively mature areas of research, whereas Clusters 1 and 3 have emerged more recently.
Figure 6 presents a visualization of the predominant themes within the field, with the red bars indicating periods of heightened citation activity. The initial keyword to emerge in 2010 was “apnea syndrome”. Subsequently, concerns such as “c reactive protein” and “insulin resistance” about sleep disorders, as well as “epidemiology” and “quality of life” assessments, gained traction from 2005 to 2012. The focus between 2009 and 2016 shifted towards gender-specific research (“men”) and therapeutic interventions (“positive airway pressure”). The period from 2015 to 2016 saw an increase in research related to “heart failure” and health within the “United States”. More recently, from 2018 to 2023, there has been a concentrated burst in the exploration of “validation” methods, the escalating concern of “T2DM”, and the implications of these conditions on “older adults”. Moreover, the enduring effects on “anxiety” levels and “sleep quality” have also been paramount, indicating a broader scope of interest in the interplay between chronic conditions and mental health, predominantly in the context of an aging population.
 |
Figure 6 Keyword burst map made with Citespace. |
Discussion
Analysis and Characteristics of the Papers
In the realm of sleep disorders and diabetes research, review articles constitute a substantial 22.92% of all publications, a figure that is significantly higher than that observed in other research domains. This suggests that researchers in this field tend to engage more in in-depth summarization and synthesis based on existing studies compared to other research areas. Thanks to previous research achievements, research related to sleep and diabetes is expected to further increase and deepen in the coming years, reflecting the scientific community’s growing understanding of the relationship between sleep disorders and diabetes. In the span from 2003 to 2023, the field of sleep disorders and diabetes witnessed the publication of 816 articles, highlighting a burgeoning interest in this intersection of medical research. Initially, up until 2010, the field experienced modest productivity, with annual outputs not surpassing 20 papers. This trend shifted markedly in 2011, with publication numbers entering a phase of rapid increase, notwithstanding a notable dip in 2014. This decrease is thought to be influenced by factors such as delays in the publication process, which subsequently contributed to a significant rebound in 2015, illustrating the sometimes volatile nature of academic publishing rhythms. The culmination of this upward trajectory was observed in 2023, with a peak of 98 papers.
This ascending trajectory is primarily fueled by advancements in research areas like Endocrinology Metabolism, Medicine General Internal, and Clinical Neurology. The surge in endocrinology and metabolism studies correlates with the escalating global prevalence of metabolic disorders such as diabetes.3 “Medicine General Internal” reflects a multidisciplinary expansion, likely driven by integrative approaches to complex, chronic diseases that require comprehensive medical insights.37–39 Meanwhile, the notable increase in “Clinical Neurology” publications can be attributed to the aging population, advances in neuroimaging and molecular biology, and a heightened focus on neurodegenerative diseases.40,41 Collectively, these trends underscore a shift towards addressing chronic health challenges, underpinned by technological advancements and the growing need for specialized medical knowledge in response to global health dynamics.
Analysis of Published Sources
A total of 4018 authors have engaged in research on sleep disorders and diabetes. However, only 10 authors have published more than five papers, accounting for just 0.25% of the total. The majority of authors have only published a single paper related to the topic. Statistics show that 3732 authors, or 92.88% of the total authors, have contributed to only one paper, indicating that while many researchers are involved in the field, there are few high-output authors. Only a tiny number of scholars consistently focus on this area. Kao, Chia-Hung from China is the author with the highest number of publications, and his extensive research significantly contributes to understanding the relationship between nonapnea sleep disorders and T2DM. His studies reveal that individuals with nonapnea sleep disorders are at a higher risk of developing T2DM, particularly those younger than 40 and those experiencing sleep disturbances.42 Furthermore, his work extends into the effects of hypnotic use, demonstrating that patients using zolpidem, especially in conjunction with benzodiazepines, face a significantly increased risk of T2DM.43 These findings underscore the importance of considering sleep health in T2DM prevention and management, highlighting the need for careful use of hypnotics in treating sleep disorders.
