Back to Journals » Journal of Inflammation Research » Volume 17
Targeting the mTOR Pathway in Hepatocellular Carcinoma: The Therapeutic Potential of Natural Products
Authors Chen G, Zhang Y, Zhou Y, Luo H, Guan H, An B
Received 16 October 2024
Accepted for publication 24 November 2024
Published 6 December 2024 Volume 2024:17 Pages 10421—10440
DOI https://doi.org/10.2147/JIR.S501270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam Bachstetter
Guo Chen,1,* Ya Zhang,2,* Yaqiao Zhou,3 Hao Luo,3 Hongzhi Guan,3 Baiping An4
1Department of Infectious Diseases, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 2Department of Endocrinology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 3Department of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 4Department of Oncology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Baiping An, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China, Email [email protected]
Abstract: Despite advancements in cancer treatment through surgery and drugs, hepatocellular carcinoma (HCC) remains a significant challenge, as reflected by its low survival rates. The mammalian target of rapamycin (mTOR) signaling pathway plays a crucial role in regulating the cell cycle, proliferation, apoptosis, and metabolism. Notably, dysregulation leading to the activation of the mTOR signaling pathway is common in HCC, making it a key focus for in-depth research and a target for current therapeutic strategies. This review focuses on the role of the mTOR signaling pathway and its downstream effectors in regulating HCC cell proliferation, apoptosis, autophagy, cell cycle, and metabolic reprogramming. Moreover, it emphasizes the potential of natural products as modulators of the mTOR signaling pathway. When incorporated into combination therapies, these natural products have been demonstrated to augment therapeutic efficacy and surmount drug resistance. These products target key signaling pathways such as mTOR signaling pathways. Examples include 11-epi-sinulariolide acetate, matrine, and asparagus polysaccharide. Their inhibitory effects on these processes suggest valuable directions for the development of more effective HCC therapeutic strategies. Various natural products have demonstrated the ability to inhibit mTOR signaling pathway and suppress HCC progression. These phytochemicals, functioning as mTOR signaling pathway inhibitors, hold great promise as potential anti-HCC agents, especially in the context of overcoming chemoresistance and enhancing the outcomes of combination therapies.
Keywords: hepatocellular carcinoma, natural products, mTOR signaling pathway
Introduction
Hepatocellular carcinoma (HCC) is one of most common malignancies globally and poses a significant threat to human health.1 HCC is recognized as one of the top five leading causes of cancer-related mortality globally, with most patients having a 5-year survival rate of less than 20%.2 Surgical resection, local therapies, and systemic treatments remain the primary therapeutic strategies for HCC at different clinical stages.3 Despite advancements in systemic treatments for HCC, most patients show low response rates and ultimately succumb to the disease.4 Therefore, there is an urgent need to explore new antitumor agents.
Mutations in oncogenes and tumor suppressor genes are the primary pathogenic mechanisms in the development of liver cancer as they disrupt critical cellular signaling pathways. In HCC, the key pathways involved in the carcinogenic process include the mammalian target of rapamycin (mTOR), Wnt/β-catenin, and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways.5 This paper will mainly focus on the mTOR signaling pathway. The mTOR pathway is a central regulator of cell growth, proliferation, and survival.6 Dysregulation of this pathway is frequently observed in HCC, contributing to tumorigenesis and cancer progression.7 The mTOR pathway operates through two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), each playing a crucial role in cellular metabolism and response to environmental signals.8 Aberrant activation of mTOR signaling has been implicated in the development and maintenance of HCC, making it an attractive target for therapeutic intervention.
Natural products, including phytochemicals and bioactive compounds derived from medicinal plants, have gained significant attention as potential anticancer agents. These compounds often exhibit multiple mechanisms of action, including the modulation of key signaling pathways such as mTOR. Recent studies have highlighted the ability of various natural products to inhibit mTOR signaling pathway, thereby suppressing the growth and proliferation of HCC cells. The exploration of natural products as modulators of the mTOR pathway offers a promising approach for the development of novel therapeutic strategies against HCC.
mTOR Signaling Pathway
mTOR is a serine/threonine kinase and a member of the phosphoinositide 3-kinase (PI3K)-related kinase protein family. It plays a crucial role in regulating cell growth and proliferation in response to nutrient signals. mTOR exists in two distinct cellular complexes: mTORC1 and mTORC2.9 Both mTOR complexes share the mammalian lethal with SEC13 protein 8, the Tti1/Tel2 complex and the inhibitory protein DEP domain-containing mTOR-interacting protein (DEPTOR). mTORC1 additionally includes the regulatory-associated protein of mTOR (Raptor) and the inhibitory subunit proline-rich Akt substrate of 40kDa (PRAS40), while mTORC2 contains the rapamycin-insensitive companion of mTOR (Rictor) and the regulatory proteins Protor1/2 and mSin1.10 mTORC1 can be activated by cytokines, oxygen, stress signals, and nutrients. Its activation promotes lipid and protein biosynthesis, cell growth, and proliferation while inhibiting autophagy.11 In contrast, mTORC2 is not sensitive to nutrients but plays a crucial role in regulating cell metabolism, survival, and growth, as well as controlling cytoskeletal organization.12,13
mTOR Signaling Pathway and HCC
The mTOR pathway is a central intracellular signaling pathway that regulates cell cycle, proliferation, apoptosis, metabolism, and angiogenesis through interactions with various upstream and downstream molecules.11 mTOR is overexpressed in HCC, promoting tumor cell proliferation and growth.14 Studies have shown that metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is significantly upregulated in HCC and accelerates tumor cell transformation and growth by activating the Wnt/β-catenin pathway and inducing the expression of serine/arginine-rich splicing factor 1 (SRSF1).15 The coordination between mTOR complexes and epithelial-mesenchymal transition (EMT) is closely associated with HCC metastasis.16 Autophagy is an evolutionarily conserved process that plays a crucial role in maintaining homeostasis under physiological conditions. Targeting autophagy can disrupt the growth and metastasis of HCC and enhance the responsiveness of tumor cells to therapy.17 Apoptosis plays a key role in development, physiology and homeostasis.18 Additionally, mTOR inhibits apoptosis and autophagy. Research indicates that downregulation of nitrogen permease regulator like 2 (NPRL2) promotes HCC growth by inhibiting autophagy through the mTOR pathway.19 Ras-related protein Rap-2a (RAP2A) is aberrantly overexpressed in HCC tissues, enhancing tumor cell proliferation and resistance to apoptosis via activation of the mTOR signaling pathway.11 mTOR also plays a critical role in metabolic reprogramming in HCC, with increased aerobic glycolysis being a hallmark of cancer metabolism.20 Inhibiting the protein kinase B (AKT)/mTOR signaling pathway reduces aerobic glycolysis in HCC cells, ultimately leading to suppressed cellular growth.21 Lipid metabolism is a crucial energy source that supports cell growth and provides intermediates for biosynthesis in cancer cells.22 In the hypoxic tumor microenvironment, activation of the AKT/mTOR pathway drives lipogenesis and lipid accumulation during HCC progression, resulting in enhanced proliferation, viability, and angiogenesis.23 mTOR also induces cell cycle arrest, and its inhibition enhances the thermosensitivity of SMMC-7721 cells by increasing apoptosis and inducing S-phase arrest.24 Inhibiting mTOR signaling pathway can reduce tumor growth by inducing apoptosis, autophagy, and cell cycle arrest.
Natural Products as Inhibitors in HCC
Regulation of Apoptosis-Related Proteins and Pathways
In HCC treatment, regulating apoptosis-related proteins and pathways is vital. Many natural products/extracts target the mTOR signaling pathway, inducing apoptosis and impeding tumor growth (Figure 1 and Table 1). For instance, among these compounds, 4-Hydroxyderricin from Angelica sinensis inhibits HepG2 and Huh7 cell proliferation dose-dependently via mitochondrial apoptosis and cell cycle arrest by suppressing the PI3K/AKT/mTOR pathway.25,26 Similarly, Artemisia capillaris (ACE‐63) has hepatoprotective and anti-inflammatory properties27 and its ethyl acetate fraction induces apoptosis, inhibits cell growth and angiogenesis by blocking the PI3K/AKT/mTOR pathway.28 Ginkgolide C (CGC) from Ginkgo biloba exhibits anti-inflammatory and antioxidant properties and induces apoptosis and reduces tumorigenic protein expression by inhibiting this pathway.29,30 Furthermore, Withania somnifera (Solanaceae) is a medicinal plant used in Ayurvedic practices to promote health and well-being.31 Withagenin A diglucoside increases the expression of cleaved caspase-8, Bax, cleaved caspase-9, cleaved caspase-3, and PARP, while reducing Bcl-2 expression by targeting VEGFR2 and downstream signaling pathways, including ERK, PI3K, AKT, and mTOR.32 Similarly, Xanthium strumarium (XS)-5 and XS-6 effectively induce apoptosis and suppress cell growth, migration, and invasion by blocking the PI3K/AKT/mTOR pathway.33 Moreover, celastrol is a bioactive natural product isolated from the medicinal plant Tripterygium wilfordii Hook F,34 which triggers caspase-dependent apoptotic signaling by inhibiting the mTOR pathway in HCC cells.35 Additionally, Celastrus orbiculatus Thunb. extracts (COE) enhance apoptosis by downregulating mTOR and altering the expression of Bcl-2, Bcl-xL, Bax, and caspase-3.36 In addition, diosmetin inhibits HepG2 cell proliferation and induces apoptosis by suppressing the mTOR pathway.37 Further supporting this trend, anemoside B4 induces apoptosis and autophagy, with the inactivation of the PI3K/AKT/mTOR pathway.38 A further example includes arenobufagin, derived from bufadienolides in toad skin and parotid venom, which has been shown to inhibit metastasis across various cancers.39 Arenobufagin induces mitochondria-mediated apoptosis and autophagy in HCC cells through inhibition of PI3K/AKT/mTOR pathway.40 A cinchona alkaloid derivative (C1) induces apoptosis and blocks autophagy in HCC cells by suppressing the AKT/mTOR/S6K pathway.41 Salidroside, a phenylpropanoid mainly isolated from Rhodiola species, with various pharmacological effects.42 Notably, salidroside induces apoptosis by modulating mitochondrial function and autophagy by inhibiting the PI3K/AKT/mTOR pathway.43 Similarly, Ginsenoside RK1 (RK1), obtained from ginseng plants, has antioxidant, antiapoptotic, anti-inflammatory effects.44 Particularly, RK1 inhibits HCC development by activating toxic autophagy and promoting apoptosis through the AMP-activated protein kinase (AMPK)/mTOR pathway.45 Gundelia (G.) tournefortii suppresses primary HCC cell proliferation and induces apoptosis by inhibiting AKT, PI3K, and mTOR phosphorylation.46 Kahweol induces apoptosis in HCC cells by inhibiting the Src/mTOR/STAT3 signaling pathway.47 Lanatoside C inhibits HCC cell growth by inducing apoptosis via negatively regulating the AKT/mTOR pathway through PKCδ activation.48 Furthermore, licochalcone B inhibits AKT/mTOR signaling pathways, and sensitizes cancer cells to TRAIL-induced apoptosis by upregulating DR5 expression through ERK and JNK activation.49 Celastrol, pristimerin, and two novel derivatives (cel-D2 and cel-D7) specifically inhibit HCC growth, with cel-D2 and cel-D7 demonstrating lower toxicity. These compounds induce apoptosis and promote the degradation and inhibition of protein kinases in the Raf/MEK/ERK and PI3K/AKT/mTOR pathways.50 Phyllanthin enhances anti-oxidant capacity and induces caspase-dependent apoptosis by inhibiting the mTOR/PI3K signaling pathway.51 Additionally, pterostilbene induces apoptosis by inhibiting mTOR and S6K1 activation.52 Puerarin 6″-O-xyloside reduces cell viability, proliferation, and stemness, while promoting autophagy and mitochondria-dependent apoptosis, at least partially through inhibition of the PI3K/AKT/mTOR pathway.53 Rotundic Acid’s anti-HCC proliferative effects are linked to its ability to inhibit angiogenesis and induce apoptosis through modulation of AKT/mTOR and MAPK signaling pathways.54 Alnustone significantly induces apoptosis and inhibits the ROS-mediated PI3K/AKT/mTOR signaling pathway in HCC cells, with lower toxicity.55 In conclusion, a diverse range of natural products have demonstrated potential in HCC treatment by targeting the mTOR signaling pathway to regulate apoptosis. This summary provides a foundation for further research into the development of natural-product-based HCC therapies, highlighting the need for additional in vivo and clinical studies to evaluate their efficacy and safety in real-world settings.
