Back to Journals » Journal of Pain Research » Volume 17
The CT-Imaging Location of Lumbar 3 Sympathetic Radiofrequency Thermocoagulation for Sympathetic-Related Diseases Therapy
Received 10 April 2024
Accepted for publication 13 November 2024
Published 26 November 2024 Volume 2024:17 Pages 4011—4022
DOI https://doi.org/10.2147/JPR.S473078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Krishnan Chakravarthy
Ruxiang Wang,1,* Zhuoyue Gao,2,* Ling Ma1
1Department of Anesthesiology and Pain Medicine, Affiliated Hospital of Jiaxing University, Jiaxing, People’s Republic of China; 2Department of Anesthesiology, Daqing Oilfield General Hospital, Daqing, 163001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ling Ma, Email [email protected]
Purpose: The location characteristic of the lumbar 3 sympathetic trunk under Computed Tomography (CT) was discovered through 106 cases, imaging analysis after successful lumbar 3 sympathetic radiofrequency thermocoagulation operations serving the clinic and reducing the operation time.
Methods: There are 113 patients underwent bilateral L3 lumbar sympathetic thermal radiofrequency procedures in our hospital from January 2017 to January 2021, with 106 cases of successful procedure. Four operation image distances were measured: 1. The left/right distances between the needle tip (the location of needle tip was the sympathetic trunk at the same CT scan level) and the transverse process of the lumbar spine (D1l/D1r); 2. The left/right distances between the needle tip and the medial margin of the psoas major (D2l/D2r); 3. The left/right vertical distances between the needle tip and vertebral body (D3l/D3r); 4. The left/right vertical distances between ureter and vertebral body (D4l/D4r). The Perfusion Index (PI) and the plantar temperature were monitored and recorded before and after the treatment (the higher PI value and the plantar temperature indicated successful procedure). After the procedure, the patients were followed up one day, one week, two months and six months for satisfaction, complications and recurrences.
Results: The left distance (D1l) from the needle tip to the transverse costal process being 4.444± 0.7668mm, longer than the right side (D1r, P< 0.001). The left distance (D2l) being 1.260± 0.4261mm longer than the right side (D2r, P=0.0039). The left distance (D3l) was 1.634± 0.2597mm longer than the right side (D3r, P< 0.0001). The D4l and D4r both having a long distance far from needle tips (P=0.665). Both the left and right temperature and PI increased have statistical significances after treatment (P< 0.0001). There were 2(1.77%) cases experiencing numbness of big thighs, and 9(7.96%) cases of compensatory hyperhidrosis, with only 3 (2.65%) cases reverting to the original state six months later.
Conclusion: Lumbar sympathetic radiofrequency thermocoagulation is a valid treatment option for sympathetic-related disease in lower limbs, and based on our study data CT-guided percutaneous puncture lumbar sympathectomy can easy be proceed and gained more persistent effection, the left needle distance more deeper than the right side, the distance from the left side should be far from vertebral body than the right side. The distance between the needle body and the vertebral body on the left side is far away from the right side.
Keywords: sympathetic, radiofrequency thermocoagulation, CT-guided
Introduction
Patients with lower limb sympathetic nerve-related diseases such as hyperhidrosis, lower limb chills, diabetes feet and lower limb artery embolism receive modulation therapy of lumbar sympathetic nerve.1–5 Percutaneous lumbar sympathetic nerve radiofrequency therapy technology has better advantages in the maintenance of the effect and the lower incidence of complications compared with drug therapy, based on recent reports.6–8 Previous reports show that the effective rate of Radiofrequency lumbar sympathectomy was 30% to 80%,9,10 despite having longer-term procedure effect. Differences in patient selection may have been a major factor for this wide range. However, in this study, we found that the position of the inserted radiofrequency needle was very important to the effect, and difficult to locate. When we adjusted the position of the needle tip under CT guidance, the distance of 3mm often get different effect, but once the exact position was located, the effect of sympathectomy was immediately observed. Therefore, in clinical operation, the accurate location of lumbar sympathetic nerve is very important. Currently, there are no records on the exact target position at which the radiofrequency needle reached for lumbar sympathectomy.11 The objective of the study was to identify the character of sympathectomy exact target by CT-guided.
