Back to Journals » Journal of Inflammation Research » Volume 18
Study on the Influence and Mechanism of Resveratrol on Cognitive Impairment in Chronic Kidney Disease Rats Through Regulating Gut Microbiota and the TLR4/NFκB Pathway
Authors Shao B, Nong Y, Lin Y, Meng Y, Zhou Y, Huang M, Huang F, Wang J
Received 13 December 2024
Accepted for publication 16 April 2025
Published 8 May 2025 Volume 2025:18 Pages 6049—6060
DOI https://doi.org/10.2147/JIR.S510867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Binbin Shao,1 Yanfei Nong,1 Yongshuang Lin,2 Yan Meng,1 Yi Zhou,1 Meiying Huang,3 Feifan Huang,3 Jie Wang3
1Graduate School, Youjiang Medical University for Nationalities, Baise, Guangxi, 533000, People’s Republic of China; 2The First Affiliated Hospital of Guangxi University of Traditional Chinese Medicine, Nanning, 530000, People’s Republic of China; 3Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, Guangxi, 533000, People’s Republic of China
Correspondence: Jie Wang, Email [email protected]
Objective: To investigate the mechanism by which resveratrol (Res) ameliorates cognitive impairment (CI) in chronic kidney disease (CKD) rats through modulation of gut microbiota and suppression of inflammation.
Methods: A CKD model was established in rats via two intravenous injections of doxorubicin (4 mg/kg, 2 weeks apart). After 8 weeks, renal function and histopathological assessments were performed to confirm the establishment of the CKD model.Rats were divided into control, CKD, and CKD+Res groups. The CKD+Res group received intragastric Res for 6 weeks. Cognitive function was assessed using the Morris water maze. Serum Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α), and Lipopolysaccharide (LPS) levels were measured via ELISA. Histopathology evaluated kidney, colon, and hippocampal damage. Gut microbiota composition was analyzed by 16S rRNA sequencing, and hippocampal Toll-Like Receptor 4 (TLR4)/ the Nuclear Factor-κB (NFκB) pathway proteins were quantified via Western blot.
Results: CKD groups exhibited elevated 24-hour urinary albumin, serum urea nitrogen, and creatinine (P < 0.01), with glomerular atrophy. During water maze navigation (days 3– 4), CKD groups showed prolonged escape latency and increased swimming distance versus controls (P < 0.05), which Res intervention alleviated (P < 0.05). In the spatial probe test, CKD rats had fewer platform crossings and shorter target quadrant occupancy (P < 0.01; P < 0.05), both improved by Res (P < 0.05). Hippocampal neuronal damage and elevated serum IL-6, TNF-α, and LPS levels (P < 0.01) were observed in CKD rats, while Res reduced IL-6 and LPS (P < 0.05). Western blot revealed upregulated TLR4/NFκB pathway activation in the CKD group (P < 0.01), suppressed by Res (P < 0.05). Gut microbiota analysis showed increased Gram-negative bacteria in CKD rats and higher Gram-positive bacteria abundance in the Res group. LPS biosynthesis was enhanced in CKD rats (P < 0.05) but attenuated by Res.
Keywords: resveratrol, chronic kidney disease, CKD, cognitive impairment, gut microbiota, TLR4/NFκB pathway
Graphical Abstract:

Introduction
CKD is a global public health issue, and its prevalence is increasing year by year. Epidemiological data show that about 10% of adults worldwide are affected by CKD, and it has become one of the most important causes of death globally.1 Among them, 16%-18% of CKD patients have varying degrees of cognitive dysfunction, manifested as memory deficits, decreased executive function, and slowed information processing speed. It is notable that after CKD patients progress to end-stage renal disease (ESRD), the incidence of CI reaches as high as 85%.2 This neurocognitive impairment not only seriously reduces the quality of life of patients but also becomes a key challenge in interdisciplinary research due to its complexity of pathological mechanisms.