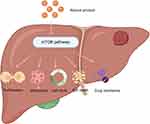 |
Figure 1 Natural products targeting mTOR pathways improve HCC. Abbreviation: mTOR, mammalian target of rapamycin. Notes: Created in BioRender. Chen, G. (2024) https://BioRnder.com/x50q835. |
 |
Table 1 Natural Products Targeting Apoptosis-Related Proteins and Pathways Improve HCC |
Regulation of Autophagy-Related Proteins and Pathways
Autophagy is crucial in the regulation of HCC, and various phytochemicals and natural products have been identified as key modulators of this process (Table 2). Among these, levo-tetrahydropalmatine (l-THP), derived from the clinical drug Corydalis yanhusuo, acts as an AMP-activated protein kinase (AMPK) activator.56 It activates the AMPK-mTOR-ULK1 and ROS-JNK-ATG cascades while impairing ERK/AKT signaling to enhance autophagy.56 Xanthoangelo also induces autophagy by activating the AMPK/mTOR signaling pathway.57 Blocking the AMPK/mTOR axis with compound C abolishes the autophagy-mediated inhibition of metastasis.57 Baicalein, a primary flavonoid extracted from the dried roots of Scutellaria baicalensis, exhibits anti-cancer properties against various malignancies.58 It suppresses mTORC1 inhibitor-induced autophagy, enhancing chemosensitivity in CD133+ tumor-initiating cells, Huh7 spheroids, and patient-derived HCC xenografts.59 Crocin, a unique water-soluble carotenoid extracted from saffron, demonstrates anticancer activity.60 Crocin induces autophagy in HCC cells via AKT/mTOR inhibition, with autophagy suppression leading to apoptosis resistance.61 Furthermore, dihydroartemisinin from Artemisia annua62,63 potentially induces autophagy in HepG2215 cells by inhibiting AKT/mTOR.64 Galangin, an extract from the ginger plant galangal, exhibits the ability to inhibit tumor cell proliferation and migration.65 Galangin stimulates autophagy in HepG2 cells through activating AMPK and inhibiting mTOR signaling pathway.66 Kaempferol in various plants induces autophagy in HCC cells via AMPK-mediated ULK1 phosphorylation and mTORC1 inhibition.67,68 Norcantharidin, a demethylated derivative of cantharidin,69 induces autophagic cell death in HCC by inhibiting c-Met/mTOR, alone or with crizotinib.70 Sinensetin from citrus fruits triggers autophagic cell death in HepG2 cells via the p53-related AMPK/mTOR pathway.71,72 Additionally, Hugan Buzure induces autophagy and apoptosis through inhibiting the PI3K/AKT/mTOR pathway, leading to HCC cell death.73 Muskone is a chemical monomer derived from musk,74 which inhibits xenograft tumor growth in mice via sestrin 2 (SESN2)/AMPK/mTOR autophagy pathways and PKR-like ER kinase (PERK)/activating transcription factor 4 (ATF4)/DNA damage-inducible transcript 3 (DDIT3) apoptosis signaling.75 Cryptotanshinone from Salvia miltiorrhiza suppresses Huh7 and MHCC97-H cell proliferation and induces autophagy and apoptosis by inhibiting PI3K/AKT/mTOR.76,77 Uvangoletin from Sarcandra glabra induces autophagy and apoptosis by inhibiting AKT/mTOR, MAPK, and TGF-β/Smad2.78 Isoliquiritigenin from liquorice induces autophagy in HCC cells by suppressing PI3K/AKT/mTOR.79,80 Isoquercitrin is widely present in vegetables, medicinal herbs and fruits,81 which triggers HCC cell death by inducing autophagy and apoptosis through AMPK activation and mTOR/p70S6K inhibition.82 Lycorine is an alkaloid isolated from plants of the Amaryllidaceae family, exhibiting potent anti-inflammatory and anti-cancer activities.83 It promotes apoptosis and autophagy in HCC by suppressing the TCRP1/AKT/mTOR pathway.84 Melittin induces autophagy and apoptosis by inhibiting the PI3K/AKT/mTOR pathway.85 Polyphyllin I is an active steroidal saponin isolated from Paris polyphylla,86 which induces autophagy by suppressing the PI3K/AKT/mTOR pathway.87 Quercetin is widely distributed across a variety of fruits and vegetables.88 It activates autophagy through AKT/mTOR inhibition and MAPK activation.89 Sarmentosin stimulates autophagy and caspase-dependent apoptosis in HCC cells by activating Nrf2 and inhibiting mTOR.90 Shikonin, a natural compound derived from the roots of Lithospermum erythrorhizon,91 induces autophagy and apoptosis in HCC cells via PI3K/AKT/mTOR pathway inhibition.92 Tenacissoside H is a medicinal monomer extracted from Marsdenia tenacissima extract, possessing antitumor properties.93 Tenacissoside H limits HCC cell proliferation and enhances radiosensitivity by inducing autophagic cell death through PI3K/AKT/mTOR inhibition.94 Berberine is an isoquinoline alkaloid isolated from Rhizoma Coptis.95 Berberine induces autophagy and apoptosis in HepG2 cells by activating AMPK and inhibiting mTORC1.96 Diosgenin is a steroidal saponin isolated from various vegetables and medicinal herbs, known for its diverse biological activities.97 Dioscin induces autophagy, apoptosis and DNA damage by inhibiting TIGAR-mediated p53, AKT/mTOR, and CDK5/ATM pathways.98 Salvianolic acid B is a natural polyphenolic acid found in Salvia miltiorrhiza, known for its remarkable anti-oxidant properties.99 Salvianolic acid B induces autophagy and apoptosis in HCC cells by inhibiting the AKT/mTOR pathway.100 Trillin is a bioactive compound extracted from Dioscorea nipponica Makino.101 Trillin induces apoptosis by inhibiting autophagy via the mTOR/signal transducer and activator of transcription 3 (STAT3) pathway.102 Brusatol induces autophagy in HCC cells by inhibiting the PI3K/AKT/mTOR pathway, effectively inhibiting cell proliferation, tumor invasion and migration.103 Collectively, these findings highlight the potential of phytochemicals and natural compounds in modulating autophagy for the treatment of HCC, offering promising avenues for therapeutic intervention.
 |
Table 2 Natural Products Targeting Autophagy-Related Proteins and Pathways Improve HCC |
Inhibition of Cell Migration and Invasion and Metastasis
HCC is an aggressive cancer with complex molecular mechanisms for proliferation, invasion, and metastasis. A variety of natural and synthetic compounds have been explored for their potential to inhibit these processes, often targeting critical signaling pathways such as mTOR (Table 3). For instance, 11-epi-sinulariolide acetate suppresses metastatic effects in HA22T cells by downregulating matrix metalloproteinase-2 (MMP-2), MMP-9, and uPA protein expression through the inhibition of focal adhesion kinase (FAK)/PI3K/AKT/mTOR signaling pathways.104 Similarly, matrine is a quinazoline alkaloid extracted from the plant Sophora flavescens.105 Matrine inhibits HCC cell proliferation and induces apoptosis by suppressing the AKT/mTOR/p70S6K and AKT/Glycogen synthase kinase 3β (GSK3β)/β-catenin signaling pathways.106 Furthermore, asparagus polysaccharide, the extract of asparagus, is a polysaccharide that has been identified as the primary active component of asparagus.107 Asparagus polysaccharide inhibits the proliferation, migration, and invasion of SK-Hep1 and Hep-3B cells and suppresses p-AKT, p-mTOR expression.107,108 Additionally, C21 steroid-enriched fraction from Marsdenia tenacissimae extraction (FR5) inhibits the proliferation and migration of HCC cells by co-inhibiting the Hippo/yes-associated protein (YAP) pathway and PI3K/PTEN/mTOR pathway.109 Moreover, compound 1a significantly inhibits the invasion and migration of HCC cells by inhibiting the PI3K/AKT/mTOR signaling pathway.110 Flaccidoxide-13-acetate inhibits HCC cell proliferation and metastasis through the inhibition of the FAK/PI3K/AKT/mTOR pathway.111 Likewise, haprolid was derived from the myxobacterium Byssovorax cruenta. Haprolid inhibits the cell proliferation, migration and invasion of HCC through the inhibition of Rb/E2F and Akt/mTOR pathways.112 Moreover, Hedyotis diffusa Willd, a herb from the Rubiaceae family,113 exhibits anti-HCC activity by inhibiting the AKT/mTOR pathway.114 Furthermore, isoviolanthin extracted from the leaves of Dendrobium officinale, inhibits the transforming growth factor β (TGF-β)/Smad and PI3K/AKT/mTOR pathways to inhibit EMT in HCC cells induced by TGF-β1.115 In addition, stachydrine, extracted from the plant Leonurus heterophyllus, has been shown to inhibit the proliferation of cancer cells.116 Stachydrine also prevents TGF-β1-induced EMT in HCC cells by inhibiting Smad2/3 and PI3K/AKT/mTOR signaling pathways.117 Similarly, stellettin B is isolated from the sponge Jaspis stellifera.118 Stellettin B suppresses HCC invasion and migration through inhibiting the FAK/PI3K/AKT/mTOR and MAPK pathways.119 Additionally, hops tetra- and hexahydro isoalpha acids (THIAA and HHIAA) reduce tumor burden in animal models and inhibit HCC cell proliferation by suppressing the NF-κB/tumor necrosis factor-α (TNF-α) pathway and mTOR activity.120 Furthermore, Silybum marianum total extract (STE), silymarin (Sm), and silibinin (Sb) significantly inhibit oxidative stress, HCC cell proliferation and PI3K/AKT/mTOR pathway.121 Additionally, berberine antagonizes the β-catenin pathway and reduces HCC cell survival by inhibiting β-catenin translation and mTOR activity.122 Furthermore, chelerythrine found in various medicinal herbs, exhibits anti-tumor activity.123 Chelerythrine inhibits cell migration through the PI3K/AKT/mTOR and MAPK pathways.124 Moreover, cinobufagin is one of the primary active components found in toad venom.125 Cinobufagin inhibits cell proliferation by blocking the AURKA/mTOR/eIF4E signaling pathway.126 Similarly, usenamine A was first isolated from the lichen Usnea longissimi.127 Usenamine A inhibits cell proliferation by downregulating the AKT/mTOR/STAT3 pathway.127 Saringosterol acetate (SSA) inhibits tumor growth and metastatic effects by inhibiting the PI3K/AKT/mTOR and TGFβ/Smad pathways.128 Furthermore, ruscogenin is derived from Radix Ophiopogon japonicus.129 Ruscogenin inhibits HCC lung metastasis by blocking the PI3K/AKT/mTOR pathway and reducing the expression of MMP-2, MMP-9, VEGF and HIF-1α.130 Similarly, cordycepin, a nucleoside found in the Cordyceps mushrooms, exhibits anti-cancer properties.131 Cordycepin inhibits inflammatory responses and oxidative stress by suppressing the PI3K/AKT/mTOR and Nrf2/heme oxygenase-1(HO-1)/NF-κB pathways.132 Portulaca oleracea L. (Purslane) (PL) exhibits protective effects against NDEA-induced HCC by modulating the same pathways.133 Additionally, mallotucin D inhibits HepG2 cell proliferation, DNA synthesis, colony formation, and HUVEC angiogenesis by inhibiting the PI3K/AKT/mTOR pathway.134 In conclusion, the compounds discussed above demonstrate significant potential in suppressing HCC progression by modulating key molecular pathways. These findings suggest that continued research into these and similar compounds may provide valuable insights into developing more effective therapeutic strategies against HCC.