Method
Patients and Imagings
A total of 113 patients (42 males and 71 females), diagnosed with lower limb sympathetic nerve-related diseases, underwent Percutaneous Radiofrequency Thermocoagulation of Sympathetic nerve (PRTS), with 106 cases successful operation at the Pain Department of the Affiliated Hospital of Jiaxing University from April 2020 to April 2021, were enrolled in the study. This study got ethical approval from the Affiliated Hospital of Jiaxing University. All patients read and signed the informed consent, with the patient's name withheld in this study. All methods are carried out in accordance with relevant ethical guidelines and regulations. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Inclusion and Exclusion
Patients inclusion criteria for analysis were as follows: (1) over 18 years old; (2) lower limb sympathetic nerve-related diseases only involved below the knee joint but not thigh; (3) visual analog scale (VAS) of ≥4; (4) Had 3 times increase in Pulse Index (PI) and 2 degrees increase in the temperature immediately after operation.
The exclusion criteria for analysis included: (1) Severe depression or mental disease; (2) Severe brain, heart, lung, liver, or kidney disease; (3) Poor blood coagulation function; (4) Puncture site or systemic infection. (5) No increase or decrease in the pulse index and the temperature after the operation.
Surgical Procedure
The patients were placed in a prone position with soft pillows under their abdomen on the table for Computed Tomography (CT) treatment (Figure 1A). The positioning grid was fixed on the bilateral lumbar 3 paravertebral sides using adhesive tape. The temperature monitoring probes were bundled on the soles of the patients, feet. Throughout the operation patients, Heart Rate (HR), Blood Pressure (NIBP), and Oxygen Saturation (SpO2) were continuously monitored (Figure 1B and C). Monitoring and recording were done using the Perfusion Index (PI) (Masimo Corp, Irvine, CA, USA) (white arrow in Figure 1C and F), and Temperature of the plantar (T) (PhysitempNTE-2A; Physitemp Instruments, Clifton, NJ, USA) (white arrow in Figure 1A and red star in Figure 1C and D and showed T value), respectively (Figure 1).
Firstly, the L3 vertebral body was marked and positioned (Figure 2a). The L3 vertebral body was first marked and placed. The anterolateral of the L3 vertebral body and the medial anterior border of the psoas major was selected as the puncture targets under the guidance of CT.8,11 The transverse process of L3 was chosen as the punctured plane, and a straight line was drawn from the tip of the transverse process to the lateral edge of the vertebral body, with the skin puncture point on this line (Figure 2b).
Secondly, 1.0% lidocaine hydrochloride was used for local anesthesia and CT guidance was used for advancing 15 cm 22-gauge needle from the skin puncture point to the target position. We started by locating the target of the needle tip on the outside of the vertebral body based on previous studies (Figure 3a). After administering intravenous analgesic drugs, we applied 95°radiofrequency thermocoagulation for 300 seconds. If the position was accurate, the lower limb temperature gradually increased 60 seconds after the PI value increased following the drop and then the rise of the PI value, which could take place in about 30 seconds. If the aforementioned PI value and temperature remain unchanged, they were not close to the nerves. Then, we adjusted the needle tip in several directions including away from and close to the vertebral body side, a deeper depth or withdrawal (Figure 3b) until PI and temperature rose. The patient's PI, T, and VAS scores were recorded before treatment (T1) and after treatment for 10 minutes (T2).
Once the needle was pulled out, the puncture point was compressed. The patient was then returned to the ward with normal vital signs after 15 min observation.
Observation Indexes
Data documented for each patient comprised:
• General information about patients.