The traditional view holds that the pathological basis of CKD-related cognitive impairment mainly stems from the direct neurotoxicity caused by the accumulation of uremic toxins. However, recent research evidence indicates that the pathogenesis involves multiple systems’ interactions such as systemic inflammation, cerebral vascular remodeling, and metabolic-endocrine disorders, forming a complex “multi-organ-brain network” pathological model.3 Among various pathological mechanisms, chronic inflammatory response is considered the pivotal link connecting renal injury and neurodegeneration. Studies have found that patients with chronic kidney disease are associated with changes in cytokines such as IL-1β, IL-6, and TNF, which have been proven to be involved in the process of cognitive dysfunction. They stimulate the permeability of the blood-brain barrier and promote neural injury.4 In recent years, the role of gut-brain axis regulation in CKD cognitive impairment has attracted much attention. As renal function progressively deteriorates, the accumulation of uremic toxins can significantly alter the composition of the intestinal microbiota, resulting in a decrease in the abundance of probiotics and an increase in the proportion of pathogenic bacteria. This microecological imbalance not only disrupts the integrity of the intestinal mucosal barrier but also leads to the translocation of bacteria and their metabolites to the systemic circulation, forming a chronic persistent low-grade inflammatory state.5,6 This “intestinal-origin inflammation” penetrates the central nervous system through the blood-brain barrier and participates in the activation of glial cells and the damage of synaptic plasticity of neurons.7 Facing the multiple pathological mechanisms of CKD-related cognitive impairment, the natural polyphenolic compound resveratrol has become a research hotspot due to its multi-effect properties such as anti-inflammatory, antioxidant and regulation of intestinal flora. Studies have found that resveratrol can inhibit the TLR4/NF-κB/STAT signaling cascade reaction and has anti-inflammatory effects on microglial cell activation triggered by Aβ.8 Additionally, resveratrol mediates the repair of intestinal barrier by inhibiting the inflammatory response mediated by TLR4/MyD88/NF-κB signaling pathway, thereby improving the motor function and neuropathy of Parkinson’s disease model mice. Currently, there is a lack of research on the impact of resveratrol on improving cognitive function in CKD patients through the intestinal flora.9 It is imperative to carry out the task of early prevention and treatment of CKD-related cognitive impairment. Therefore, we aim to verify the benefits of resveratrol in preventing and treating cognitive impairment in CKD rats, and to analyze whether the multi-effect of resveratrol can achieve a synergistic protective effect in the nervous system and intestinal system, and to explore its underlying mechanism.
Materials and Methods
Experimental Animals
In this study, 22 male Wistar rats, each weighing approximately 350 g, were obtained from Changsha Tianqin Biotechnology Co., Ltd. (Production License No. SCXK(Hunan)2022–0011). The rats were kept in the Clean Animal Room (SPF) of Youjiang Medical College for Nationalities, and the animals were treated in accordance with the Guidelines for Ethical Review of Experimental Animal Welfare (GB/T35892-2018) issued by China. The ambient temperature is maintained at 25°C, free drinking water and sterile ordinary feed are provided.They had ad libitum access to drinking water and were fed a sterile, standard rodent diet. The experimental protocol was approved by the Animal Experiment Ethics Committee of Youjiang Medical College for Nationalities (Approval No. 2023122701).
Experimental Reagents
Doxorubicin Hydrochloride Injection (50 mg/vial, MedChemExpress, Batch No. HY-15142), Resveratrol (purity≥98.0%, Xi’an Xinshengyuan Biotechnology Co., Ltd. Batch No: MM229-15),Creatinine Assay Kit, Urea Nitrogen Assay Kit, and Urine Protein Quantification Kit (Nanjing Jiancheng, Batch No. C0-35-2-1). The enzyme-linked immunosorbent assay (ELISA) kit(Ruixin Biotechnology Co., Ltd. Batch No. RX302856R), the RIPA tissue lysis buffer (Beijing Suolaibao Co., Ltd. Batch No. R0020), PAGE Gel Rapid Preparation Kit (7.5%) (Yamei Company, Batch No: PG111); TLR4, NFκB, P-NFκB Antibody (ABclonal Company, Batch No. A25687, A22331, AP1294).