 |
Table 3 Natural Products Targeting Cell Migration and Invasion and Metastasis Improve HCC |
Alteration of Cell Cycle
Several natural products have demonstrated significant anti-proliferative effects on HCC cells by inducing cell cycle arrest through mTOR signaling pathways (Table 4). For instance, celastrol modulates gut microbiota and hepatic bile acid metabolism, inhibits the interaction between farnesoid X receptor (FXR) and retinoid X receptor α (RXRα), and induces mTOR/S6K1-related G0/G1 phase cell cycle arrest.135 Hemistepsin A, a sesquiterpene lactone isolated from plants of Hemistepta lyrata Bunge (Compositae),136 induces G0/G1 phase arrest and mitochondria-related apoptosis by the activation of the AMPK/mTOR pathway.137 Linalool induces G0/G1 phase cell cycle arrest and apoptosis by generating oxidative stress and inhibiting the AKT/mTOR pathway.138 Moreover, marsdenia tenacissima (Roxb.) Wight and Arn (MT) is a well-known traditional Chinese medicine used in cancer treatment.139 Marsdenia tenacissima inhibits cell proliferation and induces autophagy, apoptosis, and S-phase cell cycle arrest by inhibiting the MIF/mTOR signaling pathway.140 Furthermore, the extract of I. baumii (EIB) induces S-phase cell cycle arrest and apoptosis by inhibiting the AMPK/mTOR/ULK1 pathway.141 β-Thujaplicin is one of the major components of Chamaecyparis obtusa.142 β-Thujaplicin causes apoptosis and S-phase arrest through ROS-mediated inhibition of the AKT/mTOR pathway and activation of the p38/ERK MAPK pathway.143 Additionally, baicalein inhibits cell proliferation by inducing S-phase and G2/M-phase cell cycle arrest via the PI3K/AKT and mTOR signaling pathway.144 Moreover, chaetocochin J inhibits HepG2 and Hep3B cell proliferation and induces G2/M phase arrest under both normoxic and hypoxic conditions by inhibiting the PI3K/AKT/mTOR/p7OS6K/4EBP1 pathway.145 Emodin is a major active component of Rheum palmatum and has demonstrated anticancer properties.146 Emodin suppresses HCC cell proliferation, induces S-phase and G2/M-phase arrest through inhibiting the PI3K/AKT signaling pathway.147 Furthermore, gamabufotalin is derived from ChanSu.148 Gamabufotalin induces G2/M phase cell cycle arrest and induces autophagy and apoptosis through inhibiting the mTOR-ULK1 signaling pathway.149 Similarly, prunetrin induces G2/M phase arrest and mitochondrial-mediated apoptosis by inhibiting the AKT/mTOR pathway and activating the MAPK pathway.150 Prunetrin causes G2/M phase cell cycle arrest by activating the caspase cascade and suppressing the AKT/mTOR pathway.151 Similarly, the petroleum ether extract of Chloranthus fortunei (PECFS) markedly inhibits HCC cell proliferation, migration, and invasion, induces G2/M phase arrest, and apoptosis by suppressing the PI3K/AKT/mTOR pathway.152 Pectolinarigenin, a natural flavonoid, suppresses HCC cell viability and induces G2/M arrest via PI3K/AKT/mTOR/ERK pathway inhibition.153,154 Oleanolic acid, a phytochemical in many edible and medicinal plants,155 induces G2/M phase cell cycle arrest and apoptosis by inhibiting the AKT/mTOR pathway.156 In summary, various natural compounds exhibit potent anti-proliferative effects on HCC cells by targeting key signaling pathways, particularly those involving mTOR. These compounds, including celastrol, hemistepsin A, and marsdenia tenacissima, among others, induce cell cycle arrest at different phases and trigger apoptosis through mechanisms such as mitochondrial dysfunction, oxidative stress, and autophagic cell death. The inhibition of the mTOR pathway appears to be a common mechanism among these compounds, underscoring the therapeutic potential of natural products in the treatment of HCC.
 |
Table 4 Natural Products Targeting Cell Cycle Improve HCC |
Metabolic Reprogramming
This part focuses on several natural products that adjust mTOR signaling via metabolism modulation (Table 5). These mechanisms are crucial in the treatment of HCC, as they not only inhibit tumor growth but also enhance the responsiveness to other therapeutic approaches. Compound K is a secondary ginsenoside with higher bioavailability and exhibits significant anti-cancer effects.157 Compound K inhibits glycolysis and AKT/mTOR/c-Myc signaling by downregulating the expression of hexokinase 2 (HK2) and pyruvate kinase isozymes M2 (PKM2).158 Similarly, osthol is a coumarin derivative extracted from Cnidium monnieri. Osthol has been found to enhance radiosensitivity in HCC by inhibiting glycolysis, likely through inhibiting the GSK-3β/AMPK/mTOR pathway.159,160 Moreover, zerumbone is isolated from the rhizomes of Zingiber zerumbet (L.) Smith.161 Zerumbone inhibits glycolysis and diverts glucose-6-phosphate from the pentose phosphate pathway potentially through the downregulation of PI3K/AKT/mTOR and STAT3 pathways.162 Likewise, morusin isolated from the roots of Morus alba, exhibit anti-angiogenic, anti-migratory, and pro-apoptotic effects.163 Morusin significantly induces G1 arrest, while attenuating the expression of p-AKT, p-mTOR, HK2, PKM2, and LDH-A through AMPK-mediated G1 arrest and antiglycolytic activity.163 Furthermore, inhibiting SREBP2 to reduce cellular cholesterol levels has been shown to improve the efficacy of lenvatinib in HCC cells, with the SREBP2 inhibitor betulin significantly enhancing lenvatinib’s anti-tumor effects, possibly through the inhibition of the mTOR/IL-1β pathway.164 Additionally, Rhizoma Paridis saponins are the primary active components of Rhizoma Paridis.165 Rhizoma Paridis saponins overcomes sorafenib resistance by inhibiting the PI3K/AKT/mTOR pathway.166 Phytosomal curcumin reduces lipid accumulation and decreases overall tumor volume and inhibits oncogenic mTOR activation.167 Overall, these natural products have significant anti-tumor potential by regulating mTOR signaling through glycolysis and lipid metabolism modulation, especially in surmounting drug resistance and increasing treatment effectiveness. More research is needed to fully understand their clinical applications in HCC treatment.
 |
Table 5 Natural Products Targeting Metabolic Reprogramming Improve HCC |
Sensitization Therapy
The therapeutic efficacy of combining natural products with conventional anti-cancer agents for HCC has been extensively studied both in vitro and in vivo (Table 6). Fucoidan, in combination with sorafenib or Avastin, markedly inhibits HCC cell viability and promotes apoptosis by downregulating the PI3K/AKT/mTOR pathways.168 Similarly, compounds such as artesunate,169 tetrandrine,170 glycyrrhizic acid,171 Pogostemon cablin,172 Huaier,173 a-Mangostin,174 ellagic acid,175 cucurbitacin E176 and amygdalin177 have been shown to enhance the anti-tumor effects of sorafenib through dual inhibition of the mTOR and other signaling pathways, effectively overcoming chemotherapy resistance. Berberine and rapamycin synergistically induces apoptosis and autophagy by inhibiting the mTOR signaling pathway.178 Berberine combined with HMQ1611 inhibits HCC growth and induces G1 phase cell cycle arrest by downregulating AKT, mTOR, and ERK/MAPK signaling pathways.179 Chlorogenic acid enhances the growth-inhibitory effects of regorafenib by suppressing the MAPK and PI3K/AKT/mTORC pathways and inhibiting the anti-apoptotic proteins.180 Similarly, catalpol and/or regorafenib significantly inhibit the PI3K/p-AKT/mTOR/NF-кB and VEGF/VEGFR2 pathways.181 The combination of aloin and metformin enhances growth inhibition and apoptosis in HCC cells by inhibiting the PI3K/AKT/mTOR pathway,182 while metformin and curcumin not only induce apoptosis but also inhibit HCC cell invasion, migration, and angiogenesis by suppressing the PI3K/AKT/mTOR/NF-κB and EGFR/STAT3 pathways.183 Doxorubicin combined with manuka honey exhibits higher cytotoxicity and enhances apoptosis through the inhibition of ERK1/2 and mTOR pathways,184 while Shufeng Jiedu Capsule, along with its active components, potentiates the anti-tumor effects of doxorubicin by suppressing the AKT/mTOR, and NF-κB pathways.185 Lachnum expolysaccharide significantly sensitizes HepG2 cells to 5-fluorouracil by inactivating Ras/Raf/MEK/ERK and PI3K/AKT/mTOR pathways,186 and curcumin further enhances the chemosensitivity of HCC cells to 5-fluorouracil, induces G2/M phase arrest, and inhibits the PI3K/AKT/mTOR pathway.187 The natural product magnolin combined with BRAF inhibitor SB590885 synergistically inhibits HCC cell proliferation by blocking the ERK/MAPK and PI3K/AKT pathways.188 Biochanin A and SB590885 inhibit HCC cell proliferation through disrupting of the ERK MAPK and the PI3K/AKT pathways.189 Ovatodiolide and antrocin enhance apoptosis and autophagy in tumor cells and effectively counteract sorafenib resistance by inhibiting the ERK1/2 and AKT/mTOR pathways.190 Oxysophocarpine sensitizes FGFR1-overexpressing HCC to lenvatinib by downregulating the AKT/mTOR and ERK signaling pathways.191 Parthenolide and arsenic trioxide enhance anti-HCC efficacy by inhibiting the PI3K/AKT/mTOR pathway.192 Lipid coated cisplatin/oleanolic acid calcium carbonate nanoparticles (CDDP/OA-LCC NPs) promote apoptosis and reduce cisplatin-induced hepatotoxicity through downregulating the PI3K/AKT/mTOR signaling pathway.193 Finally, Babaodan inhibits tumor growth and enhances the anti-HCC efficacy of camrelizumab by regulating the M1/M2 macrophage ratio and increasing CD8+ T cell abundance via the PI3K/AKT/mTOR signaling pathway.194 In summary, the combination of natural compounds with conventional anti-cancer agents has shown promising results in enhancing therapeutic efficacy against HCC. These combinations generally function by targeting key signaling pathways such as PI3K/AKT/mTOR and ERK/MAPK, leading to augmented apoptosis, autophagy, and tumor growth inhibition. Moreover, these combinations can overcome chemotherapy resistance, enhance drug sensitivity, and reduce toxicity, rendering them valuable strategies in HCC treatment.