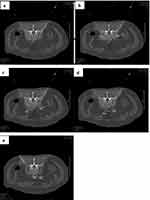
• After operation successful (Figure 4a), we measured the several data in the picture: The left/right distance between the puncture needle tip and the transverse process of the lumbar spine (D1l/D1r) (Figure 4b); The left/right distance between the puncture needle tip and the edge of the psoas major muscle (D2l/D2r) (Figure 4c); The left/right distance between the puncture needle tip and the vertebral body on the same side (D3l/D3r) (Figure 4d); The left/right vertical distance from ureter to vertebral body (D4l/D4r) (Figure 4e).
•The right/left perfusion index (PI) before and after treatment.
•The right/left plantar temperature (T) before and after treatment.
• Presence/absence of postprocedure complications.
Statistical Analysis
SPSS version 26.0 software (IBM, Chicago, USA) was employed for all data analysis. The Shapiro–Wilk test was used to determine whether the measurement data obeyed a normal distribution, and the results were expressed as the mean ± Std. Error of Mean (SEM), while the nonnormally distributed data were expressed as the median (IQR). A paired sample t-test was used to compare the differences distance value among measure data. Also, paired sample t-test were used to compare the differences PI and temperature between different time. Box Scatter Chart was used to describe the different distances, PI and temperature.
Results
Analysis of the Clinical Characteristics of Patients
In our study, 113 patients were enrolled. They included 42 males and 71 females, with mean-median IQR of 55 (44–64) years, ranged 16–78 years at the Pain Department of the Affiliated Hospital of Jiaxing University (Cold hypersensitivity in feet 99 cases, Diabetic neuropathy in feet 12 cases and Lower extremity atherosclerotic occlusive disease 2 cases) (Table 1). Successful lumbar 3 sympathetic radiofrequency thermocoagulation under CT-guided was performed on 106 (93.8%) of the patients enrolled. A total of 96 patients underwent bilateral lumbar 3 sympathetic radiofrequency thermocoagulation (SRT), and only 7 cases proceed left lumbar 3 SRT with 3 cases doing right sides (Table 2).
 |
Table 1 Baseline Characteristics of Patients |
 |
Table 2 Study Population Summary Statistics |
CT Imagings Investigation
Analysis of morphological measurements of 106 CT imaging was done. The D1l and D1r were 58.92±0.6137mm, 54.71±0.7672mm, respectively, with the differences between them statistically significant (***P < 0.001). The D1l from the needle tip to transverse costal process was 4.444±0.7668mm longer than D1r (Table 3A and B) (Figure 4b). The D2l and D2r were statistically significant (**P < 0.01), with the differences between them being 9.570±0.3190mm and 8.320±0.3297mm, respectively. The D2l from the needle tip to the edge of the psoas major muscle was 1.260±0.4261mm longer than D2r (Table 3A and C) (Figure 4c). The D3l were 6.169±0.2380mm and D3r were 4.169±0.1561mm, and the differences between them were statistically significant (***P < 0.001). The D3l from the needle tip to the vertebral body was 1.634±0.2597mm longer than D3r (Table 3A and D, Figure 4d).
 |
Table 3 The Right/Left Distance from the Puncture Needle Tip to the Different Targets of the Lumbar Spine |
Before the operation, 69 patients were given intravenous contrast agents. The vertical distance (D4l/D4r) from the bilateral ureters image displayed to the Lumbar 3 vertebral body was measured (Figure 4e). The D4l and D4r were 16.02±0.6296mm and 15.73±0.4915mm, respectively, while the left/right distances from the ureter to the vertebral body were not statistically significance (P=0.6653) (Table 4A and B).