Establishment of CKD Model Rats
A total of 22 rats were randomly assigned to the control group (n = 6) and the model group (n = 16) by random table method. The rats in the model group were administered Adriamycin via tail vein injection twice (4 mg/kg on day 0 and again on week 2), while the control group received an equal volume of normal saline. Eight weeks after the final injection, blood samples were collected from the orbital vein to measure serum creatinine and urea nitrogen levels. A 24-hour metabolic cage was used to collect urine for proteinuria assessment. One rat from the control group and two from the model group were randomly selected for renal tissue collection, followed by histopathological analysis using H&E staining to confirm the CKD model.Following model establishment, The model rats were randomly divided into the kidney disease group (CKD) with n = 7 and the resveratrol intervention group (CKD + Res) with n = 7, and the control group (control) with n = 7 using a random number tablegroup received resveratrol (100 mg/kg/day) via gavage for six weeks, while the control and CKD groups were given the same volume of pure water by gavage.
Morris Water Maze Behavioral Experiment
The Morris water maze consisted of a circular pool with a diameter of 2.0 m and a depth of 0.5 m. Patterns (square, triangle, circle, and star) were affixed to the walls of the four quadrants. A 10 cm-diameter round platform was placed in the fourth quadrant. The water temperature was maintained at 25°C. An automatic camera system tracked the swimming trajectory of the rats, and experimental data were collected via computer software. Before the formal experiment, rats were allowed to swim freely to familiarize themselves with the maze, ensuring the elimination of stress responses and evaluation of visual acuity and swimming ability. The experiment was divided into two phases: the positioning navigation task and the spatial probe task.
Positioning Navigation Task
This task was conducted over four days. The platform was submerged 1 cm below the water surface, preventing the rats from directly seeing it. Rats were placed into the pool from one of four quadrants, facing the pool wall, and trained to locate the hidden platform, thus testing their spatial learning and memory abilities. The total swimming distance and escape latency were recorded. Each rat underwent four training sessions per day over four consecutive days. Learning and memory performance was evaluated by comparing results across different days.
Spatial Probe Task
This task was performed over two days. On the first day, the round platform was removed from the pool, and on the second day, each rat was placed in the water in the second quadrant, facing the pool wall, and allowed to swim freely for 90 seconds. Parameters recorded included the swimming trajectory, the number of platform crossings, the proportion of time spent in the target quadrant, and the proportion of distance covered in the target quadrant. The experiment was repeated on the following day to assess the spatial memory of the rats regarding the original platform location.
Tissue Collection and Histopathological Analysis
After completing the water maze task, rats were placed in metabolic cages, and 24-hour urine samples were collected. Urine volume was recorded, and proteinuria was assessed. Following anesthesia, blood was collected via cardiac puncture, left at room temperature for 1 hour, and then centrifuged to obtain serum. Serum creatinine and urea nitrogen levels were measured. Kidneys, colons, and brains were excised, fixed in 4% paraformaldehyde for 24 hours, dehydrated using an automated tissue processor, and embedded in paraffin. Tissue sections (3 μm) were cut using a microtome and subjected to H&E staining.
ELISA
ELISA was performed to quantitatively measure bacterial endotoxins (LPS) and inflammation-related cytokines (IL-6, TNF-α) in serum samples. 50 μL of serum was added to each well, followed by 100 μL of HRP-conjugated specific antibody.The plate was sealed and incubated at 37°C for 1 hour.After washing, substrate solutions A and B were added, and the plate was incubated for 15 minutes in the dark. The reaction was stopped by adding 50 μL of termination solution.Absorbance was measured at 450 nm, and a standard curve was constructed for cytokine quantification.
Western Blot Analysis
Hippocampal tissue from each group was homogenized in lysis buffer containing protease and phosphatase inhibitors. The protein concentrations were measured with the BCA Protein Assay Kit, Then, proteins (50 μg/lane) were separated by 7.5% SDS-PAGE gel and transferred to the PVDF (polyvinylidene difluoride) membranes, and blocked for 30 minutes. Membranes were incubated overnight at 4°C with primary antibodies against TLR4 (1: 1000 dilution), NF-κB (1: 1000 dilution), P-NF-κB (1: 1000 dilution) and β-actin (1: 5000 dilution). Afterward, the membranes were incubated for 1 h with secondary horseradish peroxidase-conjugated antibody at room temperature. Enhanced Chemiluminescence (ECL) reagent was applied, and chemiluminescence images were captured using an imaging system. Protein expression levels were analyzed using ImageJ software.