 |
Table 6 Natural Products Improve the Treatment of HCC Through Sensitization Therapy |
Conclusion and Future Perspectives
In recent years, as the understanding of the molecular mechanisms underlying HCC has deepened, researchers have initiated the exploration of novel therapeutic modalities. Emerging studies have increasingly centered on molecular targeting of cancer cells to attain enhanced efficacy and diminished side effects. Consequently, targeting cellular signaling pathways has emerged as a prominent strategy in drug development. Among various signaling pathways, the mTOR signaling pathway plays a central role in hepatocellular carcinoma. Many natural products have emerged as potent modulators of the mTOR signaling pathway, which is critical in regulating various cellular processes such as proliferation, apoptosis, autophagy, cell cycle progression, and metabolic reprogramming in HCC. Compounds like curcumin, berberine, and celastrol have demonstrated significant anti-tumor activities by targeting mTOR and its associated pathways. These compounds can induce cell cycle arrest, promote apoptosis, and modulate autophagy, inhibiting the growth and proliferation of HCC cells. Additionally, the capacity of these natural products to influence metabolic reprogramming presents a novel approach to counteracting the metabolic adaptations frequently witnessed in cancer cells. Moreover, the combination of natural products with conventional therapies, such as chemotherapy or targeted therapy, has shown synergistic effects. These combinations not only enhance therapeutic efficacy but also reduce the likelihood of drug resistance-a significant challenge in HCC treatment. Natural products can sensitize tumor cells to chemotherapeutic agents by modulating the mTOR pathway, thereby reducing the required dose and minimizing side effects. Natural products have unique innovativeness. They act through novel mechanisms targeting specific pathways that traditional treatments usually miss. And compared with traditional treatments, these natural products have advantages in safety, as they cause fewer side effects due to their natural origin and better compatibility with the human body. Moreover, they have potential in long-term prevention and health maintenance by enhancing the body’s resistance system.
Nevertheless, notwithstanding these auspicious preclinical discoveries, the clinical application of natural products in HCC therapy confronts several formidable challenges. Firstly, many natural products exhibit low bioavailability due to their poor solubility, instability, and rapid metabolism. As a result, this limitation often results in insufficient therapeutic concentrations at the tumor site, diminishing their efficacy. Curcumin is a very promising chemopreventive agent. This has driven clinical practice to study the pharmacokinetics and efficacy of curcumin in patients. In Phase I clinical studies, it has been proven to be safe and non - toxic, even at high doses (8 g/d). However, its absorption is limited in individuals.195,196 Despite the challenges of bioavailability, clinical trials of them (used alone or in combination as anticancer drugs) have demonstrated efficacy in several disease sites.197–199 Secondly, although natural products are generally regarded as safe, their long-term utilization, especially in conjunction with other treatments, elicits concerns regarding potential toxicity and adverse effects. Therefore, comprehensive toxicological studies are necessary to ensure the safety of these compounds in clinical settings. Moreover, the variability in the composition of natural products, influenced by factors such as source, extraction methods, and storage conditions, poses a challenge for their consistent clinical application. Furthermore, the effects of natural products on the mTOR pathway can be intricate and context-dependent, with disparities observed across different cell types, tumor microenvironments, and cancer progression stages. Although numerous preclinical studies support the anti-cancer potential of natural products, clinical evidence remains limited. Few natural compounds have progressed to clinical trials, and those that have often face challenges related to efficacy, safety, and regulatory approval. Through the use of andrographolide in clinical trials, the recovery rates of different myeloma patients have achieved significant improvement.200,201 Although research has been conducted with a large amount of information on pre-clinical efficacy, clinical studies are limited in evaluating the true potential of berberine and andrographolide as cancer - modulating agents.202
To surmount these challenges and fully exploit the therapeutic potential of natural products in HCC, several strategies ought to be pursued. First and foremost, advancements in drug delivery systems, such as nanoparticle-based carriers, liposomes, and micelles, present promising remedies to enhance the bioavailability and targeted delivery of natural products. These innovations can protect the compounds from degradation, enhance their solubility, and facilitate their accumulation in tumor tissues. In addition, continued exploration of combination therapies involving natural products and conventional treatments is crucial. By identifying the optimal combinations, dosing schedules, and biomarkers for response will maximize therapeutic outcomes while minimizing side effects. Moreover, clinical trials are needed to validate the efficacy and safety of natural products in HCC patients. Additionally, the establishment of standardized protocols for the preparation, characterization, and quality control of compounds will ensure consistency in clinical applications.
In conclusion, while natural products offer a promising avenue for the treatment of HCC through the modulation of the mTOR pathway, significant challenges remain. Overcoming these challenges via innovative research and clinical development will be pivotal in unlocking the complete potential of these compounds in the battle against HCC.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge using Biorender (https://app.biorender.com/user/signin) to create the schemata (Figure 1).
Funding
The present study was financially supported by the Sichuan Administration of Traditional Chinese Medicine (2023MS419 and 2024MS555), Chengdu University of Traditional Chinese Medicine Affiliated Hospital (23YY37).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
3. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
4. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
5. Blagotinsek K, Rozman D. Targeting signalling pathways in hepatocellular carcinoma. Curr Pharm Des. 2017;23(1):170–175. doi:10.2174/1381612822666161006160005
6. Mafi S, Mansoori B, Taeb S, et al. mTOR-mediated regulation of immune responses in cancer and tumor microenvironment. Front Immunol. 2021;12:774103. doi:10.3389/fimmu.2021.774103
7. Guri Y, Colombi M, Dazert E, et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32(6):807–823.e812. doi:10.1016/j.ccell.2017.11.011
8. Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev. 2021;101(3):1371–1426. doi:10.1152/physrev.00026.2020
9. Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR Pathway in hepatocellular carcinoma. Biomedicines. 2021;9(11):1639. doi:10.3390/biomedicines9111639
10. Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–1918. doi:10.3390/ijms13021886
11. Ferrín G, Guerrero M, Amado V, Rodríguez-Perálvarez M, De la Mata M. Activation of mTOR signaling pathway in hepatocellular carcinoma. Int J Mol Sci. 2020;21(4):1266. doi:10.3390/ijms21041266
12. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi:10.1016/j.cell.2017.02.004
13. Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60(4):855–865. doi:10.1016/j.jhep.2013.11.031
14. Ge S, Huang H, Huang W, et al. PSME4 activates mTOR signaling and promotes the malignant progression of hepatocellular carcinoma. Int J Gene Med. 2022;15:885–895. doi:10.2147/IJGM.S344360
15. Malakar P, Shilo A, Mogilevsky A, et al. Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017;77(5):1155–1167. doi:10.1158/0008-5472.CAN-16-1508
16. Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–234. doi:10.1016/j.biochi.2019.08.003
17. Hashemi M, Nadafzadeh N, Imani MH, et al. Targeting and regulation of autophagy in hepatocellular carcinoma: revisiting the molecular interactions and mechanisms for new therapy approaches. Cell Commun Signaling. 2023;21(1):32. doi:10.1186/s12964-023-01053-z
18. Wang F, Wang H, Sun X, Li M. Apoptosis-induction is a novel therapeutic strategy for gastrointestinal and liver cancers. Curr Gene Ther. 2015;15(2):193–200. doi:10.2174/1566523214666141224100801
19. Wang YC, Tsai MC, Chen YS, et al. NPRL2 down-regulation facilitates the growth of hepatocellular carcinoma via the mTOR pathway and autophagy suppression. Hepatol Commun. 2022;6(12):3563–3577. doi:10.1002/hep4.2019
20. Zhong XY, Yuan XM, Xu YY, et al. CARM1 methylates GAPDH to regulate glucose metabolism and is suppressed in liver cancer. Cell Rep. 2018;24(12):3207–3223. doi:10.1016/j.celrep.2018.08.066
21. Zheng YL, Li L, Jia YX, et al. LINC01554-mediated glucose metabolism reprogramming suppresses tumorigenicity in hepatocellular carcinoma via downregulating PKM2 expression and inhibiting Akt/mTOR signaling pathway. Theranostics. 2019;9(3):796–810. doi:10.7150/thno.28992
22. Alannan M, Fayyad-Kazan H, Trézéguet V, Merched A. Targeting lipid metabolism in liver cancer. Biochemistry. 2020;59(41):3951–3964. doi:10.1021/acs.biochem.0c00477
23. Chen J, Chen J, Huang J, et al. HIF-2α upregulation mediated by hypoxia promotes NAFLD-HCC progression by activating lipid synthesis via the PI3K-AKT-mTOR pathway. Aging. 2019;11(23):10839–10860. doi:10.18632/aging.102488
24. Wang QL, Liu BO, Li XJ, Hu KP, Zhao K, Ye XM. Inhibition of mTOR promotes hyperthermia sensitivity in SMMC-7721 human hepatocellular carcinoma cell line. Exp Ther Med. 2016;11(3):961–968. doi:10.3892/etm.2016.2979
25. Li Y, Goto T, Yamakuni K, et al. 4-hydroxyderricin, as a PPARγ agonist, promotes adipogenesis, adiponectin secretion, and glucose uptake in 3T3-L1 cells. Lipids. 2016;51(7):787–795. doi:10.1007/s11745-016-4154-9
26. Gao X, Jiang Y, Xu Q, et al. 4-hydroxyderricin promotes apoptosis and cell cycle arrest through regulating PI3K/AKT/mTOR pathway in hepatocellular cells. Foods. 2021;10(9):2036. doi:10.3390/foods10092036
27. Kim J, Jung KH, Yan HH, et al. Artemisia Capillaris leaves inhibit cell proliferation and induce apoptosis in hepatocellular carcinoma. BMC Complement Altern Med. 2018;18(1):147. doi:10.1186/s12906-018-2217-6
28. Jung KH, Rumman M, Yan H, et al. An ethyl acetate fraction of Artemisia capillaris (ACE-63) induced apoptosis and anti-angiogenesis via inhibition of PI3K/AKT signaling in hepatocellular carcinoma. Phytother Res. 2018;32(10):2034–2046. doi:10.1002/ptr.6135
29. Jia L, Gong Y, Jiang X, et al. Ginkgolide C inhibits ROS-mediated activation of NLRP3 inflammasome in chondrocytes to ameliorate osteoarthritis. J Ethnopharmacol. 2024;325:117887. doi:10.1016/j.jep.2024.117887
30. Yang MH, Baek SH, Um JY, Ahn KS. Anti-neoplastic effect of ginkgolide C through modulating c-met phosphorylation in hepatocellular carcinoma cells. Int J Mol Sci. 2020;21(21):8303. doi:10.3390/ijms21218303
31. Lee BS, Yoo MJ, Kang H, et al. Withasomniferol D, a new anti-adipogenic withanolide from the roots of ashwagandha (Withania somnifera). Pharmaceuticals. 2021;14(10):1017. doi:10.3390/ph14101017
32. Lee D, Yu JS, Ha JW, et al. Antitumor potential of withanolide glycosides from ashwagandha (Withania somnifera) on apoptosis of human hepatocellular carcinoma cells and tube formation in human umbilical vein endothelial cells. Antioxidants. 2022;11(9):1761. doi:10.3390/antiox11091761
33. Kim J, Jung KH, Ryu HW, Kim DY, Oh SR, Hong SS. Apoptotic effects of Xanthium strumarium via PI3K/AKT/mTOR pathway in hepatocellular carcinoma. Evid Based Complement Alternat Med. 2019;2019:2176701. doi:10.1155/2019/2176701
34. Zhang H, Zhao X, Shang F, Sun H, Zheng X, Zhu J. Celastrol inhibits the proliferation and induces apoptosis of colorectal cancer cells via downregulating NF-κB/COX-2 signaling pathways. Anti-Cancer Agents Med Chem. 2022;22(10):1921–1932. doi:10.2174/1871520621666211103103530
35. Shen B, Chen HB, Zhou HG, Wu MH. Celastrol induces caspase-dependent apoptosis of hepatocellular carcinoma cells by suppression of mammalian target of rapamycin. J Tradit Chin Med. 2021;41(3):381–389. doi:10.19852/j.cnki.jtcm.2021.03.006
36. Qian YY, Li WY, Yan Y, et al. Celastrus orbiculatus extracts inhibit human hepatocellular carcinoma growth by targeting mTOR signaling pathways. Chin J Integr Med. 2019;25(11):845–852. doi:10.1007/s11655-019-3035-5
37. Liu J, Ren H, Liu B, Zhang Q, Li M, Zhu R. Diosmetin inhibits cell proliferation and induces apoptosis by regulating autophagy via the mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Oncol Lett. 2016;12(6):4385–4392. doi:10.3892/ol.2016.5301
38. Xue S, Zhou Y, Zhang J, et al. Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am J Transl Res. 2019;11(4):2580–2589.