 |
Table 4 There are Total 69 Patients Were Given Intravenous Contrast Agent to Show L3 Level Ureter Before Operation (4-1). The Left/Right Vertical Distance from Ureter to Vertebral Body (D4l/D4r)(4-2) |
Temperature(T)/Perfusion Index (PI) Changed Before and After the Operation
A total of 96 patients successfully underwent bilateral lumbar 3 sympathetic radiofrequency thermocoagulation. The before and after the temperature of left lumbar 3 sympathetic radiofrequency thermocoagulation were 28.55±0.1869°C and 33.07±0.1991°C respectively, with statistically significant difference (***P<0.0001). The temperature after treatment increased by 4.521°C±0.2466°C. The before and after temperatures of right lumbar 3 sympathetic radiofrequency thermocoagulation were 28.42±0.2009°C, 32.94±0.2292°C, respectively, with statistically significantly differences (***P<0.0001). The temperature after treatment increased by 4.526±0.2352°C, but there was no significant difference between the left and right temperature before treatment (P>0.05, not significant), and the same was the case for the left and right temperature after treatment. The left/right PI after the lumbar 3 sympathetic radiofrequency thermocoagulation was significantly increased compared to before the treatment (***P<0.0001). The PI after treatment increased by 6.052±0.3246 (left) and 6.178±0.3078 (right). There was no significant difference between the left and right PI before the treatment (P>0.05, not significant), with the same case for the left and right temperature after the treatment (Table 5).
 |
Table 5 Temperature/Perfusion Index (PI) Changes Before and After Operation |
There were 2 (1.77%) cases experiencing numbness of big thighs, and 9(7.96%) cases of compensatory hyperhidrosis, with only 3 (2.65%) cases reverting to the original state six months later by followed-up.
Discussion
Over two centuries of surgical practice,1–3 lumbar sympathectomy was initially developed as a treatment for Peripheral Vascular Disease (PAD).2,4,5 In recent years, lumbar sympathetic nerve modulation has been used to treat chronic pain conditions such as complex regional pain syndrome, phantom limb pain, and post-herpetic neuralgia.12–15 Therefor, the types of diseases around lumbar sympathetic associated with lumbar sympathetic modulation are extensive, and technological innovation has become an irreversible trend.16
Open or retroperitoneoscopic techniques can be used for surgical lumbar sympathectomy, and anatomical or radiologically guided techniques can be done using percutaneous chemical sympathectomy or high-temperature thermocoagulation.17–19 The operation technology transforming from open to minimally invasive requires more accurate skill and less damage. Radiofrequency thermocoagulation sympathectomy may need more accurate needle placement than percutaneous chemical sympathectomy, but it has lower complication rates and a longer effect period.6 To achieve the objective of the treatment, Continue Radiofrequency Thermocoagulation (CRFT) generates high-frequency current, induction oscillation in tissues, heats tissues, increases local temperature, and inactivates hypersensitive nerve endings caused by pathological changes through thermal effect.20 Currently, it is hypothesized that the analgesic effect of CRFT may be through the permanent blocking of neural signals through the neural pathway.21 The temperature and time of RFT vary with the location of the operation, and the temperature of CRFT was treated for 90 to 120s at 80 to 95°C.22,23 The exposed end of the radiofrequency needle expands the operating range with an increase in temperature. In our study, the primary purpose of choosing CRFT at 95°C for 300 s was to increase nerve damage and extend the effect period.
Our study contributes to new body of knowledge for accurate needle placement during lumbar 3 sympathetic radiofrequency thermocoagulation under CT-guided techniques. Radiofrequency thermocoagulation sympathectomy may need a more precise needle placement but has lower complication rates and get longer effection period compared with percutaneous chemical sympathectomy.24,25 Nevertheless, there is a lack of information on the exact location of the radiofrequency needle for the lumbar sympathetic radiofrequency thermocoagulation procedure. In our study, 106 cases of lumbar 3 sympathetic radiofrequency thermocoagulation (L3SRT) were successfully performed using CT-guided. Using imaging analysis from successful L3SRT cases, the exact location of the needle tip can be determined. Our study is the first to successfully describe the L3SRT under CT guidance while delivering exact needle data. In our study, the left distance from the needle tip to the transverse costal process was 4.444±0.7668mm longer than right side. The left distance from the needle tip to the edge of the psoas major muscle was 1.260±0.4261mm longer than the right side (D2r), while the left distance (D3l) from needle tip to the vertebral body was 1.634±0.2597mm longer than the right side (D3r). This indicated that the left needle tip on the left side is deeper and farther from the vertebral body than right side (Figure 4b–d). The puncture route we redesigned based on these features made it easier to gain sympathectomy effect and reduced the operation time.