Against the backdrop of global aging, sleep disorders and diabetes represent a worldwide health issue, yet 29.11% of countries have only published one paper on the subject. Among the top 10 countries, only China and India are developing countries, with the rest being developed nations. Furthermore, African countries have almost no participation in related research, suggesting that research on sleep disorders and diabetes in developing countries lags behind that in developed nations. Overall, the global influence of developing countries in the field of sleep disorders and diabetes is limited. Consequently, developing countries should strengthen cooperation and exchange with developed nations, adopting advanced research methodologies and technologies. Such collaborative efforts are essential for advancing research on sleep disorders and diabetes, with developed countries playing a key role in supporting and facilitating this international cooperation to narrow the existing research divide. It is also important to note that our analysis, relying on the Web of Science Core Collection, may have limitations in capturing the full spectrum of global research. For instance, countries within the Commonwealth of Independent States, such as Russia and Kazakhstan, were not prominently represented in our dataset, potentially due to language barriers or indexing biases within the database. Future research could consider expanding the data sources to include databases with broader coverage of regional publications to provide a more comprehensive global perspective.
A total of 470 journals published 816 papers, with an average of 1.74 papers per journal. 357 journals published only one paper on sleep disorders and diabetes, representing 75.96% of all journals. Only 19 journals published more than five papers, accounting for 2.33% of all journals, with Sleep Medicine leading in publication volume with 26 papers. This indicates a significant imbalance in the publication of research on sleep disorders and diabetes, with only a handful of journals focusing on advancements in this field. Among the top ten most productive journals, five were categorized under Clinical Neurology, and two under Endocrinology & Metabolism. Co-citation refers to the degree of relationship between journals, occurring when two (or more) papers are cited by one (or more) subsequent papers, thereby forming a co-citation relationship.44 We observed that among the top ten most co-cited journals, four were categorized under Clinical Neurology. This observation not only aligns with prior discussions but also underscores the pivotal role these two categories play in the overarching research landscape.
Among the top ten institutions, eight are based in the USA, with the remaining two located in China. The citation rate per paper (Citations/N) is often used as a metric to represent the scientific significance or quality of a paper. However, the average citation counts for the two Chinese institutions are 18.47 and 26.12, respectively, while institutions from the USA, particularly the University of Chicago, boast an average citation count of 152.6. This indicates that papers published by the University of Chicago are highly regarded and popular among scholars, reflecting the institution’s esteemed reputation in the field. It also suggests that the quality and appeal of these publications are high, pointing to the need for continued efforts by Chinese institutions in this area of research. Gozal, D from Stanford University and Koren, D from Harvard University are the leading authors of these papers. Their publication, “Role of sleep quality in the metabolic syndrome”, greatly enriches the research surrounding sleep disorders and diabetes45 The article highlights the critical role of sleep in maintaining metabolic balance, demonstrating how changes in sleep patterns—whether due to sleep deprivation or diseases such as sleep apnea—significantly affect the risk and severity of metabolic syndrome, thereby impacting diabetes. The paper has been cited 448 times, making it the most cited article in the field, demonstrating its significant impact on the discipline.
Hotspots and Frontiers
The analysis of high-frequency keywords reflects the hotspots of specific research fields. Through co-occurrence analysis of keywords, we identified the main research directions and hotspots for sleep disorders and diabetes, revealing the evolution of their thematic structures. Based on the clustering analysis of keywords, four clusters were ultimately formed. Then, by analyzing the top 20 keywords with the most robust citation bursts, we pinpointed the research hotspots and frontiers for sleep disorders and diabetes, with the main contents as follows:
Bidirectional Relationship Between Sleep Disorders and Diabetes
Prior research has indicated that sleep disturbances can exacerbate metabolic control in individuals with Type 1 and Type 2 diabetes, thereby fostering a detrimental cycle where diabetes can subsequently induce sleep disorders.46,47 Numerous researchers have recommended that the sleep problems of diabetes patients should be taken seriously, suggesting that optimizing sleep duration and quality could enhance glycemic control. However, there is currently a scarcity of related literature. In certain small-scale studies, shortened sleep duration has been proven to increase the prevalence of T2DM or to impair the effectiveness of treatments.48,49 Future studies are needed to undertake more extensive and prolonged randomized controlled trials to clarify the impact of sleep interventions on diabetes. Additionally, while much research has focused on the effects of insufficient sleep and sleep disruptions on T2DM patients, there is a lack of studies addressing Type 1 diabetes and excessive sleepiness, such as hypersomnia. Future research should particularly concentrate on the effects of sleep disorders on Type 1 diabetes and the potential pathophysiological impacts of hypersomnia on diabetes, while also paying particular attention to the effects of these conditions on different demographic groups, including the elderly, women, and children.