39. Wang M, Hu S, Yang J, et al. Arenobufagin inhibits lung metastasis of colorectal cancer by targeting c-MYC/Nrf2 axis. Phytomedicine. 2024;127:155391. doi:10.1016/j.phymed.2024.155391
40. Zhang DM, Liu JS, Deng LJ, et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 2013;34(6):1331–1342. doi:10.1093/carcin/bgt060
41. Jin PR, Ta YN, Chen IT, et al. Cinchona alkaloid-inspired urea-containing autophagy inhibitor shows single-agent anticancer efficacy. J Med Chem. 2021;64(19):14513–14525. doi:10.1021/acs.jmedchem.1c01036
42. Zhang N, Nao J, Dong X. Neuroprotective mechanisms of salidroside in Alzheimer’s disease: a systematic review and meta-analysis of preclinical studies. J Agric Food Chem. 2023;71(46):17597–17614. doi:10.1021/acs.jafc.3c06672
43. Jiang B, Feng L, Yang T, et al. Combination of chloroquine diphosphate and salidroside induces human liver cell apoptosis via regulation of mitochondrial dysfunction and autophagy. Mol Med Rep. 2023;27(2). doi:10.3892/mmr.2022.12924
44. She L, Sun J, Xiong L, et al. Ginsenoside RK1 improves cognitive impairments and pathological changes in Alzheimer’s disease via stimulation of the AMPK/Nrf2 signaling pathway. Phytomedicine. 2024;122:155168. doi:10.1016/j.phymed.2023.155168
45. Wu H, Qu L, Bai X, et al. Ginsenoside Rk1 induces autophagy-dependent apoptosis in hepatocellular carcinoma by AMPK/mTOR signaling pathway. Food Chem Toxicol. 2024;186:114587. doi:10.1016/j.fct.2024.114587
46. Amer J, Salhab A, Jaradat N, et al. Gundelia tournefortii inhibits hepatocellular carcinoma progression by lowering gene expression of the cell cycle and hepatocyte proliferation in immunodeficient mice. Biomed Pharmacother. 2022;156:113885. doi:10.1016/j.biopha.2022.113885
47. Seo HY, Lee SH, Lee JH, Lee JH, Jang BK, Kim MK. Kahweol induces apoptosis in hepatocellular carcinoma cells by inhibiting the Src/mTOR/STAT3 signaling pathway. Int J Mol Sci. 2021;22(19):10509. doi:10.3390/ijms221910509
48. Chao MW, Chen TH, Huang HL, et al. Lanatoside C, a cardiac glycoside, acts through protein kinase Cδ to cause apoptosis of human hepatocellular carcinoma cells. Sci Rep. 2017;7:46134. doi:10.1038/srep46134
49. Zhang YY, Feng PP, Wang HF, et al. Licochalcone B induces DNA damage, cell cycle arrest, apoptosis, and enhances TRAIL sensitivity in hepatocellular carcinoma cells. Chem Biol Interact. 2022;365:110076. doi:10.1016/j.cbi.2022.110076
50. Wei W, Wu S, Wang X, et al. Novel celastrol derivatives inhibit the growth of hepatocellular carcinoma patient-derived xenografts. Oncotarget. 2014;5(14):5819–5831. doi:10.18632/oncotarget.2171
51. You Y, Zhu F, Li Z, et al. Phyllanthin prevents diethylnitrosamine (DEN) induced liver carcinogenesis in rats and induces apoptotic cell death in HepG2 cells. Biomed Pharmacother. 2021;137:111335. doi:10.1016/j.biopha.2021.111335
52. Khalil MI, Agamy AF, Elshewemi SS, Sultan AS, Abdelmeguid NE. Pterostilbene induces apoptosis in hepatocellular carcinoma cells: biochemical, pathological, and molecular markers. Saudi J Biol Sci. 2023;30(8):103717.
53. Li L, Liu JD, Gao GD, Zhang K, Song YW, Li HB. Puerarin 6″-O-xyloside suppressed HCC via regulating proliferation, stemness, and apoptosis with inhibited PI3K/AKT/mTOR. Cancer Med. 2020;9(17):6399–6410. doi:10.1002/cam4.3285
54. Roy G, Guan S, Liu H, Zhang L. Rotundic acid induces DNA damage and cell death in hepatocellular carcinoma through AKT/mTOR and MAPK pathways. Front Oncol. 2019;9:545. doi:10.3389/fonc.2019.00545
55. Wang L, Cheng L, Ma L, et al. Alnustone inhibits the growth of hepatocellular carcinoma via ROS- mediated PI3K/Akt/mTOR/p70S6K axis. Phytother Res. 2022;36(1):525–542.
56. Yin X, Li W, Zhang J, et al. AMPK-mediated metabolic switching is high effective for phytochemical Levo-Tetrahydropalmatine (l-THP) to Reduce Hepatocellular Carcinoma Tumor Growth. Metabolites. 2021;11(12):811. doi:10.3390/metabo11120811
57. Yang X, Xie J, Liu X, et al. Autophagy induction by xanthoangelol exhibits anti-metastatic activities in hepatocellular carcinoma. Cell Biochem Funct. 2019;37(3):128–138. doi:10.1002/cbf.3374
58. Lai JQ, Zhao LL, Hong C, et al. Baicalein triggers ferroptosis in colorectal cancer cells via blocking the JAK2/STAT3/GPX4 axis. Acta Pharmacol Sin. 2024;45(8):1715–1726. doi:10.1038/s41401-024-01258-z
59. Wu R, Murali R, Kabe Y, et al. Baicalein targets GTPase-mediated autophagy to eliminate liver tumor-initiating stem cell-like cells resistant to mTORC1 inhibition. Hepatology. 2018;68(5):1726–1740. doi:10.1002/hep.30071
60. Tang Y, Yang H, Yu J, et al. Network pharmacology-based prediction and experimental verification of the involvement of the PI3K/Akt pathway in the anti-thyroid cancer activity of crocin. Arch Biochem Biophys. 2023;743:109643. doi:10.1016/j.abb.2023.109643
61. Yao C, Liu BB, Qian XD, et al. Crocin induces autophagic apoptosis in hepatocellular carcinoma by inhibiting Akt/mTOR activity. Onco Targets Ther. 2018;11:2017–2028. doi:10.2147/OTT.S154586
62. Shi X, Wang L, Ren L, et al. Dihydroartemisinin, an antimalarial drug, induces absent in melanoma 2 inflammasome activation and autophagy in human hepatocellular carcinoma HepG2215 cells. Phytother Res. 2019;33(5):1413–1425. doi:10.1002/ptr.6332
63. Hao L, Guo Y, Peng Q, et al. Dihydroartemisinin reduced lipid droplet deposition by YAP1 to promote the anti-PD-1 effect in hepatocellular carcinoma. Phytomedicine. 2022;96:153913. doi:10.1016/j.phymed.2021.153913
64. Zou J, Ma Q, Sun R, et al. Dihydroartemisinin inhibits HepG2.2.15 proliferation by inducing cellular senescence and autophagy. BMB Rep. 2019;52(8):520–524. doi:10.5483/BMBRep.2019.52.8.058
65. Wu B, Xu C, Ding HS, et al. Galangin inhibits neointima formation induced by vascular injury via regulating the PI3K/AKT/mTOR pathway. Food Funct. 2022;13(23):12077–12092. doi:10.1039/D2FO02441A
66. Zhang H, Li N, Wu J, et al. Galangin inhibits proliferation of HepG2 cells by activating AMPK via increasing the AMP/TAN ratio in a LKB1-independent manner. Eur J Pharmacol. 2013;718(1–3):235–244. doi:10.1016/j.ejphar.2013.08.026
67. Yao YX, Yu YJ, Dai S, et al. Kaempferol efficacy in metabolic diseases: molecular mechanisms of action in diabetes mellitus, obesity, non-alcoholic fatty liver disease, steatohepatitis, and atherosclerosis. Biomed Pharmacother. 2024;175:116694. doi:10.1016/j.biopha.2024.116694
68. Han B, Yu YQ, Yang QL, Shen CY, Wang XJ. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget. 2017;8(49):86227–86239. doi:10.18632/oncotarget.21043
69. Pan MS, Cao J, Fan YZ. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. ChinMed. 2020;15:55. doi:10.1186/s13020-020-00338-6
70. Sun CY, Zhu Y, Li XF, et al. Norcantharidin alone or in combination with crizotinib induces autophagic cell death in hepatocellular carcinoma by repressing c-Met-mTOR signaling. Oncotarget. 2017;8(70):114945–114955. doi:10.18632/oncotarget.22935
71. Li X, Li Y, Wang Y, et al. Sinensetin suppresses angiogenesis in liver cancer by targeting the VEGF/VEGFR2/AKT signaling pathway. Exp Ther Med. 2022;23(5):360. doi:10.3892/etm.2022.11287
72. Kim SM, Ha SE, Lee HJ, et al. Sinensetin induces autophagic cell death through p53-related AMPK/mTOR signaling in hepatocellular carcinoma HepG2 cells. Nutrients. 2020;12(8):2462.
