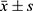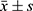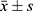Back to Journals » Journal of Inflammation Research » Volume 18
Study on the Clinical Efficacy of Combined Therapy with Minocycline Hydrochloride Ointment and Tinidazole for Chronic Periodontitis: Retrospective Study
Received 8 February 2025
Accepted for publication 21 March 2025
Published 2 April 2025 Volume 2025:18 Pages 4641—4649
DOI https://doi.org/10.2147/JIR.S514806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Qirong Wu,1,* Zhengyao Cao,2,* Sisi Wu3
1Department of Prosthetics, Wuxi Stomatological Hospital, Wuxi, 214001, People’s Republic of China; 2Department of Stomatology Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, 214023, People’s Republic of China; 3Department of Periodontics, Wuxi Stomatology Hospital, Wuxi, 214001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Sisi Wu, Email [email protected]
Objective: To analyze the clinical efficacy of combined minocycline hydrochloride and tinidazole therapy for chronic periodontitis (CP).
Methods: A retrospective analysis was conducted on clinical data from 93 CP patients admitted to our hospital from January 2021 to January 2023. All patients met the inclusion and exclusion criteria. They were divided into a control group (n=46) and an observation group (n=47). All patients received full-mouth ultrasonic debridement. On this basis, patients in the control group received weekly subgingival minocycline hydrochloride ointment (Sunstar®) injections, while patients in the observation group received combined 500 mg tinidazole tablets twice daily × 4 weeks. Periodontal parameters and gingival crevicular fluid (GCF) biomarkers were assessed at baseline and 3-month follow-up.
Results: The results showed that the total effective rate of treatment in the observation group (91.49%) was significantly higher than that in the control group (73.91%) (P< 0.05). The periodontal indicators (Plaque Index, Gingival Bleeding Index, Periodontal Pocket Depth), inflammatory factor indicators (C-reactive protein, Tumor Necrosis Factor-α, Interleukin-1β), Matrix Metalloproteinase-9 (MMP-9), and Secretory Immunoglobulin A (SIgA) levels of both groups decreased significantly after treatment. Moreover, these indicators in the observation group were significantly lower than those in the control group (P< 0.05). There was no significant difference in the incidence of adverse reactions between the two groups (P> 0.05).
Conclusion: The combination therapy of minocycline hydrochloride and tinidazole significantly improved the clinical efficacy for CP patients. Compared with minocycline hydrochloride alone, the addition of tinidazole further improved patients’ periodontal health, reduced the inflammatory response and MMP-9, SIgA levels, and did not increase the risk of adverse reactions. This suggests good drug safety and clinical promotion value.
Keywords: minocycline hydrochloride, tinidazole, chronic periodontitis, periodontal health, inflammatory factors, clinical efficacy
Introduction
Chronic periodontitis (CP) is an inflammatory disease affecting the tissues surrounding the teeth, primarily involving the interaction between dental plaque and the host immune system.1 When dental plaque accumulates at the gingival margin and is not adequately removed, bacterial metabolic by-products and toxins can trigger an inflammatory response in the host immune system, leading to gingival tissue swelling, bleeding, the formation of periodontal pockets, and alveolar bone resorption.2 If left untreated, CP can progress to periodontal disease, and in severe cases, it can lead to tooth mobility, loss, and even impact systemic health, increasing the risk of chronic diseases such as cardiovascular diseases and diabetes.3
Recent studies have identified key biomarkers associated with CP progression, including elevated levels of inflammatory mediators (C-reactive protein, TNF-α, IL-1β) and Matrix Metalloproteinase-9 (MMP-9), which contribute to tissue degradation, as well as reduced Secretory Immunoglobulin A (SIgA) reflecting impaired mucosal immunity.4–6 Clinical assessments such as Plaque Index (PLI), Gingival Bleeding Index (GBI), and Periodontal Pocket Depth (PPD) remain gold standards for evaluating therapeutic outcomes.7
Traditional treatment methods mainly include mechanical debridement and drug therapy. Mechanical debridement involves cleaning the tooth surface and subgingival scaling to remove dental plaque and calculus, serving as one of the fundamental treatments for CP.8 However, a meta-analysis by Sälzeret al revealed that mechanical debridement alone achieves only 58% PPD reduction in moderate CP cases, with 32% of patients requiring adjunctive pharmacological intervention.9