Gut Microbiota Sequencing
Colon tissues were collected in a sterile environment, and fecal samples were transferred to sterile tubes and stored at −80°C. After quality control, high-throughput 16S RNA gene sequencing was performed on the V3-V4 region. The data were analyzed using α-diversity and β-diversity metrics, Species difference analysis(LEfSe) and Prediction analysis of biological metabolic functional.
Statistical Analysis
Data analysis was accomplished by GraphPad Prism 10 software. Quantitative data that conformed to normal distribution were expressed as mean ± standard deviation (SD). Independent sample t-test was used for comparison between two groups, and one-way analysis of variance was used for comparison among multiple groups. P < 0.05 was considered statistically significant.
Results
Construction of CKD Model Rats
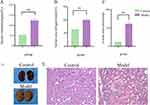
Renal Function and Pathology in the Model Group:Compared to the control group, rats in the model group exhibited significantly elevated 24-hour urinary albumin, serum urea nitrogen, and serum creatinine levels (P< 0.01)(Figure 1A–C). The kidneys of the Model group showed abnormal appearance, with irregular surfaces and pale color(Figure 1D).Histopathological examination demonstrated distinct renal morphological alterations between experimental groups. Renal tissues from control rats maintained intact glomerular architecture with normal tubular morphology. In contrast, CKD model animals exhibited extensive pathological changes, including: (1) marked tubular epithelial cell degeneration and necrosis accompanied by tubular lumen dilatation and significant loss of functional nephrons; (2) glomerular atrophy with the cystic cavities expanded; (3) prominent interstitial fibrosis with multifocal inflammatory cell infiltration. Additionally, abundant eosinophilic proteinaceous casts were observed within tubular lumens, indicative of severe proteinuria. (Figure 1E). These findings confirm the successful establishment of the CKD model.
The Effects of Res on the Cognitive Behavior and Hippocampal Region of Rats
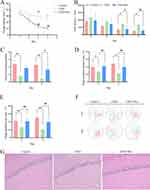
Effects of resveratrol on cognitive behavior and hippocampus in rats during the localization voyage, from the third day to the fourth day of training, the escape latency and total distance of the CKD model group were longer than those of the control group (P<0.05).Compared with the CKD group, the escape latency and total distance of the medication group were shortened (P<0.05) (Figure 2A and B). In the space exploration period, the CKD group had a significantly increased number of platform crossings (P<0.01) and a significantly decreased proportion of target quadrant distance (P<0.01) and target quadrant time (P<0.05) compared with the control group on the first and second days. Compared with the CKD group, the medication group had a relative increase in the number of platform crossings on the 6th day (P<0.05), the proportion of distance in the target quadrant(P<0.01), and the proportion of time in the target quadrant(P<0.01)(Figure 2C–E). The observation of the trajectory map of the three groups of rats in the spatial exploration experiment period showed that the rats in the control group and the medication group showed reentry activities in the central area and the target quadrant area when looking for the platform trajectory, and the frequency of accurate crossing the platform area was more common, while the rats in the CKD group showed marginal exploration when looking for the platform trajectory, and the accurate crossing the platform was less common (Figure 2F). Histopathological examination showed that the nerve cells in the control group were dense and arranged neatly; In the CKD group, the number of cells in the hippocampal tissue was slightly reduced. The arrangement of pyramidal cells was looser, with irregular gaps between cells. There were neuronal degeneration and some cell bodies were deeply stained with nuclear pyknosis.The cells in the medication group were more compact and arranged more neatly than those in the model group, and the damage of nerve cells was reduced(Figure 2G).