73. Meng X, Li M, Qiao M, Li X, Yang J, Hu J. Hugan buzure induces autophagy and apoptosis in hepatocellular carcinoma by inhibiting PI3K/Akt/mTOR signaling pathway. Evid Based Complement Alternat Med. 2022;2022:1618491. doi:10.1155/2022/1618491
74. Liu YJ, Xu JJ, Yang C, et al. Muscone inhibits angiotensin II-induced cardiac hypertrophy through the STAT3, MAPK and TGF-β/SMAD signaling pathways. Mol Biol Rep. 2023;51(1):39. doi:10.1007/s11033-023-08916-1
75. Qi W, Li Z, Yang C, et al. Inhibitory mechanism of muscone in liver cancer involves the induction of apoptosis and autophagy. Oncol Rep. 2020;43(3):839–850. doi:10.3892/or.2020.7484
76. Li Z, Shen Y, Xin J, et al. Cryptotanshinone alleviates radiation-induced lung fibrosis via modulation of gut microbiota and bile acid metabolism. Phytother Res. 2023;37(10):4557–4571. doi:10.1002/ptr.7926
77. Luo Y, Song L, Wang X, et al. Uncovering the mechanisms of cryptotanshinone as a therapeutic agent against hepatocellular carcinoma. Front Pharmacol. 2020;11:1264. doi:10.3389/fphar.2020.01264
78. Shen J, Zhu X, Wu Z, Shi Y, Wen T. Uvangoletin, extracted from Sarcandra glabra, exerts anticancer activity by inducing autophagy and apoptosis and inhibiting invasion and migration on hepatocellular carcinoma cells. Phytomedicine. 2022;94:153793. doi:10.1016/j.phymed.2021.153793
79. Wang H, Jia X, Zhang M, et al. Isoliquiritigenin inhibits virus replication and virus-mediated inflammation via NRF2 signaling. Phytomedicine. 2023;114:154786. doi:10.1016/j.phymed.2023.154786
80. Song L, Luo Y, Li S, et al. ISL induces apoptosis and autophagy in hepatocellular carcinoma via downregulation of PI3K/AKT/mTOR pathway in vivo and in vitro. Drug Des Devel Ther. 2020;14:4363–4376. doi:10.2147/DDDT.S270124
81. Li M, Zhang C, Li X, Lv Z, Chen Y, Zhao J. Isoquercitrin promotes the osteogenic differentiation of osteoblasts and BMSCs via the RUNX2 or BMP pathway. Connective Tissue Res. 2019;60(2):189–199. doi:10.1080/03008207.2018.1483358
82. Shui L, Wang W, Xie M, et al. Isoquercitrin induces apoptosis and autophagy in hepatocellular carcinoma cells via AMPK/mTOR/p70S6K signaling pathway. Aging. 2020;12(23):24318–24332. doi:10.18632/aging.202237
83. Liang Q, Cai W, Zhao Y, et al. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol Res. 2020;158:104884. doi:10.1016/j.phrs.2020.104884
84. Yu H, Qiu Y, Pang X, et al. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR axis inactivation in human hepatocellular carcinoma. Mol Cancer Ther. 2017;16(12):2711–2723. doi:10.1158/1535-7163.MCT-17-0498
85. Lv C, Chen J, Huang F, Fang F, Li B. Melittin inhibits the proliferation migration and invasion of HCC cells by regulating ADAMTS9-AS2 demethylation. Toxicon. 2023;222:106996. doi:10.1016/j.toxicon.2022.106996
86. Mao F, Wu A. Polyphyllin I alleviates lipopolysaccharide-induced inflammation reduces pyroptosis in BEAS-2B and HPAEC cells by inhibiting NF-κB signaling. Allergol Immunopathol. 2022;50(4):23–30. doi:10.15586/aei.v50i4.591
87. Shi YM, Yang L, Geng YD, Zhang C, Kong LY. Polyphyllin I induced-apoptosis is enhanced by inhibition of autophagy in human hepatocellular carcinoma cells. Phytomedicine. 2015;22(13):1139–1149. doi:10.1016/j.phymed.2015.08.014
88. Di Petrillo A, Orrù G, Fais A, Fantini MC. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. 2022;36(1):266–278. doi:10.1002/ptr.7309
89. Ji Y, Li L, Ma YX, et al. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J Nutr Biochem. 2019;69:108–119. doi:10.1016/j.jnutbio.2019.03.018
90. Jiang Z, Gao L, Liu C, Wang J, Han Y, Pan J. Sarmentosin induces autophagy-dependent apoptosis via activation of Nrf2 in hepatocellular carcinoma. J Clin Transl Hepatol. 2023;11(4):863–876. doi:10.14218/JCTH.2022.00312
91. Yan C, Li Q, Sun Q, et al. Promising nanomedicines of shikonin for cancer therapy. Int j Nanomed. 2023;18:1195–1218. doi:10.2147/IJN.S401570
92. Zhang J, Shang L, Jiang W, Wu W. Shikonin induces apoptosis and autophagy via downregulation of pyrroline-5-carboxylate reductase1 in hepatocellular carcinoma cells. Bioengineered. 2022;13(3):7904–7918. doi:10.1080/21655979.2022.2052673
93. Hong ZS, Zhuang HB, Qiu CZ, et al. Tenacissoside H induces apoptosis and inhibits migration of colon cancer cells by downregulating expression of GOLPH3 gene. Evid Based Complement Alternat Med. 2020;2020:2824984. doi:10.1155/2020/2824984
94. Lin J, Ruan J, Zhu H, Chen Z, Chen J, Yu H. Tenacissoside H induces autophagy and radiosensitivity of hepatocellular carcinoma cells by PI3K/Akt/mTOR signaling pathway. Dose-Response. 2021;19(2):15593258211011023. doi:10.1177/15593258211011023
95. Chen Y, Li K, Zhao H, et al. Integrated lipidomics and network pharmacology analysis to reveal the mechanisms of berberine in the treatment of hyperlipidemia. J Transl Med. 2022;20(1):412. doi:10.1186/s12967-022-03623-0
96. Yu R, Zhang ZQ, Wang B, Jiang HX, Cheng L, Shen LM. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Can Cell Inter. 2014;14:49. doi:10.1186/1475-2867-14-49
97. Zhong Y, Liu J, Sun D, et al. Dioscin relieves diabetic nephropathy via suppressing oxidative stress and apoptosis, and improving mitochondrial quality and quantity control. Food Funct. 2022;13(6):3660–3673. doi:10.1039/D1FO02733F
98. Mao Z, Han X, Chen D, et al. Potent effects of dioscin against hepatocellular carcinoma through regulating TP53-induced glycolysis and apoptosis regulator (TIGAR)-mediated apoptosis, autophagy, and DNA damage. Br J Pharmacol. 2019;176(7):919–937. doi:10.1111/bph.14594
99. Wang D, Lu X, Wang E, Shi L, Ma C, Tan X. Salvianolic acid B attenuates oxidative stress-induced injuries in enterocytes by activating Akt/GSK3β signaling and preserving mitochondrial function. Eur J Pharmacol. 2021;909:174408. doi:10.1016/j.ejphar.2021.174408
100. Gong L, Di C, Xia X, et al. AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int j Oncol. 2016;49(6):2538–2548. doi:10.3892/ijo.2016.3748
101. Tan H, He Q, Li R, Lei F, Lei X. Trillin reduces liver chronic inflammation and fibrosis in carbon tetrachloride (CCl4) induced liver injury in mice. Immunol invest. 2016;45(5):371–382. doi:10.3109/08820139.2015.1137935
102. Zhan G, Wei T, Xie H, et al. Autophagy inhibition mediated by trillin promotes apoptosis in hepatocellular carcinoma cells via activation of mTOR/STAT3 signaling. Naunyn-Schmiedeberg’s Arch Pharmacol. 2024;397(3):1575–1587. doi:10.1007/s00210-023-02700-5
103. Ye R, Dai N, He Q, et al. Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed Pharmacother. 2018;105:962–973. doi:10.1016/j.biopha.2018.06.065
104. Lin JJ, Su JH, Tsai CC, Chen YJ, Liao MH, Wu YJ. 11-epi-Sinulariolide acetate reduces cell migration and invasion of human hepatocellular carcinoma by reducing the activation of ERK1/2, p38MAPK and FAK/PI3K/AKT/mTOR signaling pathways. Mar Drugs. 2014;12(9):4783–4798. doi:10.3390/md12094783
105. Zhang F, Zhang H, Qian W, et al. Matrine exerts antitumor activity in cervical cancer by protective autophagy via the Akt/mTOR pathway in vitro and in vivo. Oncol Lett. 2022;23(4):110. doi:10.3892/ol.2022.13230
106. Liu Y, Qi Y, Bai ZH, et al. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol Sin. 2017;38(1):120–132. doi:10.1038/aps.2016.104
107. Cheng W, Cheng Z, Xing D, Zhang M. Asparagus polysaccharide suppresses the migration, invasion, and angiogenesis of hepatocellular carcinoma cells partly by targeting the HIF-1α/VEGF signalling pathway in vitro. Evid Based Complement Alternat Med. 2019;2019:3769879. doi:10.1155/2019/3769879
108. Cheng W, Cheng Z, Weng L, Xing D, Zhang M. Asparagus Polysaccharide inhibits the Hypoxia-induced migration, invasion and angiogenesis of Hepatocellular Carcinoma Cells partly through regulating HIF1α/VEGF expression via MAPK and PI3K signaling pathway. J Cancer. 2021;12(13):3920–3929. doi:10.7150/jca.51407
109. Zhang Y, Li K, Ying Y, et al. C21 steroid-enriched fraction refined from Marsdenia tenacissima inhibits hepatocellular carcinoma through the coordination of Hippo-Yap and PTEN-PI3K/AKT signaling pathways. Oncotarget. 2017;8(66):110576–110591. doi:10.18632/oncotarget.22833
110. Deng X, Luo T, Li Z, et al. Design, synthesis and anti-hepatocellular carcinoma activity of 3-arylisoquinoline alkaloids. Eur J Med Chem. 2022;228:113985. doi:10.1016/j.ejmech.2021.113985
111. Wu YJ, Wei WC, Dai GF, Su JH, Tseng YH, Tsai TC. Exploring the mechanism of flaccidoxide-13-acetate in suppressing cell metastasis of hepatocellular carcinoma. Mar Drugs. 2020;18(6). doi:10.3390/md18060314
112. Xing J, Bhuria V, Bui KC, et al. Haprolid inhibits tumor growth of hepatocellular carcinoma through Rb/E2F and Akt/mTOR inhibition. Cancers. 2020;12(3):615. doi:10.3390/cancers12030615
113. Li H, Lai Z, Yang H, Peng J, Chen Y, Lin J. Hedyotis diffusa Willd. inhibits VEGF‑C‑mediated lymphangiogenesis in colorectal cancer via multiple signaling pathways. Oncol Rep. 2019;42(3):1225–1236. doi:10.3892/or.2019.7223
114. Huang L, Xu H, Wu T, Li G. Hedyotis diffusa Willd. Suppresses hepatocellular carcinoma via downregulating AKT/mTOR pathways. Evid Based Complement Alternat Med. 2021;2021:5210152. doi:10.1155/2021/5210152
115. Xing S, Yu W, Zhang X, et al. Isoviolanthin extracted from dendrobium officinale reverses TGF-β1-mediated epithelial-mesenchymal transition in hepatocellular carcinoma cells via deactivating the TGF-β/Smad and PI3K/Akt/mTOR signaling pathways. Int J Mol Sci. 2018;19(6):1556. doi:10.3390/ijms19061556
116. Jung TW, Kim H, Park SY, et al. Stachydrine alleviates lipid-induced skeletal muscle insulin resistance via AMPK/HO-1-mediated suppression of inflammation and endoplasmic reticulum stress. J Endocrinol Invest. 2022;45(11):2181–2191. doi:10.1007/s40618-022-01866-8