Medications also play a significant role in the treatment of CP, with minocycline hydrochloride being a broad-spectrum antibiotic with both antibacterial and anti-inflammatory properties, widely used in the treatment of CP.10 Tinidazole, on the other hand, is an antiprotozoal drug with potent antibacterial effects, effectively eliminating bacteria and parasites in gingival tissues.11 Minocycline hydrochloride has demonstrated significant anti-biofilm effects in CP treatment, with a randomized trial showing 1.8 mm PPD reduction at 6 months (vs 1.2 mm placebo; p<0.05).12 Conversely, tinidazole exhibits unique efficacy against anaerobic pathogens – a Phase III study reported 73% reduction in anaerobic bacterial load compared to baseline.13 Notably, synergistic interactions between tetracyclines and nitroimidazoles have been observed in vitro, with combination therapy achieving 99.9% biofilm eradication versus 85% with monotherapies.14 Despite these findings, clinical evidence remains scarce: a systematic review identified only two small-scale trials (total n=127) evaluating this combination, highlighting insufficient data on inflammatory biomarker modulation and long-term safety.15
Given these knowledge gaps, we hypothesize that combined minocycline-tinidazole therapy will demonstrate superior clinical efficacy over conventional approaches through dual mechanisms: 1) enhanced microbial clearance via complementary antimicrobial spectra, and 2) modulation of host inflammatory responses. To test this hypothesis, we conducted a retrospective cohort analysis evaluating both standard periodontal parameters (PLI, GBI, PPD) and molecular biomarkers (CRP, TNF-α, IL-1β, MMP-9, SIgA). The retrospective design was selected to enable analysis of longitudinal data from a patient cohort (n=93) with 6-month follow-up, addressing the statistical limitations of previous prospective studies.
Objects and Methods
Study Objects
A retrospective analysis was conducted on the clinical data of 93 patients with chronic periodontitis (CP) admitted to our hospital from January 2021 to January 2023. Diagnosis of CP followed the 2017 World Workshop Classification criteria,16 requiring: ① Periodontal pocket depth (PPD) >3 mm with clinical attachment loss (CAL) ≥2 mm at ≥2 non-adjacent teeth; ② Radiographic evidence of horizontal/vertical bone loss (assessed via periapical X-rays using Planmeca ProMax® 3D unit, exposure parameters: 66 kVp, 8 mA); ③ Bleeding on probing (BOP) ≥30% sites; ④ Tooth mobility grade ≤2 (Miller’s classification).
Inclusion criteria: ① Patients meeting the above CP diagnostic criteria, with at least 2 affected teeth; ② Patients retaining at least 20 teeth in the oral cavity; ③ Patients aged 18 years or older, of any gender; ④ Patients who had not received any CP-related treatment intervention within the recent month; ⑤ Patients with stable tooth roots (absence of root fractures or periapical lesions confirmed radiographically) in the affected area; ⑥ Patients with complete and authentic clinical data available for analysis.
Exclusion criteria: ① Exclusion of those who had received CP-related treatment interventions recently; ② Exclusion of those with severe functional impairment of vital organs; ③ Exclusion of those with abnormalities in immune function, coagulation function, hematopoietic function, etc.; ④ Exclusion of those with malignant tumor diseases; ⑤ Exclusion of those with severe infection or bleeding tendencies; ⑥ Exclusion of those allergic or contraindicated to the drugs or methods used in this study; ⑦ Exclusion of those with concomitant cognitive or consciousness disorders; ⑧ Exclusion of current smokers (>5 cigarettes/day) or tobacco users within past 6 months. Based on the treatment interventions received by the patients, they were divided into a control group (n=46) and an observation group (n=47). All patients received routine supra-gingival cleaning and subgingival scaling intervention upon admission. Patients in the control group were treated with minocycline hydrochloride ((Sunstar INC, Japan; H20100244)) on top of the aforementioned intervention, while patients in the observation group were treated with tinidazole (manufactured by Guangzhou Baiyunshan Pharmaceutical Group Co., Ltd. Baiyunshan Pharmaceutical Factory, National Drug Approval No.: H44021435, Specification: 0.5 g/tablet) in addition to the treatment received by the control group patients.