The duration of effective maintenance after CRFT is a concern. Multivariate logistic regression analysis by Luo et al26 showed that operation time was one of the risk factors affecting the success rate of the Radiofrequency Thermocoagulation of the Lumbar Sympathetic Nerve. Most of the time spent in surgery is primarily to locate the nerves. These are exactly the challenges that need to be solved in our study. Previous studies on standard radiofrequency thermocoagulation of nerves indicated that over a year after the operation, the total effect rate was 86.67%–100%.27–29 Radiofrequency themolucation, which is accurate and controllable, is used by CRFT to destroy the sympathetic nerve. It might reduce the extent of tissue injury and the likelihood of postoperative complications. In this study, only two patients experienced lateral femoral cutaneous numbness during the two-year follow-up after the operation which subsided without intervention. Only one patient developed genitofemoral neuralgia, which might be related to the distance from the lateral femoral cutaneous nerve and genitofemoral nerve to the lumbar sympathetic trunk in various patients. Feigl et al observed that the gap between the genitofemoral nerve and the lumbar sympathetic trunk was 0–28 mm at the level of L3/4, while some of them <5 mm.8 Consequently, chemical lumbar sympathectomy can very easily damage the genitofemoral nerve. Only 3 (2.65%) cases reverting to their original state in our study after half a year of follow-up.
In our study, the Lumbar 3 sympathetic nerve was primarily selected because it is far away from the kidney at this level, and based on imaging observation, there is a large space for the nerve making it easier to avoid the inferior vena cava and abdominal aorta. Additionally, 69 patients received an intravenous contrast agent before the operation to show L3 level ureter in our study. The left and right vertical distances (D4l and D4r) from the ureter to the vertebral body were 16.02±0.6296 and 15.73±0.4915, respectively. The left/right distances from the ureter to the vertebral body were not statistically significant. These showed that puncture at this level can avoid ureteral injury which had not been previously reported (Figure 4e, Table 4). In future studies, we will expect other measurement data from other segments.
After half a year of follow-up in our study, 2 (1.77%) cases experienced numbness on big thighs, and 9 (7.96%) cases had compensatory hyperhidrosis, with only 3 (2.65%) cases reverting to their original state. All these translate to long effection and fewer side effects after radiofrequency treatment of the lumbar sympathetic nerve.
Limitations
There are some limitations in our study. Firstly, only the sympathetic nerve of lumbar 3 was enrolled in this research, and Lumbar 2 and Lumbar 4 were not included in the study. The results inform the clinical practice is more suitable for the radiofrequency surgery of sympathetic nerve under CT-guided, and not fit for X-ray. In addition, the single-center, facility nature and retrospective nature of the review produces moderate selection bias. Furthermore future multicenter, multisegmental nerve data can give more clinical guidance.
Conclusions
Previous imaging analysis revealed that lumbar sympathetic radiofrequency thermocoagulation requires a more accurate target. The higher sympathetic nerve precision positions, fewer adjacent nerve injuries and better control set it apart from chemical sympathectomy. Our retrospective study revealed several anatomical characteristics of the L3 sympathetic nerve under CT that can provide an accurate target for the sympathetic radiofrequency thermocoagulation pathway. Based on our study data CT-guided percutaneous puncture lumbar sympathectomy can easy be proceed (Figure 1) and gained more persistent effection, the left needle distance more deeper than the right side, the distance from the left side should be far from vertebral body than the right side. The distance between the needle body and the vertebral body on the left side is far away from the right side.