Pathophysiological Mechanisms of Sleep Disorders and Diabetes Mellitus
Previous research has illuminated the mechanisms through which sleep disorders impact diabetes, highlighting the roles of sympathetic nervous system activation, hormonal imbalances, and appetite regulation disruptions. These factors contribute to glucose intolerance, insulin resistance, and β-cell dysfunction, linking sleep disturbances directly to metabolic health challenges.45,50,51 Conversely, diabetes can adversely affect sleep quality through rapid changes in blood sugar levels and hyperglycemia, leading to extended sleep latency, reduced sleep efficiency, and daytime fatigue.52 Additionally, obesity, a common factor in Type 2 diabetes, can lead to sleep-related breathing disorders such as Obstructive Sleep Apnea (OSA), exacerbating sleep disturbances.36,53 Yet, the effects of excessive sleep on diabetes remain underexplored, pointing to a significant research gap in understanding the bidirectional relationship between sleep and metabolic health.
In addition to these established connections, emerging research has begun to spotlight the role of microRNAs (miRNAs) in the interplay between sleep disorders and diabetes.54–56 For example, miRNA-17-5p and miRNA-126 have been highlighted for their roles in metabolic dysregulation and endothelial dysfunction, respectively. miRNA-17-5p is downregulated in patients with obstructive sleep apnea and is associated with increased β-cell death through upregulation of TXNIP, directly impacting the pathophysiology of diabetes.57–59 Similarly, the downregulation of miRNA-126 leads to endothelial dysfunction and diabetic retinopathy, highlighting the interconnected pathways between sleep disorders and diabetes at the molecular level.60,61 These findings underscore the potential of miRNAs as non-invasive markers for early diabetes detection and therapeutic targets, yet the direct impact of sleep disorders on miRNA expression warrants further investigation.
The intricate relationship extends to the gut microbiome’s influence on sleep and metabolic health.62–64 For instance, certain Lactobacillus and Bifidobacterium strains have been identified for their potential to improve sleep quality and metabolic outcomes, through the production of short-chain fatty acids (SCFAs) like butyrate, which modulate the gut-brain axis and inflammatory responses.65 Additionally, specific molecules such as tryptophan, which is metabolized by gut bacteria into serotonin and subsequently melatonin, highlight the microbial contribution to sleep regulation mechanisms.66–68 Significantly, the role of melatonin extends to diabetes management, as observed in studies where melatonin’s influence on insulin resistance and beta-cell function comes to the fore.69,70 Specifically, melatonin receptors MT1 and MT2 have been linked to glucose regulation, where impaired melatonin signaling due to sleep disturbances can exacerbate or contribute to the onset of type 2 diabetes by disrupting insulin secretion and increasing insulin resistance.71 Despite these advances, the field remains in its infancy, with a need for more detailed studies on the causal relationships between specific microbial species, their metabolic products, and sleep health.
Impact of Sleep Disorder Interventions on Diabetes Management
Current interventions for sleep disorders include exercise therapy, cognitive behavioral therapy (CBT), surgical treatments, device-based therapies (such as CPAP), and pharmacological treatments, offering various approaches to managing the intricate relationship between sleep disorders and diabetes.4,72–75 Some studies have reported improvements in insulin sensitivity and glucose control in obese patients with severe Obstructive Sleep Apnea (OSA) following CPAP treatment.76,77 However, findings have been inconsistent, particularly among patients with manifest diabetes, possibly due to variations in disease stage, CPAP usage patterns, and patient adherence.72,78,79 Similarly, pharmacological interventions such as suvorexant, liraglutide, and SGLT2 inhibitors have shown potential benefits for sleep disorder and diabetes management, yet their cumulative effects warrant further investigation.80–82 The current body of evidence calls for more extensive, randomized clinical trials with long-term follow-ups to assess the direct effects of various treatments on sleep disorders and their metabolic consequences. Such studies should strive for rigorous control of confounding factors, including treatment adherence and duration, to elucidate the causal pathways and potential differential effects based on diabetes status (prediabetes vs overt diabetes). Investigating the synergistic effects of combined interventions on sleep quality and metabolic health could offer novel insights into integrated management approaches for these interrelated conditions.
The study of sleep disorders and diabetes holds profound implications for practical clinical applications and research. By unraveling the intricate mechanisms underlying these conditions, we can develop more effective, personalized interventions, improving patient outcomes. Understanding this relationship deepens our insights into the systemic nature of these diseases, guiding the development of integrated treatment strategies that address both sleep and metabolic health. Furthermore, this knowledge informs public health strategies aimed at preventing the onset and progression of diabetes through sleep hygiene and intervention programs. Ultimately, advancing this field of study not only contributes to our scientific knowledge but also paves the way for novel therapeutic avenues, enhancing the quality of life for individuals affected by sleep disorders and diabetes.