Effects of Res on the Intestine and Gut Microbiota of Rats
Effect of resveratrol on intestinal tract and diversity of bacterial flora in rats. Histopathological examination showed that the intestinal wall of rats in the control group was smooth, and the intestinal villi were arranged neatly and closely. In the CKD group, the colonic tissue structure was disordered, the upper end of the intestinal villi was defective, the submucosal structure was separated and thickened, the epithelial cells of the intestinal villi were rare and arranged disorderly, and inflammatory cell infiltration was observed in the interstitium. In the medication group, intestinal villus epithelial cells and goblet cells were arranged neatly, and some villi were absent(Figure 3A). 16s rRNA sequencing was performed on the colonic contents of rats in each group, and α diversity analysis showed that Chao1 (richness of the microbiota), observed_otus (number of bacterial species/strains), and Shannon (diversity and stability of the microbiota) were significantly different among the groups (P < 0.05)(Figure 3B). The results of β diversity analysis showed that there were differences in microbiota structure between groups(Figure 3C).Species difference analysis (LEfSe) showed that: There were differences in the intestinal flora of rats in each group. The relative abundance of Prevotella9, Bacteroides, Prevotella1, Helicobacter and Parabacteroides increased in the CKD group, and Gram-negative bacteria were the dominant bacteria. The relative abundance of Clostridiumsensustricto1, Bifidobacterium and Romboutsia increased in the medication group, and Gram-positive bacteria were the main dominant bacteria(Figure 3D). Through the analysis of functional prediction of the microbiota: it was found that compared with the control group, the biosynthesis function of LPS was significantly enhanced in the CKD group, while the synthetic ability in the treatment group was relatively lower.(P < 0.05) (Figure 3E).
Effects of RES on Inflammatory Responses in Rats
Resveratrol significantly attenuated systemic responses in model rats. Serum analysis revealed that animals in the CKD group exhibited markedly elevated levels of pro-inflammatory mediators, with IL-6, TNF-α and LPS concentrations significantly exceeding those in control animals (P<0.01).Resveratrol administration substantially mitigated these inflammatory alterations, reducing serum IL-6, TNF-α,and LPS levels compared to CKD group (P<0.05)(Figure 4A–C).Notably, hippocampal tissue analysis demonstrated changes in key inflammatory signaling molecules. Relative to control group, the expression levels of TLR4, NFκB and P-NFκB protein in the CKD group were increased (P<0.01). Compared with the CKD group, the protein expression of TLR4, NFκB and p-NFκB in the medication group was down-regulated (P<0.05) (Figure 4D).The coordinated downregulation of both circulating inflammatory cytokines and central nervous system inflammatory markers suggests systemic anti-inflammatory effects of resveratrol intervention.
Discussion
Cognitive impairment (CI) is a prevalent complication of chronic kidney disease (CKD), with its prevalence increasing as CKD progresses severely impacts patients’ daily functioning and their ability to perform self-care activities.10,11 CKD is recognized for its significant role in immune activation and inflammation-related mechanisms in neurological disorders.12 Recent studies have established a close relationship between chronic systemic inflammation in CKD and alterations in the gut microbiota.13 As renal function deteriorates, the accumulation of uremic toxins induces gut microbiota dysbiosis, characterized by changes in bacterial diversity and abundance.14 This imbalance leads to intestinal barrier dysfunction, increased permeability, and the translocation of gut-derived uremic toxins (GDUT) into systemic circulation, triggering a chronic inflammatory state.Increasing evidence suggests that the gut-brain axis plays a critical role in neuronal development, brain function, and cognitive regulation.15,16 Gut-derived metabolites, such as short-chain fatty acids, neurotransmitters, and their precursors, influence brain function through the circulation.17–19 Furthermore, endotoxins like LPS produced by gram-negative bacteria activate peripheral immune responses, including immune cell activation and cytokine release, which promote immune cell infiltration into the brain, triggering central nervous system (CNS) inflammation.20–22
Gut microbiota modulation is increasingly regarded as a promising therapeutic strategy for managing CKD and its complications. With growing insights into the gut microbiota-kidney-brain axis, regulating the gut microbiota may hold significant potential for the early prevention and treatment of CKD-associated neurological disorders.23 Resveratrol, a polyphenolic compound with potent anti-inflammatory and immunomodulatory properties, has been shown to protect the intestinal barrier, prevent intestinal inflammation, and modulate the gut microbiota.22,24 However, its potential role in regulating gut microbiota to improve cognitive function in CKD remains unclear.
In this study, a rat model of CKD was established using adriamycin injection, and various renal parameters, including serum creatinine, urea nitrogen, 24-hour urine protein quantification, and renal histopathology, were assessed.25 The Morris water maze (MWM) test, a classic behavioral test to evaluate learning and memory, was used to assess cognitive function in the rats.26 Our results showed that after 3 and 4 days of positioning navigation training, the CKD rats exhibited longer travel distances and latencies to locate the target platform compared to the control group, indicating a decline in their learning and memory capabilities. However, resveratrol treatment significantly improved cognitive function, as evidenced by better performance in the spatial probe test, including increased platform crossings, higher proportion of time and distance in the target quadrant, and a more accurate search pattern.