The protocol was approved by the ethics committee of Wuxi Stomatology Hospital. Informed consent was obtained from all study participants. All the methods were carried out in accordance with the Declaration of Helsinki.
Methods
Mechanical Debridement Protocol: All patients received ultrasonic scaling (EMS Piezon® Master 700, Switzerland) and Gracey curettes (Hu-Friedy, USA) for subgingival scaling. Tooth contour modification referred to selective grinding of traumatic occlusal contacts using Dental Prescale® film (Fuji, Japan). Post-debridement, subgingival irrigation was performed with alternating 3% H₂O₂ and 0.9% NaCl via blunt cannula (Vista Dental, USA).
Control Group
Both groups were treated for one month. Patients in the control group were treated with minocycline hydrochloride on top of routine interventions by injecting minocycline hydrochloride ointment (manufactured by Sunstar INC, Japan, Approval No.: H20100244) into the bottom of the periodontal pockets until slight overflow, once a week.
Observation Group
Patients in the observation group were treated with tinidazole in addition to the treatment received by the control group patients. The methods of routine intervention and minocycline hydrochloride treatment in the observation group were consistent with those in the control group, and tinidazole tablets (manufactured by Guangzhou Baiyunshan Pharmaceutical Group Co., Ltd. Baiyunshan Pharmaceutical Factory, National Drug Approval No.: H44021435, Specification: 0.5 g/tablet) were orally administered at 1.0 g/dose, once daily.
Outcome Measures
Clinical Treatment Effect
Effective: ① Complete resolution of bleeding/pain;② PPD reduction ≥2 mm; ③ Alveolar bone stability (radiographic bone level change <0.5 mm); Valid: ① ≥50% reduction in bleeding/pain severity (VAS); ② PPD reduction 1–2 mm; ③ Bone stability as above; Ineffective: After treatment, there was no significant change or even worsening of clinical symptoms, inflammation, and X-ray examination results compared to before treatment. The total effective rate of treatment = (Effective + Valid) cases / Total cases × 100%.
Periodontal Index Levels
The periodontal indices observed in this study included Plaque Index (PLI), Gingival Bleeding Index (GBI), and Periodontal Pocket Depth (PPD), evaluated before and after treatment. Evaluation criteria are as follows: ① PLI: Using a probe and visual examination, inspect the labial, buccal, and lingual surfaces near the gingival margin of the affected teeth. Score 0: No plaque; 1: Thin plaque visible, not visible to the naked eye but detectable by the probe; 2: Moderate amount of plaque visible, covering less than 2/3 of the tooth surface; 3: Plaque covering ≥2/3 of the tooth surface. ② GBI: Score 0: Gingiva healthy, no signs of inflammation such as redness or swelling; 1: Slight gingival swelling, slight redness, no bleeding on probing; 2: Gingival swelling and redness, slight bleeding on probing; 3: Gingival redness, swelling, or ulceration, bleeding upon probing. ③ PPD: Measured with Williams probe (Hu-Friedy, USA; tip diameter 0.4 mm).