117. Chen X, Yan N. Stachydrine inhibits TGF-β1-induced epithelial-mesenchymal transition in hepatocellular carcinoma cells through the TGF-β/Smad and PI3K/Akt/mTOR signaling pathways. Anti-Cancer Drugs. 2021;32(8):786–792. doi:10.1097/CAD.0000000000001066
118. Kuo TJ, Jean YH, Shih PC, et al. Stellettin B-induced oral cancer cell death via endoplasmic reticulum stress-mitochondrial apoptotic and autophagic signaling pathway. Int J Mol Sci. 2022;23(15):8813. doi:10.3390/ijms23158813
119. Tsai TC, Wu WT, Lin JJ, Su JH, Wu YJ. Stellettin B isolated from Stelletta Sp. reduces migration and invasion of hepatocellular carcinoma cells through reducing activation of the MAPKs and FAK/PI3K/AKT/mTOR signaling pathways. Int J Cell Biol. 2022;2022:4416611. doi:10.1155/2022/4416611
120. Stärkel P, De Saeger C, Delire B, et al. Tetrahydro ISO-alpha acids and hexahydro iso-alpha acids from hops inhibit proliferation of human hepatocarcinoma cell lines and reduce diethylnitrosamine induced liver tumor formation in rats. Nutr Cancer. 2015;67(5):748–760. doi:10.1080/01635581.2015.1032429
121. Yassin NYS, AbouZid SF, El-Kalaawy AM, Ali TM, Almehmadi MM, Ahmed OM. Silybum marianum total extract, silymarin and silibinin abate hepatocarcinogenesis and hepatocellular carcinoma growth via modulation of the HGF/c-Met, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways. Biomed Pharmacother. 2022;145:112409. doi:10.1016/j.biopha.2021.112409
122. Vishnoi K, Ke R, Saini KS, et al. Berberine represses β-catenin translation involving 4E-BPs in hepatocellular carcinoma cells. Mol Pharmacol. 2021;99(1):1–16. doi:10.1124/molpharm.120.000029
123. Yang B, Zhang D, Qian J, Cheng Y. Chelerythrine suppresses proliferation and metastasis of human prostate cancer cells via modulating MMP/TIMP/NF-κB system. Mol Cell Biochem. 2020;474(1–2):199–208. doi:10.1007/s11010-020-03845-0
124. Zhu Y, Pan Y, Zhang G, et al. Chelerythrine inhibits human hepatocellular carcinoma metastasis in vitro. Biol Pharm Bull. 2018;41(1):36–46. doi:10.1248/bpb.b17-00451
125. Zhang H, Jian B, Kuang H. Pharmacological effects of cinobufagin. Med Sci Monit. 2023;29:e940889. doi:10.12659/MSM.940889
126. Jin X, Wang J, Zou S, et al. Cinobufagin triggers defects in spindle formation and cap-dependent translation in liver cancer cells by inhibiting the AURKA-mTOR-eIF4E axis. Am J Chin Med. 2020;48(3):651–678. doi:10.1142/S0192415X20500330
127. Yang A, Huang H, Xie J, et al. Interfering with the AKT/mTOR/STAT3/ID1 signaling axis with usenamine A restrains the proliferative and invasive potential of human hepatocellular carcinoma cells. ChinMed. 2024;19(1):4. doi:10.1186/s13020-023-00875-w
128. Kim EA, Lee JH, Heo SJ, Jeon YJ. Saringosterol acetate isolated from Hizikia fusiforme, an edible brown alga, suppressed hepatocellular carcinoma growth and metastasis in a zebrafish xenograft model. Chem Biol Interact. 2021;335:109362. doi:10.1016/j.cbi.2020.109362
129. Ruan Q, Wang C, Zhang Y, Sun J. Ruscogenin attenuates cartilage destruction in osteoarthritis through suppressing chondrocyte ferroptosis via Nrf2/SLC7A11/GPX4 signaling pathway. Chem Biol Interact. 2024;388:110835. doi:10.1016/j.cbi.2023.110835
130. Hua H, Zhu Y, Song YH. Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2018;101:115–122. doi:10.1016/j.biopha.2018.02.031
131. Khan MA, Tania M. Cordycepin and kinase inhibition in cancer. Drug Discovery Today. 2023;28(3):103481. doi:10.1016/j.drudis.2022.103481
132. Zeng Y, Lian S, Li D, et al. Anti-hepatocarcinoma effect of cordycepin against NDEA-induced hepatocellular carcinomas via the PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathway in mice. Biomed Pharmacother. 2017;95:1868–1875. doi:10.1016/j.biopha.2017.09.069
133. Guoyin Z, Hao P, Min L, Wei G, Zhe C, Changquan L. Antihepatocarcinoma effect of Portulaca oleracea L. in mice by PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathway. Evid Based Complement Alternat Med. 2017;2017:8231358. doi:10.1155/2017/8231358
134. Dai X, Sun F, Deng K, et al. Mallotucin D, a clerodane diterpenoid from croton crassifolius, suppresses HepG2 cell growth via inducing autophagic cell death and pyroptosis. Int J Mol Sci. 2022;23(22):14217.
135. Zeng D, Zhang L, Luo Q. Celastrol-regulated gut microbiota and bile acid metabolism alleviate hepatocellular carcinoma proliferation by regulating the interaction between FXR and RXRα in vivo and in vitro. Front Pharmacol. 2023;14:1124240. doi:10.3389/fphar.2023.1124240
136. Jin L, Kim EY, Chung TW, et al. Hemistepsin A suppresses colorectal cancer growth through inhibiting pyruvate dehydrogenase kinase activity. Sci Rep. 2020;10(1):21940. doi:10.1038/s41598-020-79019-1
137. Baek SY, Hwang UW, Suk HY, Kim YW. Hemistepsin A inhibits cell proliferation and induces G0/G1-phase arrest, cellular senescence and apoptosis via the AMPK and p53/p21 signals in human hepatocellular carcinoma. Biomolecules. 2020;10(5):713. doi:10.3390/biom10050713
138. Rodenak-Kladniew B, Castro A, Stärkel P, De Saeger C, García de Bravo M, Crespo R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018;199:48–59. doi:10.1016/j.lfs.2018.03.006
139. Yi B, Zhang S, Yan S, et al. Marsdenia tenacissima enhances immune response of tumor infiltrating T lymphocytes to colorectal cancer. Front Immunol. 2023;14:1238694. doi:10.3389/fimmu.2023.1238694
140. Lin S, Sheng Q, Ma X, et al. Marsdenia tenacissima extract induces autophagy and apoptosis of hepatocellular cells via MIF/mToR signaling. Evid Based Complement Alternat Med. 2022;2022:7354700. doi:10.1155/2022/7354700
141. Yang Y, He P, Li N. The antitumor potential of extract of the oak bracket medicinal mushroom Inonotus baumii in SMMC-7721 tumor cells. Evid Based Complement Alternat Med. 2019;2019:1242784. doi:10.1155/2019/1242784
142. Ko J, Bao C, Park HC, et al. β-Thujaplicin modulates estrogen receptor signaling and inhibits proliferation of human breast cancer cells. Biosci Biotechnol Biochem. 2015;79(6):1011–1017. doi:10.1080/09168451.2015.1008978
143. Zhang G, He J, Ye X, et al. β-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death Dis. 2019;10(4):255. doi:10.1038/s41419-019-1492-6
144. Bie B, Sun J, Li J, et al. Baicalein, a natural anti-cancer compound, alters microRNA expression profiles in Bel-7402 human hepatocellular carcinoma cells. Cell Physiol Biochem. 2017;41(4):1519–1531. doi:10.1159/000470815
145. Hu P, Hu L, Chen Y, et al. Chaetocochin J exhibits anti-hepatocellular carcinoma effect independent of hypoxia. Bioorg Chem. 2023;139:106701. doi:10.1016/j.bioorg.2023.106701
146. Dai G, Wang D, Ma S, et al. ACSL4 promotes colorectal cancer and is a potential therapeutic target of emodin. Phytomedicine. 2022;102:154149. doi:10.1016/j.phymed.2022.154149
147. Qin B, Zeng Z, Xu J, et al. Emodin inhibits invasion and migration of hepatocellular carcinoma cells via regulating autophagy-mediated degradation of snail and β-catenin. BMC Cancer. 2022;22(1):671. doi:10.1186/s12885-022-09684-0
148. Jiang N, Li S, Meng L, et al. Gamabufotalin inhibits colitis-associated colorectal cancer by suppressing transcription factor STAT3. Eur J Pharmacol. 2024;966:176372. doi:10.1016/j.ejphar.2024.176372
149. Liu L, Shi D, Xia ZY, et al. Gamabufotalin induces apoptosis and cytoprotective autophagy through the mTOR signaling pathway in hepatocellular carcinoma. J Natural Prod. 2023;86(4):966–978. doi:10.1021/acs.jnatprod.2c01155
150. Abusaliya A, Bhosale PB, Kim HH, et al. Investigation of prunetrin induced G2/M cell cycle arrest and apoptosis via Akt/mTOR/MAPK pathways in hepatocellular carcinoma cells. Biomed Pharmacother. 2024;174:116483. doi:10.1016/j.biopha.2024.116483
151. Abusaliya A, Jeong SH, Bhosale PB, et al. Mechanistic action of cell cycle arrest and intrinsic apoptosis via inhibiting Akt/mTOR and activation of p38-MAPK signaling pathways in Hep3B liver cancer cells by Prunetrin-A flavonoid with therapeutic potential. Nutrients. 2023;15(15):3407. doi:10.3390/nu15153407
152. Gong X, Zhou Y, Wu P, et al. The petroleum ether extracts of Chloranthus fortunei (A. Gray) Solms-Laub. With bioactivities: a rising source in HCC drug treatment. J Ethnopharmacol. 2024;333:118414. doi:10.1016/j.jep.2024.118414
153. Lee HJ, Venkatarame Gowda Saralamma V, Kim SM, et al. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients. 2018;10(8):1043. doi:10.3390/nu10081043
154. Wu T, Dong X, Yu D, Shen Z, Yu J, Yan S. Natural product pectolinarigenin inhibits proliferation, induces apoptosis, and causes G2/M phase arrest of HCC via PI3K/AKT/mTOR/ERK signaling pathway. Onco Targets Ther. 2018;11:8633–8642. doi:10.2147/OTT.S186186
155. Triaa N, Znati M, Ben Jannet H, Bouajila J. Biological activities of novel oleanolic acid derivatives from bioconversion and semi-synthesis. Molecules. 2024;29(13):3091. doi:10.3390/molecules29133091
156. Wang X, Bai H, Zhang X, et al. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis. 2013;34(6):1323–1330. doi:10.1093/carcin/bgt058
157. Liu M, Zhang Y, Zhang A, et al. Compound K is a potential clinical anticancer agent in prostate cancer by arresting cell cycle. Phytomedicine. 2023;109:154584. doi:10.1016/j.phymed.2022.154584
158. Shin N, Lee HJ, Sim DY, et al. Apoptotic effect of compound K in hepatocellular carcinoma cells via inhibition of glycolysis and Akt/mTOR/c-Myc signaling. Phytother Res. 2021;35(7):3812–3820. doi:10.1002/ptr.7087
159. Huang H, Xue J, Xie T, Xie ML. Osthole increases the radiosensitivity of hepatoma cells by inhibiting GSK-3β/AMPK/mTOR pathway-controlled glycolysis. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;396(4):683–692. doi:10.1007/s00210-022-02347-8