Comparison with Other Bibliometric Studies
When contextualized alongside recent bibliometric analyses in diabetes research, this study reveals distinct mechanistic pathways underlying sleep-disordered metabolism. Huang et al (2022) highlighted exercise modalities (eg, aerobic and resistance training) as central to improving insulin sensitivity, whereas our analysis identifies circadian misalignment and sleep fragmentation as independent risk factors exacerbating glycemic dysregulation—a bidirectional relationship less emphasized in physical activity-focused reviews.20 Similarly, studies on diabetes-biological clock interactions and gut microbiota prioritized molecular pathways or microbial metabolites, respectively, whereas our work underscores the clinical relevance of sleep architecture alterations in metabolic homeostasis.21,22 These comparative insights reinforce the necessity of integrating sleep health into multidisciplinary diabetes management frameworks, complementing existing lifestyle and microbiome-targeted strategies.
Limitation
Our bibliometric analysis, primarily utilizing articles from the WoSCC database, encounters notable limitations. The exclusive use of WoSCC could result in the omission of significant studies published in non-SCI journals or other databases, potentially leaving out key insights. One of the inherent challenges with bibliometric analyses lies in their reliance on citation metrics, which do not directly measure the quality of individual research contributions. Given the temporal nature of citations, recent articles often accumulate fewer citations than their older counterparts, not necessarily reflecting their relevance or impact but rather the shorter timeframe they have been available for citation. This aspect can introduce biases in the analysis, potentially skewing the perceived importance or focus of recent studies. Furthermore, limitations such as the specific scope of our search criteria, database biases, and language biases may restrict the comprehensiveness of our analysis. These factors emphasize the importance of expanding the database selection and including multilingual studies in future research to achieve a more holistic view.
Conclusion
This study conducted a detailed bibliometric analysis of sleep disorders and diabetes, evaluating literature information across different years, countries, institutions, authors, and journals, and analyzed the development of themes and future research hotspots. We found that from 2003 to 2023, there has been an increasing interest in the relationship between sleep disorders and diabetes. The majority of the research outputs originated from the United States, with prominent academic institutions such as Johns Hopkins University taking a leading position. Key research hotspots have emerged, focusing on the bidirectional relationship and pathophysiological mechanisms between the conditions, underscoring the necessity for targeted intervention studies. This analysis not only maps out the current landscape but also serves as a guide for future research, indicating a pivot towards a holistic approach in managing and understanding these interlinked health issues.
Acknowledgments
Ziquan Jiang and Jianhui Tan are co-first authors for this study. We express our sincere gratitude to all members of our research team at the Department of Thyroid, Breast, and Hernia Surgery, General Surgery, The Second Affiliated Hospital of Shantou University Medical College, for their invaluable support and collaboration throughout this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Special Fund Project of Guangdong Science and Technology (210728156901524, 210728156901519), Medical Scientific Research Foundation of Guangdong Province, China (A2023481), Shantou Medical Science and Technology Planning Project (grant number, 220518116490772, 220518116490933). Their financial assistance made it possible to undertake this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Lin X, Xu Y, Pan X. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790. doi:10.1038/s41598-020-71908-9
3. Global Report on Diabetes. (2016).
4. Schipper SBJ, Van Veen MM, Elders PJM, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia. 2021;64(11):2367–2377. doi:10.1007/s00125-021-05541-0
5. Antza C, Kostopoulos G, Mostafa S, Nirantharakumar K, Tahrani A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2022;252(2):125–141. doi:10.1530/JOE-21-0155
6. Mohammadi S, Moosaie F, Saghazadeh A, Mahmoudi M, Rezaei N. Metabolic profile in patients with narcolepsy: a systematic review and meta-analysis. Sleep Med. 2021;81:268–284. doi:10.1016/j.sleep.2021.02.040
7. Khalil M, Power N, Graham E, Deschênes SS, Schmitz N. The association between sleep and diabetes outcomes – a systematic review. Diabet Res Clin Pract. 2020;161:108035. doi:10.1016/j.diabres.2020.108035