Gingival Crevicular Inflammatory Parameters
Gingival crevicular fluid (GCF) collection and processing: Pre- and post-treatment GCF was collected from the mesiobuccal and distolingual sulci of the target teeth using sterile Periopaper® strips (Oraflow, USA). Strips were inserted into the sulcus until mild resistance was felt, left in place for 30 seconds, and immediately transferred to 1.5 mL Eppendorf tubes containing 200 μL phosphate-buffered saline (PBS; Thermo Fisher, USA; Cat 10010023). Centrifugation protocol: Samples were centrifuged at 3,000 × g for 15 min at 4°C (Centrifuge: Eppendorf 5430R, Germany) to separate cellular debris. The supernatant after centrifugation was aliquoted and stored at −80°C until analysis. ELISA quantification: CRP, TNF-α, and IL-1β levels were measured using Human ELISA Kits (R&D Systems, USA; Cat DCRP00/DY210/DLB50) following manufacturer protocols. Absorbance was read at 450 nm with a BioTek Synergy H1 microplate reader (Agilent, USA). Inter-assay coefficients of variation (CV): CRP <8%, TNF-α <7%, IL-1β <9% (verified through three independent experiments).
Matrix Metalloproteinase-9 (MMP-9) and Secretory Immunoglobulin A (SIgA) Levels
GCF collection: As described in Results, with duplicate strips collected for MMP-9/SIgA analysis. Sample preparation: supernatant after centrifugation (200 μL) was treated with Protease Inhibitor Cocktail (Sigma-Aldrich, USA; Cat P8340) at 1:100 dilution. Assay methodology: MMP-9 was quantified using the Human MMP-9 Quantikine ELISA Kit (Abcam, UK; Cat ab219372) with a detection range of 0.156–10 ng/mL. SIgA was measured via the Human SIgA ELISA Kit (Abcam, UK; Cat ab213968) with a detection range of 1.56–100 μg/mL. Quality control: Each plate included manufacturer-provided standards and internal controls (CV <10%). Data normalization: Analyte concentrations were normalized to total protein content measured by BCA Assay (Pierce, USA; Cat 23225).
Incidence of Adverse Reactions
Adverse reactions observed in this study included dizziness, headache, nausea, vomiting, insomnia, dizziness, skin reactions, gastrointestinal reactions, etc. The occurrence of the above adverse reactions was uniformly recorded by relevant medical staff in our hospital. Monitored via CTCAE v5.0 criteria.17
Statistical Analysis
GraphPad Prism 8 was used for graphing, and SPSS 22.0 was used for data analysis. Continuous data were described using ( ) and analyzed using the t-test; categorical data were described using n (%) and analyzed using the chi-square test. P < 0.05 was considered statistically significant.
) and analyzed using the t-test; categorical data were described using n (%) and analyzed using the chi-square test. P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
Comparison The baseline characteristics of the two groups of patients were comparable, with no significant differences observed in the comparison (P > 0.05). Refer to Table 1 for details.
 |
Comparison of Clinical Treatment Effects
The total effective rate of treatment in the observation group (91.49%) was significantly higher than that in the control group (73.91%) (P < 0.05). Refer to Table 2 for details.
 |
Table 2 Comparison of Clinical Treatment Effects [n (%)] |
Comparison of Periodontal Index Levels
As shown in Figure 1, the levels of PLI, GBI, and PPD in both groups of patients decreased significantly after treatment compared to before treatment, and the levels of PLI, GBI, and PPD in the observation group were significantly lower than those in the control group (P < 0.05).
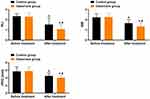 |
Figure 1 Comparison of Periodontal Index Levels ( Note: Compared to before treatment, *P < 0.05; between groups, #P < 0.05. |
Comparison of Inflammatory Factor Index Levels
As shown in Figure 2, the levels of CRP, TNF-α, and IL-1β in both groups of patients decreased significantly after treatment compared to before treatment, and the levels of CRP, TNF-α, and IL-1β in the observation group were significantly lower than those in the control group (P < 0.05).