Ethics Approval
This study got ethical approval (2022-LY-360) from the Affiliated Hospital of Jiaxing University. Our study complies with the Declaration of Helsinki.
Informed consent: All patients read and signed the informed consent before the operation, with the patient's name withheld in this study.
Acknowledgments
Ruxiang Wang and Zhuoyue Gao are co-first authors and contributed equally to this work. This work was supported by the Affiliated Hospital of Jiaxing University. The authors declare no conflicts of interest with regard to the work.
Funding
This study was supported by the Jiaxing City Science and Technology Plan(2021AD30143), Natural Science Foundation of Zhejiang Province of China (LY20H090020, LGF20H090021, LQ19H090007), Clinical Medicine Special Fund Project of Zhejiang Medical Association (2022ZYC-Z35), Jiaxing Key Laboratory of Neurology and Pain Medicine, Jiaxing Provinces and Cities Jointly Cultivate Discipline --General Surgery (Minimally Invasive) (2023-PYXK-001) and Zhejiang Provincial Clinical Key Specialties-Anesthesiology (2023-ZJZK-001).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fontaine R. History of lumbar sympathectomy from its origin to the present. Acta Chir Belg. 1977;76(1):3–16.
2. Langeron P, Bastide G. Lumbar sympathectomy for arteritis. A reliable and (almost) seventy year-old technique. Chirurgie. 1992;118(9):522–528.
3. Ewing M. The history of lumbar sympathectomy. Surgery. 1971;70(5):790–796.
4. Ferket BS, Spronk S, Colkesen EB, et al. Systematic review of guidelines on peripheral artery disease screening. Am J Med. 2012;125(2):198–208.e3. doi:10.1016/j.amjmed.2011.06.027
5. Wong PF, Chong LY, Mikhailidis DP, et al. Antiplatelet agents for intermittent claudication. Cochrane Database Syst Rev. 2013;11:CD001272. doi:10.1002/14651858
6. Ding Y, Yao P, Hongxi L, et al. Evaluation of combined radiofrequency and chemical blockade of multi-segmental lumbar sympathetic ganglia in painful diabetic peripheral neuropathy. J Pain Res. 2018;Volume 11:1375–1382. doi:10.2147/JPR.S175514
7. Lantsberg L, Goldman M, Khoda J. Should chemical sym pathectomy precede below knee amputation? Int Surg. 1996;81(1):85–87.
8. Feigl GC, Dreu M, Ulz H, et al. Susceptibility of the genitofemoral and lateral femoral cutaneous nerves to complications from lumbar sympathetic blocks: is there a morphological reason? †. British Journal of Anaesthesia. 2014;112(6):1098–1104. doi:10.1093/bja/aet552
9. Straube S, Derry S, Moore RA, et al. Cervico-thoracic or lumbar sympathectomy for neuropathic pain and complex regional pain syndrome. Cochrane Database Syst Rev. 2013;9:CD002918. doi:10.1002/14651858
10. Zacharias NA, Karri J, Garcia C, et al. Interventional radiofrequency treatment for the sympathetic nervous system: a review article. Pain Ther. 2021;10(1):115–141. doi:10.1007/s40122-020-00227-8
11. Murata Y, Takahashi K, Yamagata M, et al. Variations in the number and position of human lumbar sympathetic ganglia and rami communicantes. Clin Anat. 2003;16(2):108–113. doi:10.1002/ca.10069
12. Abramov R. Lumbar sympathetic treatment in the management of lower limb pain. Curr Pain Headache Rep. 2014;18(4):403. doi:10.1007/s11916-014-0403-x
13. Spiegel MA, Hingula L, Chen GH, et al. The use of L2 and L3 lumbar sympathetic blockade for cancer-related pain, an experience and recommendation in the oncologic population. Pain Med. 2020;21(1):176–184. doi:10.1093/pm/pnz142
14. Lee Y, Lee CJ, Choi E, et al. Lumbar sympathetic block with botulinum toxin type A and type B for the complex regional pain syndrome. Toxins;2018:10. doi:10.3390/toxins11010010
15. Duong S, Bravo D, Todd KJ, et al. Treatment of complex regional pain syndrome: an updated systematic review and narrative synthesis. Can J Anaesth. 2018;65:658–684. doi:10.1007/s12630-018-1091-5
16. Ruiz-Aragon J, Marquez Calderon S. Effectiveness of lumbar sympathectomy in the treatment of occlusive peripheral vascular disease in lower limbs: systematic review. Med Clin. 2010;134(11):477–482. doi:10.1016/j.medcli.2009.09.039
17. Lee YC, You YK, Lee S, et al. Evaluation of blood perfusion using laser Doppler flowmetry during endoscopic lumbar sympathectomy in patients with plantar hyperhidrosis: a retrospective observational study. Sci Rep. 2022;12(1):11456. doi:10.1038/s41598-022-14778-7
18. Hur KJ, Moon HW, Park YH. Retroperitoneoscopic lumbar sympathectomy for the treatment of primary plantar hyperhidrosis. BMC Surg. 2021;21(1):397. doi:10.1186/s12893-021-01393-y.