8. Darien I. International Classification of Sleep Medicine. American Academy of Sleep Medicine; 2014.
9. Dong J, Yu X, Wang Y, Zhang H, Guo R. Obstructive sleep apnea and cognition: insights gleaned from bibliometric analysis. Front Psychiatry. 2023;14. doi:10.3389/fpsyt.2023.1259251
10. Cross NE, Memarian N, Duffy SL. Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. Eur Respir J. 2018;52(1):1800740. doi:10.1183/13993003.00740-2018
11. Inoue K, Semba E, Yamakawa T, Terauchi Y. Associations of impaired glucose tolerance and sleep disorders with mortality among the US general population. BMJ Open Diabetes Res Care. 2021;9(1):e002047. doi:10.1136/bmjdrc-2020-002047
12. Wang J, Campos AI, Rentería ME, Xu L. Causal associations of sleep apnea, snoring with cardiovascular diseases, and the role of body mass index: a two-sample Mendelian randomization study. Eur J Prev Cardiol. 2023;30(7):552–560. doi:10.1093/eurjpc/zwad005
13. Liu S, Wang X, Zheng Q, Gao L, Sun Q. Sleep Deprivation and Central Appetite Regulation. Nutrients. 2022;14(24):5196. doi:10.3390/nu14245196
14. Chaput J-P, McHill AW, Cox RC. The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. 2023;19(2):82–97. doi:10.1038/s41574-022-00747-7
15. Meyer EJ, Wittert GA. Approach the Patient With Obstructive Sleep Apnea and Obesity. J Clin Endocrinol Metab. 2024;109(3):e1267–e1279. doi:10.1210/clinem/dgad572
16. Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. doi:10.1016/j.smrv.2015.10.002
17. Kokol P, Blažun Vošner H, Završnik J. Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Info Libr J. 2021;38(2):125–138. doi:10.1111/hir.12295
18. van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
19. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci. 2004;101(suppl_1):5303–5310. doi:10.1073/pnas.0307513100
20. Huang K, Zhu J, Xu S, Zhu R, Chen X. Bibliometric and Visualized Analysis of 2011–2020 Publications on Physical Activity Therapy for Diabetes. Front Med. 2022;9:1.
21. Zhang L. A bibliometric study of global trends in diabetes and gut flora research from 2011 to 2021. Front Endocrinol. 2022;13:2.
22. Zhong D, Cheng H, Li H, Kong X. A Bibliometric Study from 1992 to 2023 on the Relationship between Biological Clock and Diabetes. Endocr Metab Immune Disord Drug Targets. 2024;24:1.
23. Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes. Chest. 2017;152(5):1070–1086. doi:10.1016/j.chest.2017.05.009
24. Ogilvie RP, Patel SR. The Epidemiology of Sleep and Diabetes. Curr Diab Rep. 2018;18(10):82. doi:10.1007/s11892-018-1055-8
25. Laverty B, Puthezhath Jayanandan S, Smyth S. Understanding the relationship between sleep and quality of life in type 2 diabetes: a systematic review of the literature. J Health Psychol. 2023;28(8):693–710. doi:10.1177/13591053221140805
26. Freeman LC. Centrality in social networks conceptual clarification. Soc Networks. 1978;1(3):215–239. doi:10.1016/0378-8733(78)90021-7
27. Koren D, Dumin M, Gozal D. Role of sleep quality in the metabolic syndrome. Diabetes Metab Syndr Obes. 2016;9:281–310. doi:10.2147/DMSO.S95120
28. Manna P, Jain SKO. Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: causes and Therapeutic Strategies. Metab Syndr Relat Disord. 2015;13(10):423–444. doi:10.1089/met.2015.0095
29. Briançon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7. doi:10.1186/s13098-015-0018-3
30. Samanta S. Physiological and pharmacological perspectives of melatonin. Arch Physiol Biochem. 2022;128(5):1346–1367. doi:10.1080/13813455.2020.1770799
31. Kember AJ, Elangainesan P, Ferraro ZM, Jones C, Hobson SR. Common sleep disorders in pregnancy: a review. Front Med Lausanne. 2023;10:3.
32. Grandner MA, Alfonso-Miller P, Fernandez-Mendoza J, Shetty S, Shenoy S, Combs D. Sleep: important considerations for the prevention of cardiovascular disease. Curr Opin Cardiol. 2016;31(5):551–565. doi:10.1097/HCO.0000000000000324
33. Hariri A, Mirian M, Zarrabi A. The circadian rhythm: an influential soundtrack in the diabetes story. Front Endocrinol. 2023;14. doi:10.3389/fendo.2023.1156757
34. Cardinali DP, Pagano ES, Scacchi Bernasconi PA, Reynoso R, Scacchi P. Disrupted chronobiology of sleep and cytoprotection in obesity: possible therapeutic value of melatonin. Neuro Endocrinol Lett. 2011;32(5):588–606.