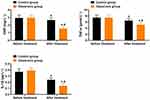 |
Figure 2 Comparison of Inflammatory Factor Index Levels ( Note: Compared to before treatment, *P < 0.05; between groups, #P < 0.05. |
Comparison of MMP-9 and SIgA Levels
As shown in Figure 3, the levels of MMP-9 and SIgA in both groups of patients decreased significantly after treatment compared to before treatment, and the levels of MMP-9 and SIgA in the observation group were significantly lower than those in the control group (P < 0.05).
 |
Figure 3 Comparison of MMP-9 and SIgA Levels ( Note: Compared to before treatment, *P < 0.05; between groups, #P < 0.05. |
Comparison of Adverse Reactions
There is no significant difference in the incidence of adverse reactions between the two groups of patients (P > 0.05). See Table 3 for details.
 |
Table 3 Comparison of Adverse Reactions [n (%)] |
Discussion
CP is not only a common oral disease but also considered a window to systemic health. Studies18 have shown that CP is closely associated with various systemic health problems, including cardiovascular diseases, diabetes, preterm birth, and low birth weight infants. This is because the gingival inflammation caused by CP can lead to the destruction of gingival tissue, allowing oral bacteria and their toxins to enter the bloodstream, thereby triggering systemic inflammation and affecting the health of other organs. Therefore, actively treating CP is crucial for maintaining oral health, preventing tooth loss, and reducing systemic health risks. Historically, the main treatment for CP in clinical practice has been mechanical cleaning, which involves dental professionals using ultrasonic or manual tools to remove dental plaque and calculus, thereby helping patients improve oral function.19 However, due to the complex and varied morphology of periodontal pockets and tooth roots, mechanical cleaning often cannot completely remove dental plaque and calculus. Moreover, this method also fails to control anaerobic bacteria in periodontal pockets, resulting in overall unsatisfactory effects in CP treatment. In recent years, with the deepening of clinical research on CP, some studies20 have attempted to use local sustained-release drugs for CP treatment. Minocycline hydrochloride ointment is one of the commonly used local sustained-release drugs. As a broad-spectrum antibiotic, it can effectively inhibit bacterial growth and reproduction without being affected by food, thus demonstrating high safety.21 However, research22 has found that the use of minocycline hydrochloride alone, while effective in inhibiting the activity of anaerobic bacteria and improving periodontal inflammation, is relatively weak in blocking bacterial DNA transcription. It struggles to completely address the rapid reproduction and spread of stubborn anaerobic bacteria, resulting in slow efficacy and inadequate outcomes. Therefore, seeking more effective treatment strategies to improve patient outcomes has become an urgent need in the current treatment of periodontal diseases.
In recent years, the combined use of multiple drugs has become a research hotspot for improving the treatment efficacy of CP. The results of this study showed that the total effective rate of treatment in the observation group (91.49%) was significantly higher than that in the control group (73.91%). The levels of PLI, GBI, and PPD in the observation group after treatment were significantly lower than those in the control group, and there was no significant difference in the incidence of adverse reactions between the two groups. These research findings are consistent with previous related studies,23,24 indicating that the combination of tinidazole with minocycline hydrochloride can further enhance the efficacy of CP treatment, improve oral health-related indicators in patients, and does not increase the risk of adverse drug reactions in patients. The observed synergistic effect likely stems from complementary antimicrobial targeting. Minocycline hydrochloride, as a tetracycline derivative, inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit.25 In contrast, tinidazole’s nitroimidazole structure generates free radicals that disrupt DNA helix stability in anaerobic pathogens.26 Crucially, our findings align with Thilagar et al (2023)27 who demonstrated in vitro that this combination reduces biofilm metabolic activity by 89% versus 67% with monotherapies, through dual inhibition of quorum sensing (lasR gene) and efflux pump (mexB gene) expression. However, studies28 have confirmed that excessive use of tinidazole can easily lead to a series of adverse reactions such as headaches, dizziness, nausea, etc.
Therefore, clinicians should master the indications for use during clinical practice and minimize the dosage of the drug as much as possible while ensuring efficacy.