160. Huang H, Xue J, Xie ML, Xie T. Osthole inhibits GSK-3β/AMPK/mTOR pathway-controlled glycolysis and increases radiosensitivity of subcutaneous transplanted hepatocellular carcinoma in nude mice. Strahlentherapie und Onkologie. 2024;200(5):444–452. doi:10.1007/s00066-023-02173-8
161. Biji M, Prabha B, Lankalapalli RS, Radhakrishnan KV. Transition metal/Lewis acid catalyzed reactions of zerumbone for diverse molecular motifs. Chem Rec. 2021;21(12):3943–3953. doi:10.1002/tcr.202100206
162. Wani NA, Zhang B, Teng KY, et al. Reprogramming of glucose metabolism by zerumbone suppresses hepatocarcinogenesis. Mol Cancer Res. 2018;16(2):256–268. doi:10.1158/1541-7786.MCR-17-0304
163. Cho AR, Park WY, Lee HJ, et al. Antitumor effect of morusin via G1 arrest and antiglycolysis by AMPK activation in hepatocellular cancer. Int J Mol Sci. 2021;22(19):10619. doi:10.3390/ijms221910619
164. Fan M, Chen Z, Shao W, et al. SREBP2 inhibitor betulin sensitizes hepatocellular carcinoma to lenvatinib by inhibiting the mTOR/IL-1β pathway. Acta Biochim Biophys Sin. 2023;55(9):1479–1486. doi:10.3724/abbs.2023122
165. Han Y, Pan L, Ran S, et al. Rhizoma Paridis saponins ameliorates hepatic fibrosis in rats by downregulating expression of angiogenesis‑associated growth factors. Mol Med Rep. 2019;19(5):3548–3554. doi:10.3892/mmr.2019.10006
166. Yao J, Man S, Dong H, Yang L, Ma L, Gao W. Combinatorial treatment of Rhizoma Paridis saponins and sorafenib overcomes the intolerance of sorafenib. J Steroid Biochem Mol Biol. 2018;183:159–166. doi:10.1016/j.jsbmb.2018.06.010
167. Teng CF, Yu CH, Chang HY, et al. Chemopreventive effect of phytosomal curcumin on hepatitis B virus-related hepatocellular carcinoma in A transgenic mouse model. Sci Rep. 2019;9(1):10338. doi:10.1038/s41598-019-46891-5
168. Abdollah MRA, Ali AA, Elgohary HH, Elmazar MM. Antiangiogenic drugs in combination with seaweed fucoidan: a mechanistic in vitro and in vivo study exploring the VEGF receptor and its downstream signaling molecules in hepatic cancer. Front Pharmacol. 2023;14:1108992. doi:10.3389/fphar.2023.1108992
169. Jing W, Shuo L, Yingru X, et al. Artesunate promotes sensitivity to sorafenib in hepatocellular carcinoma. Biochem Biophys Res Commun. 2019;519(1):41–45. doi:10.1016/j.bbrc.2019.08.115
170. Niu B, Wei S, Sun J, Zhao H, Wang B, Chen G. Deciphering the molecular mechanism of tetrandrine in inhibiting hepatocellular carcinoma and increasing sorafenib sensitivity by combining network pharmacology and experimental evaluation. Pharm Biol. 2022;60(1):75–86. doi:10.1080/13880209.2021.2017468
171. Hu Y, Luo Z, Cai S, Xie Q, Zheng S. Glycyrrhizic acid attenuates sorafenib resistance by inducing ferroptosis via targeting mTOR signaling in hepatocellular carcinoma. Scand J Gastroenterol. 2024;59(6):730–736. doi:10.1080/00365521.2024.2315317
172. Huang XF, Sheu GT, Chang KF, Huang YC, Hung PH, Tsai NM. Pogostemon cablin triggered ROS-induced DNA damage to arrest cell cycle progression and induce apoptosis on human hepatocellular carcinoma in vitro and in vivo. Molecules. 2020;25(23):5639. doi:10.3390/molecules25235639
173. Zhang Z, Shen C, Zhou F. The natural medicinal fungus Huaier promotes the anti-hepatoma efficacy of sorafenib through the mammalian target of rapamycin-mediated autophagic cell death. Med Oncol. 2022;39(12):221. doi:10.1007/s12032-022-01797-7
174. Wang -T-T, Hong Y-F, Chen Z-H, et al. Synergistic effects of α-Mangostin and sorafenib in hepatocellular carcinoma: new insights into α-mangostin cytotoxicity. Biochem Biophys Res Commun. 2021;558:14–21. doi:10.1016/j.bbrc.2021.04.047
175. Tan Z, Li X, Chen X, et al. Ellagic acid inhibits tumor growth and potentiates the therapeutic efficacy of sorafenib in hepatocellular carcinoma. Heliyon. 2024;10(1):e23931. doi:10.1016/j.heliyon.2023.e23931
176. Üremiş MM, Üremiş N, Türköz Y. Cucurbitacin E shows synergistic effect with sorafenib by inducing apoptosis in hepatocellular carcinoma cells and regulates Jak/Stat3, ERK/MAPK, PI3K/Akt/mTOR signaling pathways. Steroids. 2023;198:109261. doi:10.1016/j.steroids.2023.109261
177. El-Sewedy T, Salama AF, Mohamed AE, et al. Hepatocellular carcinoma cells: activity of Amygdalin and Sorafenib in targeting AMPK /mTOR and BCL-2 for anti-angiogenesis and apoptosis cell death. BMC Complement Med Therap. 2023;23(1):329. doi:10.1186/s12906-023-04142-1
178. Guo N, Yan A, Gao X, et al. Berberine sensitizes rapamycin‑mediated human hepatoma cell death in vitro. Mol Med Rep. 2014;10(6):3132–3138. doi:10.3892/mmr.2014.2608
179. Dai B, Ma Y, Yang T, et al. Synergistic effect of berberine and HMQ1611 impairs cell proliferation and migration by regulating Wnt signaling pathway in hepatocellular carcinoma. Phytother Res. 2019;33(3):745–755. doi:10.1002/ptr.6267
180. Refolo MG, Lippolis C, Carella N, Cavallini A, Messa C, D’Alessandro R. Chlorogenic acid improves the regorafenib effects in human hepatocellular carcinoma cells. Int J Mol Sci. 2018;19(5):1518. doi:10.3390/ijms19051518
181. El-Hanboshy SM, Helmy MW, Abd-Alhaseeb MM. Catalpol synergistically potentiates the anti-tumour effects of regorafenib against hepatocellular carcinoma via dual inhibition of PI3K/Akt/mTOR/NF-κB and VEGF/VEGFR2 signaling pathways. Mol Biol Rep. 2021;48(11):7233–7242. doi:10.1007/s11033-021-06715-0
182. Sun R, Zhai R, Ma C, Miao W. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med. 2020;9(3):1141–1151. doi:10.1002/cam4.2723
183. Zhang HH, Zhang Y, Cheng YN, et al. Metformin incombination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol Carcinog. 2018;57(1):44–56. doi:10.1002/mc.22718
184. Al Refaey HR, Newairy AA, Wahby MM, et al. Manuka honey enhanced sensitivity of HepG2, hepatocellular carcinoma cells, for Doxorubicin and induced apoptosis through inhibition of Wnt/β-catenin and ERK1/2. Biol Res. 2021;54(1):16. doi:10.1186/s40659-021-00339-1
185. Xia J, Rong L, Sawakami T, et al. Shufeng Jiedu Capsule and its active ingredients induce apoptosis, inhibit migration and invasion, and enhances doxorubicin therapeutic efficacy in hepatocellular carcinoma. Biomed Pharmacother. 2018;99:921–930. doi:10.1016/j.biopha.2018.01.163
186. Zong S, Li J, Yang L, et al. Mechanism of bioactive polysaccharide from Lachnum sp. acts synergistically with 5-fluorouracil against human hepatocellular carcinoma. J Cell Physiol. 2019;234(9):15548–15562. doi:10.1002/jcp.28202
187. Jin M, Kong L, Han Y, Zhang S. Gut microbiota enhances the chemosensitivity of hepatocellular carcinoma to 5-fluorouracil in vivo by increasing curcumin bioavailability. Phytother Res. 2021;35(10):5823–5837. doi:10.1002/ptr.7240
188. Wang W, Xiao Y, Li S, et al. Synergistic activity of magnolin combined with B-RAF inhibitor SB590885 in hepatocellular carcinoma cells via targeting PI3K-AKT/mTOR and ERK MAPK pathway. Am J Transl Res. 2019;11(6):3816–3824.
189. Xiao Y, Gong Q, Wang W, et al. The combination of Biochanin A and SB590885 potentiates the inhibition of tumour progression in hepatocellular carcinoma. Can Cell Inter. 2020;20:371.
190. Chen MY, Hsu CH, Setiawan SA, et al. Ovatodiolide and antrocin synergistically inhibit the stemness and metastatic potential of hepatocellular carcinoma via impairing ribosome biogenesis and modulating ERK/Akt-mTOR signaling axis. Phytomedicine. 2023;108:154478. doi:10.1016/j.phymed.2022.154478
191. Zhao Z, Song J, Zhang D, Wu F, Tu J, Ji J. Oxysophocarpine suppresses FGFR1-overexpressed hepatocellular carcinoma growth and sensitizes the therapeutic effect of lenvatinib. Life Sci. 2021;264:118642. doi:10.1016/j.lfs.2020.118642
192. Yi J, Gong X, Yin XY, et al. Parthenolide and arsenic trioxide co-trigger autophagy-accompanied apoptosis in hepatocellular carcinoma cells. Front Oncol. 2022;12:988528. doi:10.3389/fonc.2022.988528
193. Khan MW, Zhao P, Khan A, et al. Synergism of cisplatin-oleanolic acid co-loaded calcium carbonate nanoparticles on hepatocellular carcinoma cells for enhanced apoptosis and reduced hepatotoxicity. Int j Nanomed. 2019;14:3753–3771. doi:10.2147/IJN.S196651
194. Liu C, Lin X, Huang M, et al. Babaodan inhibits cell proliferation and metastasis and enhances anti-tumor effects of camrelizumab by inhibiting M2 phenotype macrophages in hepatocellular carcinoma. J Ethnopharmacol. 2024;334:118540. doi:10.1016/j.jep.2024.118540
195. Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi:10.1158/1078-0432.CCR-04-0744
196. Kanai M, Otsuka Y, Otsuka K, et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol. 2013;71(6):1521–1530. doi:10.1007/s00280-013-2151-8
197. Bayet-Robert M, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9(1):8–14. doi:10.4161/cbt.9.1.10392
198. Mahammedi H, Planchat E, Pouget M, et al. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology. 2016;90(2):69–78. doi:10.1159/000441148
199. Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62(8):1137–1141. doi:10.1080/01635581.2010.513802
200. Gao H, Wang J. Andrographolide inhibits multiple myeloma cells by inhibiting the TLR4/NF-κB signaling pathway. Mol Med Rep. 2016;13(2):1827–1832. doi:10.3892/mmr.2015.4703
201. Gunn EJ, Williams JT, Huynh DT, et al. The natural products parthenolide and andrographolide exhibit anti-cancer stem cell activity in multiple myeloma. Leukemia Lymphoma. 2011;52(6):1085–1097. doi:10.3109/10428194.2011.555891
202. Zhang Y, Li X, Zou D, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93(7):2559–2565. doi:10.1210/jc.2007-2404
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