35. von Deneen KM, Garstka MA. Neuroimaging perspective in targeted treatment for type 2 diabetes melitus and sleep disorders. Intelligent Medicine. 2022;2(4):209–220. doi:10.1016/j.imed.2022.05.003
36. Meyer EJ, Wittert GA. Approach the Patient With Obstructive Sleep Apnea and Obesity. J Clin Endocrinol Metab. 2024;109(3):e1267–e1279.
37. Ioja S, Weir ID, Rennert NJ. Relationship between Sleep Disorders and the Risk for Developing Type 2 Diabetes Mellitus. Postgrad Med. 2012;124(4):119–129. doi:10.3810/pgm.2012.07.2573
38. Bargues-Navarro G, Ibáñez-del Valle V, El Mlili N, Cauli O. Salivary Biomarkers Associated with Psychological Alterations in Patients with Diabetes: a Systematic Review. Medicina. 2022;58(8):1091. doi:10.3390/medicina58081091
39. Fernández-Torres R, Ruiz-Muñoz M, Pérez-Belloso AJ, García-Romero J, Gónzalez-Sánchez M. Is There an Association between Sleep Disorders and Diabetic Foot? A Scoping Review. J Clin Med. 2021;10(11):2530. doi:10.3390/jcm10112530
40. Gaines J, Vgontzas AN, Fernandez-Mendoza J, Bixler EO. Obstructive sleep apnea and the metabolic syndrome: the road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med Rev. 2018;42:211–219. doi:10.1016/j.smrv.2018.08.009
41. Olivares MJ, Toledo C, Ortolani D. Sleep dysregulation in sympathetic-mediated diseases: implications for disease progression. Sleep. 2022;45(11). doi:10.1093/sleep/zsac166
42. Lai Y-J, Lin C-L, Lin M-C. Population-based cohort study on the increase in the risk for type 2 diabetes mellitus development from nonapnea sleep disorders. Sleep Med. 2013;14(9):913–918. doi:10.1016/j.sleep.2013.03.024
43. Lin C-L, Yeh M-C, Harnod T, Lin C-L, Kao C-H. Risk of Type 2 Diabetes in Patients With Nonapnea Sleep Disorders in Using Different Types of Hypnotics. Medicine. 2015;94(38):e1621. doi:10.1097/MD.0000000000001621
44. Small H. Co‐citation in the scientific literature: a new measure of the relationship between two documents. Journal of the American Society for Information Science. 1973;24(4):265–269. doi:10.1002/asi.4630240406
45. Gozal D, Dumin M, Koren D. Role of sleep quality in the metabolic syndrome. Diabetes Metab Syndr Bes Volume. 2016;9:281–310.
46. Song Y, Chang Z, Song C, et al. Association of sleep quality, its change and sleep duration with the risk of type 2 diabetes mellitus: findings from the English longitudinal study of ageing. Diabetes Metab Res Rev. 2023;39(6). doi:10.1002/dmrr.3669.
47. Chattu V, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare. 2018;7(1):1. doi:10.3390/healthcare7010001
48. Tuomilehto H, Peltonen M, Partinen M. Sleep Duration, Lifestyle Intervention, and Incidence of Type 2 Diabetes in Impaired Glucose Tolerance. Diabetes Care. 2009;32(11):1965–1971. doi:10.2337/dc08-1980
49. Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial Impact of Sleep Extension on Fasting Insulin Sensitivity in Adults with Habitual Sleep Restriction. Sleep. 2015;38(5):707–715. doi:10.5665/sleep.4660
50. Greenlund IM, Carter JR. Sympathetic neural responses to sleep disorders and insufficiencies. Am J Physiol Heart Circ Physiol. 2022;322(3):H337–H349. doi:10.1152/ajpheart.00590.2021
51. Mosavat M, Mirsanjari M, Arabiat D, Smyth A, Whitehead L. The Role of Sleep Curtailment on Leptin Levels in Obesity and Diabetes Mellitus. Obes Facts. 2021;14(2):214–221. doi:10.1159/000514095
52. Zhu B, Abu Irsheed GM, Martyn-Nemeth P, Reutrakul S. Type 1 Diabetes, Sleep, and Hypoglycemia. Curr Diab Rep. 2021;21(12):55. doi:10.1007/s11892-021-01424-1
53. Pugliese G, Barrea L, Laudisio D. Sleep Apnea, Obesity, and Disturbed Glucose Homeostasis: epidemiologic Evidence, Biologic Insights, and Therapeutic Strategies. Curr Obes Rep. 2020;9(1):30–38.