CP patients generally exhibit varying degrees of inflammatory reactions, which, as the condition progresses, can lead to the entry of CRP, TNF-α, and IL-1β from gingival crevicular fluid into the patient’s bloodstream, affecting the development of the disease.29 Inflammatory stimuli can prompt the body to release large amounts of free radicals, resulting in varying degrees of oxidative stress reactions. The accumulation of oxygen free radicals damages the small blood vessels in the gingival tissues, increasing the susceptibility of the patient’s periodontal tissues, reducing immunity, promoting invasion by anaerobic bacteria and toxins. The extensive generation of oxygen free radicals can activate osteoclasts, inhibit the activity of osteoblasts, destroy periodontal tissues, and have a significant impact on the repair of periodontal tissues.30 The results of this study show that the levels of CRP, TNF-α, and IL-1β in both groups of patients decreased significantly after treatment compared to before treatment. Furthermore, the levels of CRP, TNF-α, and IL-1β in the observation group were significantly lower than those in the control group. These results suggest that the combination of minocycline hydrochloride and Tinidazole can effectively reduce the levels of inflammatory factors in CP patients. The reason for this may be that minocycline hydrochloride can eliminate oxygen free radicals, alleviate oxidative stress reactions in the body, and relieve clinical symptoms. Additionally, both minocycline hydrochloride and Tinidazole have potent antibacterial effects. Their combined use can better inhibit pathogenic bacteria in periodontal pockets, reduce stimulation to periodontal tissues, decrease the release of inflammatory factors and oxidative stress reactions, thereby alleviating inflammation damage to periodontal tissues.
MMP-9 is a member of the matrix metalloproteinase family that participates in the destruction of periodontal tissues by acting on denatured collagenase and affecting fibronectin and elastase.31 After inflammatory reactions occur in periodontal tissues, multinucleated cells and osteoclasts can produce MMP-9. MMP-9 expression is observed in both CP and aggressive periodontitis (AP), and its expression level is closely related to the development of the patient’s condition.32 SIgA is a glycoprotein that exists widely in human secretions in the form of dimers. It is synthesized by local epithelial cells of mucous membranes and plays an important role in the body’s anti-infection mechanisms.33 In CP patients, the proportions of T and B lymphocytes are in a state of imbalance, which can reduce the body’s immune function, promote infections, stimulate immune responses in oral mucosa, increase SIgA secretion, and play a protective role in the oral cavity.34 The results of this study show that the levels of MMP-9 and SIgA in both groups of patients decreased significantly after treatment compared to before treatment. Additionally, the levels of MMP-9 and SIgA in the observation group were significantly lower than those in the control group, indicating that the combination therapy of minocycline hydrochloride ointment and Tinidazole further reduced the expression levels of MMP-9 and SIgA. The reason for this may be related to the correction of patient’s inflammatory response and the restoration of normal immune function with the combined use of the two drugs, but the specific mechanism is not yet fully understood.
It is worth noting that although this study has achieved some positive results in analyzing the clinical efficacy of minocycline hydrochloride combined with Tinidazole in the treatment of CP, there are still some limitations in the study itself, such as: ① Small sample size: The sample size included in this study is relatively small, which may affect the reliability and generalizability of the study. Additionally, a small sample size may limit the statistical significance of certain results, leading to bias. ② Limitations of study design: This study is a retrospective analysis, which may involve potential information bias and treatment selection bias. Randomized controlled trials (RCTs) or prospective study designs may be more convincing. ③ Single-center study: This study was conducted in a single hospital, which may limit the external validity of the study. Differences between different medical institutions may affect the generalizability of the study results. ④ Inadequate consideration of individual differences in patients: This study did not deeply consider individual differences in patients, such as their lifestyle habits, baseline health conditions, etc., which may have some impact on treatment outcomes. In summary, although this study has presented some beneficial results, there are also some shortcomings that need to be addressed. In future research, we will strive to address these deficiencies by increasing the sample size, improving the study design, and strengthening consideration of potential influencing factors, to make the research results more reliable and comprehensive.