Further analysis revealed that CKD leads to a shift in the number of gram-negative bacteria in the gut.27 Bacterial LPS, a potent endotoxin produced in the outer membrane of Gram-negative bacteria, causes a chronic immune response associated with inflammation.28 Clinical studies have found that Gram-negative bacteria in the intestinal microbiota of T2DM-CKD patients are dysregulated, leading to increased serum levels of LPS.29 Immune cells recognize lipid A of LPS, which induces a series of inflammatory responses, produces microbial-specific molecular signals that bind to immune cell surface receptor complexes including TLR4, and induces the activation of NF-κB, which plays a key role in the signaling transduction of pro-inflammatory cytokines.30 The imbalance of intestinal flora may be involved in TLR4/NF-κB signaling pathway to regulate the brain immune system, and further play a role in various nervous system diseases.31 LPS, the most potent pro-inflammatory neurotoxin known, has been found to be prevalent in Parkinson’s disease (PD) patients in clinical studies. LPS, the endotoxin released by Gram-negative bacteria, interacts with TLR4 on macrophages to stimulate downstream inflammatory cascades in the gut and brain.32 Studies on the gut microbiota of Alzheimer’s disease(AD) patients have found that fecal microbial diversity is reduced, the abundance of beneficial bacterial taxa such as Bifidobacterium is decreased, and the abundance of potentially pathogenic microorganisms such as Bacteroides is increased.33 Other studies have found that Clostridium butyricum can improve cognitive dysfunction in Alzheimer’s disease mice by inhibiting TLR4 signaling pathway of gut-brain axis.34 In a mouse model of Parkinson’s disease, resveratrol intervention showed that repairing the intestinal barrier by inhibiting the inflammatory response mediated by TLR4/MyD88/NF-κB signaling pathway could improve neuropathy.
Interestingly, resveratrol has been shown to exert neuroprotective effects by modulating this gut-brain axis. In our study, resveratrol intervention alleviated colonic pathology in CKD rats, reducing structural disorganization and inflammatory cell infiltration. 16s RNA sequencing results showed that there were significant differences in the intestinal flora of rats in each group.
In the CKD group, Prevotella9, Bacteroides, Prevotella1, Helicobacter and Parabacteroides were the dominant bacteria with high abundance, and they were basically Gram-negative bacteria. Resveratrol intervention synergistically increased the abundance of Clostridiumsensustricto1, Bifidobacterium and Romboutsia, showing that Gram-positive bacteria were dominant, and beneficial bacteria mainly consisting of Bifidobacterium were enhanced. It has been found that probiotics Bifidobacterium breve can effectively improve LPS-induced depressive behavior in mice by reducing the levels of inflammatory cytokines, inhibiting the level of brain-derived neurotrophic factor in the prefrontal cortex and the decline of neuronal cell viability.35 Lactobacillus NK41 and Bifidobacterium longum NK4 were found to attenuate cognitive impairment and neuroinflammation by inducing NFκB inhibition of LPS-producing gut bacteria.36 Our research findings reveal that the functional prediction analysis of the microbiota indicates that the microbiota of the rats in the CKD group has enhanced the biosynthesis of LPS, and high concentrations of LPS have also been detected in the serum. Based on the above results, it is indicated that chronic nephropathy leads to intestinal-origin uremic toxins and dysregulation of microbiota metabolism, increasing the release of inflammatory factors such as LPS. However, the intervention with resveratrol plays a role in inhibiting the biosynthesis of LPS and reducing its function, and also reduces the levels of pro-inflammatory cytokines IL-6 and TNF-α in the serum. Further experiments have found that the expression of TLR4 in the hippocampus of the rats in the treatment group is significantly decreased, and the activation of the inflammatory pathways NF-κB and P-NF-κB is reduced. These changes are related to the reduction of neuronal damage and the improvement of cognitive function.This study provided a new therapeutic strategy for alleviating cognitive impairment after chronic kidney disease, but the clinical translation of resveratrol is currently rather challenging due to factors such as low bioavailability. Although it shows potential in the fields of antioxidant and anti-tumor, more high-quality clinical studies are still needed to verify its efficacy and safety.