54. Karuga FF, Jaromirska J, Malicki M. The role of microRNAs in pathophysiology and diagnostics of metabolic complications in obstructive sleep apnea patients. Front Mol Neurosci. 2023;16. doi:10.3389/fnmol.2023.1208886
55. Zhang Y, Bai R, Liu C. MicroRNA single‐nucleotide polymorphisms and diabetes mellitus: a comprehensive review. Clin Genet. 2019;95(4):451–461.
56. Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9(9):513–521. doi:10.1038/nrendo.2013.86
57. Masutani H, Yoshihara E, Masaki S, Chen Z, Yodoi J. Thioredoxin binding protein (TBP)-2/Txnip and α-arrestin proteins in cancer and diabetes mellitus. J Clin Biochem Nutr. 2011;50(1):23–34. doi:10.3164/jcbn.11-36SR
58. Yan YR, Zhang L, Lin YN. Chronic intermittent hypoxia-induced mitochondrial dysfunction mediates endothelial injury via the TXNIP/NLRP3/IL-1β signaling pathway. Free Radic Biol Med. 2021;165:401–410. doi:10.1016/j.freeradbiomed.2021.01.053
59. Pan Q, Xu X, He W. Enrichment of miR-17-5p enhances the protective effects of EPC-EXs on vascular and skeletal muscle injury in a diabetic hind limb ischemia model. Biol Res. 2023;56(1):16. doi:10.1186/s40659-023-00418-5
60. Barutta F, Bruno G, Matullo G. MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta Diabetol. 2017;54(2):133–139. doi:10.1007/s00592-016-0915-4
61. He Y, Li Y, Zhai Z. Relationship of miRNA-126 and miRNA-122 expression with type 2 diabetes mellitus and related glucose metabolism parameters: a systematic review and meta-analysis. Exp Ther Med. 2022;24(5):652. doi:10.3892/etm.2022.11589
62. Lau WL, Tran T, Rhee CM, Kalantar-Zadeh K, Vaziri ND. Diabetes and the Gut Microbiome. Semin Nephrol. 2021;41(2):104–113. doi:10.1016/j.semnephrol.2021.03.005
63. Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340. doi:10.1016/j.smrv.2020.101340
64. Wang Z, Wang Z, Lu T. The microbiota-gut-brain axis in sleep disorders. Sleep Med Rev. 2022;65:101691. doi:10.1016/j.smrv.2022.101691
65. Zhang SL. Human and rat gut microbiome composition is maintained following sleep restriction. Proc Natl Acad Sci. 2017;114:4.
66. Cryan JF, O’Riordan KJ, Cowan CSM. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99(4):1877–2013. doi:10.1152/physrev.00018.2018
67. Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int J Mol Sci. 2021;22(6):2973. doi:10.3390/ijms22062973
68. Zhang Y, Bai R, Liu C. MicroRNA single‐nucleotide polymorphisms and diabetes mellitus: a comprehensive review. Clin Genet. 2019;95(4):451–461. doi:10.1111/cge.13491
69. Farid A, Moussa P, Youssef M, Haytham M, Shamy A, Safwat G. Melatonin relieves diabetic complications and regenerates pancreatic beta cells by the reduction in NF-kB expression in streptozotocin induced diabetic rats. Saudi J Biol Sci. 2022;29(7):103313. doi:10.1016/j.sjbs.2022.103313
70. Wajid F, Poolacherla R, Mim FK, Bangash A, Rutkofsky IH. Therapeutic potential of melatonin as a chronobiotic and cytoprotective agent in diabetes mellitus. J Diabetes Metab Disord. 2020;19(2):1797–1825. doi:10.1007/s40200-020-00585-2
71. Xia A-Y, Zhu H, Zhao Z-J. Molecular Mechanisms of the Melatonin Receptor Pathway Linking Circadian Rhythm to Type 2 Diabetes Mellitus. Nutrients. 2023;15(6):1406. doi:10.3390/nu15061406
72. Pugliese G, Barrea L, Laudisio D. Sleep Apnea, Obesity, and Disturbed Glucose Homeostasis: epidemiologic Evidence, Biologic Insights, and Therapeutic Strategies. Curr Obes Rep. 2020;9(1):30–38. doi:10.1007/s13679-020-00369-y