Conclusion
The clinical efficacy of minocycline hydrochloride combined with Tinidazole in the treatment of CP patients is significant. Compared to minocycline hydrochloride alone, the combination with Tinidazole can further improve the periodontal health of patients, reduce the inflammatory response, and levels of MMP-9 and SIgA. Furthermore, the combined use of Tinidazole did not increase the risk of adverse reactions in patients, indicating ideal drug safety and worth clinical promotion and application.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kavarthapu A, Gurumoorthy K. Linking chronic periodontitis and oral cancer: a review. Oral Oncol. 2021;121:105375. doi:10.1016/j.oraloncology.2021.105375
2. Wang RP, Ho Y-S, Leung WK, et al. Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav Immun. 2019;81:63–73. doi:10.1016/j.bbi.2019.07.002
3. Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018;130(1):98–104. doi:10.1080/00325481.2018.1396876
4. Zhang H, Liu L, Jiang C, Pan K, Deng J, Wan C. MMP9 protects against LPS-induced inflammation in osteoblasts. Innate Immun. 2020;26(4):259–269. doi:10.1177/1753425919887236
5. Agrawal S, Taneja S, Shetty D, Gopikrishna V, Bhalla VK. Evaluating the concentration of MMP-9 and TNF- α in pulpal blood at various stages of pulpal inflammation in diabetics: a cross sectional study. Europ Endodontic J. 2023;8(4):286–292. doi:10.14744/eej.2023.41736
6. Kochumon S, Al-Sayyar A, Jacob T, et al. TGF-β and TNF-α interaction promotes the expression of MMP-9 through H3K36 dimethylation: implications in breast cancer metastasis. Front Immunol. 2024;15:1430187. doi:10.3389/fimmu.2024.1430187
7. Lee TH, Chen JL, Tsai MM, et al. protective effects of sophoraflavanone G by inhibiting TNF-α-induced MMP-9-mediated events in brain microvascular endothelial cells. Int J mol Sci. 2023;25(1):283. doi:10.3390/ijms25010283
8. Sato H, Ishihata H, Kameyama Y, et al. Professional mechanical tooth cleaning method for dental implant surface by agar particle blasting. Materials. 2021;14(22):6805. doi:10.3390/ma14226805
9. Sälzer S, Graetz C, Dörfer CE, et al. Contemporary practices for mechanical oral hygiene to prevent periodontal disease. Periodontol 2000. 2020;84(1):35–44. doi:10.1111/prd.12332
10. Chen Q, Yan W, Geng N. The efficacy of minocycline hydrochloride combined with multiple antibiotic paste in elderly patients with chronic periodontitis and concomitant pulp lesions. Evid Based Complement Alternat Med. 2022;2022:7604741. doi:10.1155/2022/7604741
11. Tinidazole, in LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
12. Tsimpiris A, Tsolianos I, Grigoriadis A, Tsimtsiou Z, Goulis DG, Grigoriadis N. Association of chronic periodontitis with multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 2023;77(104874). doi:10.1016/j.msard.2023.104874
13. Martínez Nieto M, De Leon Rodríguez ML, Anaya Macias RDC, Lomelí Martínez SM. Periodontitis and chronic kidney disease: a bidirectional relationship based on inflammation and oxidative stress. World Jl Clin Cases. 2024;12(35):6775–6781. doi:10.12998/wjcc.v12.i35.6775
14. Agnihotri R, Gaur S. C3 targeted complement therapy for chronic periodontitis - a scoping review. J Inter Soc Prev Commun Dentis. 2022;12(5):500–505. doi:10.4103/jispcd.JISPCD_161_22
15. Pakarinen S, Saarela RKT, Välimaa H, et al. Home-applied dual-light photodynamic therapy in the treatment of stable chronic periodontitis (HOPE-CP)-three-month interim results. Dentistry Journal. 2022;10(11):206. doi:10.3390/dj10110206
16. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Period. 2018;89(Suppl 1):S173–S182. doi:10.1002/JPER.17-0721
17. Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–3676. doi:10.1007/s00520-016-3297-9
18. Kumar S. Evidence-based update on diagnosis and management of gingivitis and periodontitis. Dent Clin North Am. 2019;63(1):69–81. doi:10.1016/j.cden.2018.08.005
19. Heidari Z, Moudi B, Mahmoudzadeh-Sagheb H. Immunomodulatory factors gene polymorphisms in chronic periodontitis: an overview. BMC Oral Health. 2019;19(1):29. doi:10.1186/s12903-019-0715-7
20. Qiu X, Xu S, Hao Y, et al. Biological effects on tooth root surface topographies induced by various mechanical treatments. Colloids Surf B Biointerfaces. 2020;188:110748. doi:10.1016/j.colsurfb.2019.110748
21. Yang Z, Liang X, Jiang X, et al. Development and evaluation of minocycline hydrochloride-loaded in situ cubic liquid crystal for intra-periodontal pocket administration. Molecules. 2018;23(9):2275.