Conclusion
Our research findings indicate that resveratrol may improve the cognitive dysfunction in CKD rats by regulating the intestinal microbiota and inhibiting the TLR4/NF-κB signaling pathway. These results provide new insights into the potential therapeutic benefits of resveratrol as an adjunctive treatment for cognitive dysfunction in CKD, supporting the protective role of the “intestinal-kidney-brain” axis. With the increasing prevalence of CKD patients and the concomitant prevalence of cognitive impairment, it is imperative to improve the quality of life of patients and treat CI. This requires more specific mechanism studies and clinical trial explorations.
Data Sharing Statement
Special thanks to the laboratory of Youjiang Medical College for Nationalities for providing the experimental platform.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The author (s) declares financial support for research, authorship, and/or publication of this article. This study was supported by the Guangxi Science and Natural Science Foundation of China (No. 2019JJA140110).
Disclosure
The authors declare that they have no competing interests.
References
1. Tang Y, Jiang J, Zhao Y, Du D. Aging and chronic kidney disease: epidemiology, therapy, management and the role of immunity. Clin Kidney J. 2024;17(9):sfae235. doi:10.1093/ckj/sfae235
2. Xie Z, Tong S, Chu X, Feng T, Geng M. chronic kidney disease and cognitive impairment: the kidney-brain axis. Kidney Dis. 2022;8(4):275–285. doi:10.1159/000524475
3. Yan Q, Liu M, Xie Y, et al. Kidney-brain axis in the pathogenesis of cognitive impairment. Neurobiol Dis. 2024;200:106626. doi:10.1016/j.nbd.2024.106626
4. Chagas YW, Vaz DCP, Simoes-E-Silva AC. Neuroinflammation in kidney disease and dialysis. Behav Brain Res. 2025;483:115465. doi:10.1016/j.bbr.2025.115465
5. Lau WL, Vaziri ND. The leaky gut and altered microbiome in chronic kidney disease. J Ren Nutr. 2017;27(6):458–461. doi:10.1053/j.jrn.2017.02.010
6. Olivier V, Dunyach-Remy C, Lavigne JP, Moranne O. Micro-inflammation and digestive bacterial translocation in chronic kidney disease. Nephrol Ther. 2018;14(3):135–141. doi:10.1016/j.nephro.2017.10.005
7. Goyal D, Ali SA, Singh RK. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110112. doi:10.1016/j.pnpbp.2020.110112
8. Capiralla H, Vingtdeux V, Zhao H, et al. Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J Neurochem. 2012;120(3):461–472. doi:10.1111/j.1471-4159.2011.07594.x
9. Gui J, Sun X, Wen S, Liu X, Qin B, Sang M. Resveratrol protects dopaminergic neurons in a mouse model of Parkinson’s disease by regulating the gut-brain axis via inhibiting the TLR4 signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2024;44(2):270–279. doi:10.12122/j.issn.1673-4254.2024.02.09
10. Tsuruya K, Yoshida H. Cognitive impairment and brain atrophy in patients with chronic kidney disease. J Clin Med. 2024;13(5):1401. doi:10.3390/jcm13051401
11. Wang F, Ma WB, Liu YW, Guo Q, Yu Y. Mechanism of impaired cognitive function in patients with chronic kidney disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022;44(6):1082–1088. doi:10.3881/j.issn.1000.503X.14291
12. Kim DS, Kim SW, Gil HW. Emotional and cognitive changes in chronic kidney disease. Korean J Intern Med. 2022;37(3):489–501. doi:10.3904/kjim.2021.492
13. Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the Gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol. 2019;9:206. doi:10.3389/fcimb.2019.00206
14. Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron Clin Pract. 2015;130(2):92–98.