73. Alshehri MM, Alenazi AM, Alothman SA. Using Cognitive Behavioral Therapy for Insomnia in People with Type 2 Diabetes, Pilot RCT Part I: sleep and Concomitant Symptom. Behav Sleep Med. 2021;19(5):652–671. doi:10.1080/15402002.2020.1831501
74. Tsunoda T, Yamada M, Akiyama T. The Effects of Ramelteon on Glucose Metabolism and Sleep Quality in Type 2 Diabetic Patients With Insomnia: a Pilot Prospective Randomized Controlled Trial. J Clin Med Res. 2016;8(12):878–887. doi:10.14740/jocmr2754w
75. Kothari V, Cardona Z, Chirakalwasan N, Anothaisintawee T, Reutrakul S. Sleep interventions and glucose metabolism: systematic review and meta-analysis. Sleep Med. 2021;78:24–35. doi:10.1016/j.sleep.2020.11.035
76. Salord N, Fortuna AM, Monasterio C. A Randomized Controlled Trial of Continuous Positive Airway Pressure on Glucose Tolerance in Obese Patients with Obstructive Sleep Apnea. Sleep. 2016;39(1):35–41. doi:10.5665/sleep.5312
77. Martínez-Cerón E, Barquiel B, Bezos A-M. Effect of Continuous Positive Airway Pressure on Glycemic Control in Patients with Obstructive Sleep Apnea and Type 2 Diabetes. A Randomized Clinical Trial. Am J Respir Crit Care Med. 2016;194(4):476–485. doi:10.1164/rccm.201510-1942OC
78. Sivam S, Phillips CL, Trenell MI. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012;40(4):913–918. doi:10.1183/09031936.00177011
79. Weinstock TG, Wang X, Rueschman M. A Controlled Trial of CPAP Therapy on Metabolic Control in Individuals with Impaired Glucose Tolerance and Sleep Apnea. Sleep. 2012;35(5):617–625. doi:10.5665/sleep.1816
80. Furukawa S, Miyake T, Senba H. The effectiveness of dapagliflozin for sleep-disordered breathing among Japanese patients with obesity and type 2 diabetes mellitus. Endocr J. 2018;65(9):953–961. doi:10.1507/endocrj.EJ17-0545
81. Yoshikawa F, Shigiyama F, Ando Y. Chronotherapeutic efficacy of suvorexant on sleep quality and metabolic parameters in patients with type 2 diabetes and insomnia. Diabet Res Clin Pract. 2020;169:108412. doi:10.1016/j.diabres.2020.108412
82. Jiang W, Li W, Cheng J, Li W, Cheng F. Efficacy and safety of liraglutide in patients with type 2 diabetes mellitus and severe obstructive sleep apnea. Sleep Breathing. 2023;27(5):1687–1694. doi:10.1007/s11325-022-02768-y
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Gender Differences in Relation to Body Composition, Insulin Resistance, and Islet Beta Cell Function in Newly Diagnosed Diabetic or Pre-Diabetic Patients
Ma M, Jiang T, Wen Z, Zhang D, Xiu L
Diabetes, Metabolic Syndrome and Obesity 2023, 16:723-732
Published Date: 10 March 2023
The Role of the AIM2 Gene in Obesity-Related Glucose and Lipid Metabolic Disorders: A Recent Update
Zhang Y, Xuan X, Ye D, Liu D, Song Y, Gao F, Lu S
Diabetes, Metabolic Syndrome and Obesity 2024, 17:3903-3916
Published Date: 21 October 2024
Gender Differences of Visceral Fat Area to Hip Circumference Ratio for Insulin Resistance
Cao H, Huang X, Luo B, Shi W, Li H, Shi R
Diabetes, Metabolic Syndrome and Obesity 2024, 17:3935-3942
Published Date: 22 October 2024
Exploring Research Trends and Mechanisms: Maternal Diabetes and Neural Tube Defects (1991–2023)
Cao L, Xi Y, Jing Z, Bao Z, Bai B, Lian X, Zhang X, Di J, Liu F
Journal of Multidisciplinary Healthcare 2025, 18:1107-1121
Published Date: 25 February 2025
Prevention of Hepatalin-Dependent Insulin Resistance Induced by a High Sucrose Diet Using a Synergistic Combination of S-Adenosyl Methionine, Vitamin E and Vitamin C (SAMEC) in Virgin and Pregnant Sprague Dawley Rats
Lovat NEJ, Chowdhury K, Legare DJ, Lautt WW
Journal of Experimental Pharmacology 2025, 17:193-205
Published Date: 15 May 2025