19. Nesargikar PN, Ajit MK, Eyers PS, et al. Lumbar chemical sympathectomy in peripheral vascular disease: does it still have a role? Int J Surg. 2009;7(2):145–149. doi:10.1016/j.ijsu.2009.01.004
20. Wang Z, Wang Z, Li K, et al. Radiofrequency thermocoagulation for the treatment of trigeminal neuralgia. Exp Ther Med. 2021;23(1):17. doi:10.3892/etm.2021.10939
21. Kırcelli A, Demirçay E, Özel Ö, et al. Radiofrequency thermocoagulation of the ganglion impar for coccydynia management: long-term effects. Pain Pract. 2019;19(1):9–15. doi:10.1111/papr.12698
22. Grandhi RK, Kaye AD, Abd-Elsayed A. Systematic review of radiofrequency ablation and pulsed radiofrequency for management of cervicogenic headaches. Curr Pain Headache Rep. 2018;22(3):18. doi:10.1007/s11916-018-0673-9
23. Wang T, Xu S, He Q, et al. Efficacy and safety of radiofrequency thermocoagulation with different puncture methods for treatment of V1 trigeminal neuralgia: a prospective study. Pain Physician. 2021;24:145–152.
24. Jiachun Tao MD, Bing Huang MD, Jiayi Tang MD, et al. Comparison of efficacy and safety of lumbar sympathetic radiofrequency thermocoagulation versus chemical lumbar sympathectomy in the treatment of cold hypersensitivity in the hands and feet: a retrospective study. Pain Physician. 2022;25:357–364.
25. Oh TK, Kim NW, Yim J, et al. Effect of radiofrequency thermocoagulation of thoracic nerve roots in patients with cancer and intractable chest wall pain. Pain Physician. 2018;21:323–329.
26. Luo G, Zhu J, Chen R, et al. Risk factors affecting the success rate of radiofrequency thermocoagulation of lumbar sympathetic nerve. Pain Physician. 2021;24:1075–1083.
27. Zhu J, Luo G, Qiuli H, et al. Evaluation of the efficacy of unipolar and bipolar spinal dorsal root ganglion radiofrequency thermocoagulation in the treatment of postherpetic neuralgia. Korean J Pain. 2022;35(1):114–123. doi:10.3344/kjp.2022.35.1.114
28. Elawamy A, Abdalla EEM, Shehata GA. Effects of pulsed versus conventional versus combined radio radiofrequency for the treatment of trigeminal neuralgia: a prospective study. Pain Physician. 2017;20:873–881.
29. Song L, He L, Pei Q, et al. CT-guided percutaneous radiofrequency thermocoagulation for glos sopharyngeal neuralgia: a retrospective clinical study of 117 cases. Clin Neurol Neurosurg. 2019;178:42–45. doi:10.1016/j.clineuro.2019.01.013
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.