15. Pan I, Issac PK, Rahman MM, Guru A, Arockiaraj J. Gut-brain axis a key player to control gut dysbiosis in neurological diseases. mol Neurobiol. 2024;61(12):9873–9891. doi:10.1007/s12035-023-03691-3
16. Liang X, Fu Y, Cao WT, et al. Gut microbiome, cognitive function and brain structure: a multi-omics integration analysis. Transl Neurodegener. 2022;11(1):49. doi:10.1186/s40035-022-00323-z
17. Chen C, Liao J, Xia Y, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71(11):2233–2252. doi:10.1136/gutjnl-2021-326269
18. Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021;13(6):2099.
19. Mou Y, Du Y, Zhou L, et al. Gut microbiota interact with the brain through systemic chronic inflammation: implications on neuroinflammation, neurodegeneration, and aging. Front Immunol. 2022;13:796288. doi:10.3389/fimmu.2022.796288
20. McCarthy GM, Bridges CR, Blednov YA, Harris RA. CNS cell-type localization and LPS response of TLR signaling pathways. F1000Res. 2017;6:1144. doi:10.12688/f1000research.12036.1
21. Kim HS, Kim S, Shin SJ, et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: pathologic roles and therapeutic implications. Transl Neurodegener. 2021;10(1):49. doi:10.1186/s40035-021-00273-y
22. Prakash V, Bose C, Sunilkumar D, Cherian RM, Thomas SS, Nair BG. Resveratrol as a promising nutraceutical: implications in gut microbiota modulation, inflammatory disorders, and colorectal cancer. Int J mol Sci. 2024;25(6):3370. doi:10.3390/ijms25063370
23. Feng Z, Wang T, Dong S, et al. Association between gut dysbiosis and chronic kidney disease: a narrative review of the literature. J Int Med Res. 2021;49(10):675853092. doi:10.1177/03000605211053276
24. Drabinska N, Jarocka-Cyrta E. Crosstalk between resveratrol and gut barrier: a review. Int J mol Sci. 2022;23(23):15279. doi:10.3390/ijms232315279
25. Huang M, Li G, Tan J, et al. Effects of chronic kidney disease on cognitive function and alpha-klotho expression in hippocampus. Transl Androl Urol. 2022;11(8):1157–1168. doi:10.21037/tau-22-465
26. Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp. 2011(53):2920
27. Yang X, Cai S, Gong J, et al. Characterization of gut microbiota in patients with stage 3-4 chronic kidney disease: a retrospective cohort study. Int Urol Nephrol. 2024;56(5):1751–1762. doi:10.1007/s11255-023-03893-7
28. Maldonado RF, Sa-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev. 2016;40(4):480–493. doi:10.1093/femsre/fuw007
29. Salguero MV, Al-Obaide M, Singh R, Siepmann T, Vasylyeva TL. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18(5):3461–3469. doi:10.3892/etm.2019.7943
30. Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45(12):e66. doi:10.1038/emm.2013.97
31. Singh S, Sahu K, Singh C, Singh A. Lipopolysaccharide induced altered signaling pathways in various neurological disorders. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(3):285–294. doi:10.1007/s00210-021-02198-9
32. Roy R, Kumar D, Bhattacharya P, Borah A. Modulating the biosynthesis and TLR4-interaction of lipopolysaccharide as an approach to counter gut dysbiosis and Parkinson’s disease: role of phyto-compounds. Neurochem Int. 2024;178:105803. doi:10.1016/j.neuint.2024.105803
33. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi:10.1038/s41598-017-13601-y
34. Su Y, Wang D, Liu N, Yang J, Sun R, Zhang Z. Clostridium butyricum improves cognitive dysfunction in ICV-STZ-induced Alzheimer’s disease mice via suppressing TLR4 signaling pathway through the gut-brain axis. PLoS One. 2023;18(6):e286086. doi:10.1371/journal.pone.0286086
35. Sushma G, Vaidya B, Sharma S, et al. Bifidobacterium breve Bif11 supplementation improves depression-related neurobehavioural and neuroinflammatory changes in the mouse. Neuropharmacology. 2023;229:109480. doi:10.1016/j.neuropharm.2023.109480
36. Ma X, Kim JK, Shin YJ, et al. Alleviation of cognitive impairment-like behaviors, neuroinflammation, colitis, and gut dysbiosis in 5xFAD transgenic and aged mice by lactobacillus mucosae and bifidobacterium longum. Nutrients. 2023;15(15):3381. doi:10.3390/nu15153381
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.