22. Yang C, Wang X, Wang Y. Effect of diode laser combined with minocycline hydrochloride in nonsurgical periodontal therapy: a randomized clinical trial. BMC Oral Health. 2022;22(1):71. doi:10.1186/s12903-022-02106-4
23. Miyazawa H, Nakajima T, Horimizu M, et al. Impact of local drug delivery of minocycline on the subgingival microbiota during supportive periodontal therapy: a randomized controlled pilot study. Dent J. 2020;8(4). doi:10.3390/dj8040123.
24. Yuan Y, Xu J. Application analysis of minocycline hydrochloride ointment combined with tinidazole in the treatment of chronic periodontitis. Minerva Surg. 2024;79(1):123–125. doi:10.23736/S2724-5691.21.09427-2
25. Akhondian S, Fatemi K, Ebrahim Zadeh N, et al. Necroptosis has a crucial role in the development of chronic periodontitis. J Oral Biol Craniof Res. 2023;13(4):465–470. doi:10.1016/j.jobcr.2023.05.010
26. Tan L, He Y, Wang T, Gao X, Fan W, Fan B. A Mendelian randomization study between chronic periodontitis and non-alcoholic fatty liver disease. J Periodontal Res. 2024;59(2):346–354. doi:10.1111/jre.13218
27. Thilagar S, Theyagarajan R, Mugri MH, et al. Periodontal treatment for chronic periodontitis with rheumatoid arthritis. Int Dental J. 2022;72(6):832–838. doi:10.1016/j.identj.2022.04.008
28. Pereira Sousa JC, Kogawa AC. Overview of analytical methods for evaluating tinidazole. J AOAC Int. 2023;106(2):309–315. doi:10.1093/jaoacint/qsac142
29. Van Dyke TE, Sima C. Understanding resolution of inflammation in periodontal diseases: is chronic inflammatory periodontitis a failure to resolve? Periodontol 2000. 2020;82(1):205–213. doi:10.1111/prd.12317
30. Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–440. doi:10.1038/s41577-020-00488-6
31. Arshad R, Ismail WA, Zara B, et al. Salivary MMP-9 levels in chronic periodontitis patients with type-II diabetes mellitus. Molecules. 2022;27(7). doi:10.3390/molecules27072174.
32. Li X, Lu J, Teng W, et al. Quantitative evaluation of MMP-9 and TIMP-1 promoter methylation in chronic periodontitis. DNA Cell Biol. 2018;37(3):168–173. doi:10.1089/dna.2017.3948
33. Akhi R, Nissinen AE, Wang C, et al. Salivary IgA antibody to malondialdehyde-acetaldehyde associates with mild periodontal pocket depth. Oral Dis. 2022;28(8):2285–2293. doi:10.1111/odi.13936
34. Piasetska L, Luchynskiy M, Oshchypko R, et al. THE STATE OF LOCAL IMMUNITY IN PERSONS WITH PERIODONTAL DISEASES ON A BACKGROUND OF DIFFERENT PHYCHOPHYSIOLOGICAL REACTIONS OF MALADAPTATION. Georgian Med News. 2020;(303):63–66.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.