Back to Journals » Clinical Ophthalmology » Volume 18
The Effect of Prism Presentation Order on Near Vertical Fusional Vergence Ranges of Normal Young Adults
Authors Gantz L , Shneor E , Shaw N, Doron R
Received 25 August 2024
Accepted for publication 8 October 2024
Published 26 November 2024 Volume 2024:18 Pages 3473—3484
DOI https://doi.org/10.2147/OPTH.S492994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Liat Gantz, Einat Shneor, Natalie Shaw, Ravid Doron
Department of Optometry and Vision Science, Hadassah Academic College, Jerusalem, Israel
Correspondence: Liat Gantz, Email [email protected]
Purpose: Fusional vergence ranges (FVR) quantify the oculomotor system’s ability to overcome heterophoria, playing a critical role in diagnosis and treatment. This study investigated the effect of prism order on near vertical FVR using the smooth and step methods.
Methods: Normal participants were randomly assigned to either the smooth or step testing method and to Base-Up (BU, infravergence) first or Base Down (BD, supravergence) first prism type. After an hour, they crossed over to the alternative testing method and prism-type. The mean of two consecutive measurements of break and recovery values for one eye in each of the 16 experimental conditions were compared using the Friedman test with post-hoc Bonferroni correction, and non-parametric Bland and Altman analysis.
Results: The mean break/recovery values of 27 participants (mean age: 22.5± 2.0, range: 20– 29, 20 female) when BU or BD were measured first were BU: 4.20± 1.15Δ/3.07± 1.04Δ and BD: 4.20± 1.21Δ/3.00± 0.96Δ for step, and BU: 4.31± 0.98Δ/2.97± 0.85Δ and BD: 4.15± 1.14Δ/2.70± 0.87Δ for smooth methods, respectively. When measured second, they were BU: 3.74± 1.02Δ/2.63± 0.93Δ and BD: 3.85± 1.09Δ/2.64± 1.06Δ for step, BU: 3.91± 0.99Δ/2.73± 0.93Δ and BD: 3.81± 1.04Δ/2.54± 1.04Δ for smooth, respectively. The Friedman test found a significant effect of prism order on break values of the smooth (p< 0.001) and step (p< 0.02) methods, and recovery values of the step method (p< 0.005), but post-hoc tests showed no significant differences. Mean differences were below 0.50Δ indicating clinical insignificance.
Conclusion: Unlike horizontal FVR, prism order does not affect near vertical FVR using the smooth and step. This simplifies clinical assessment and suggests that horizontal and vertical fusion systems may be treated as separate entities.
Plain Language Summary: This study examined whether the order in which prisms are presented affects the measurement of near vertical fusional vergence ranges—a measure of the ability of the eye’s muscles to move in order to avoid double vision. Researchers tested 27 participants using two methods (smooth and step) and presented the prisms in different orders: Base-Up (BU) first or Base-Down (BD) first. Findings show that the order of prism presentation did not significantly affect the results, meaning that the measurements remained consistent regardless of whether BU or BD was tested first. This suggests that, unlike the effect of prism order on horizontal eye muscle movements, vertical eye muscle movement measurements are not influenced by the sequence in which tests are conducted. These results are important because they simplify clinical assessments – and clinicians can confidently measure vertical fusional vergence without worrying about the order of prism presentation. Findings also imply that the systems controlling horizontal and vertical eye movements may function independently of each other.
Keywords: vertical fusional vergence ranges, vertical fusional amplitudes, vertical fusional reserves, vertical heterophoria, vertical prism vergence ranges, vertical motor fusion
Introduction
Fusional vergence ranges describe the ability of the reflexive oculomotor components of the visual system to overcome retinal disparity in order to maintain fusion and avoid diplopia.1,2 The fusional vergence system should be able to compensate for the heterophoria in order to allow binocular single vision.3 Thus, for exophoria, sufficient convergence ranges are necessary, whereas for esophoria sufficient divergence ranges are necessary.4 When the vergence range is able to overcome the heterophoria, the heterophoria is considered compensated.5 However, when it is unable to overcome the heterophoria, it is considered decompensated and is associated with symptoms of asthenopia.6
The fusional vergence range is measured clinically both in the horizontal and vertical directions. Reduced ranges may indicate underlying vergence dysfunction or an inability to overcome deviations.7 These disorders can be accompanied by symptoms of diplopia, blurred vision, headaches, asthenopia, inability to concentrate during sustained visual tasks, sleepiness, and loss of place during reading.8 Further, fusional vergence ranges can be used to prescribe prisms in the management of ocular deviations.7
Fusional vergence ranges may be measured using a prism bar, rotary prisms or the synoptophore,9–13 each of which examine different aspects of fusion.14,15 The synoptophore, and rotary Risley prisms measure smooth vergence.16,17 The prism bar measures the step vergence.16,17 Smooth vergences describe a gradual, incrementally progressing step size; whereas step vergences describe non-gradual progression in the step sizes. The step method is considered to emulate natural viewing conditions.18 A recent systematic review concluded that the normative values for step vergence testing vary with age with considerable differences between adults and children.17
Vergence or prism adaptation describes the change that occurs in vergence eye movements as a result of a retinal disparity induced by placement of a prism in front of the eye.19 Thus, vergence adaptation from the clinical testing of fusional vergence ranges could affect subsequent measurements. This has been demonstrated in horizontal fusional vergence ranges.18,20–22 Divergence ranges have been shown to be significantly reduced when measured after convergence ranges.20 This has been attributed to a strong convergence response, which may continue even after the stimulus is no longer present.18,20,21 It has also been suggested that the vergence direction that is measured second is biased by the vergence direction that is measured first.20
Therefore, it was suggested that the testing paradigm should first measure the direction of vergence that compensates for the heterophoria finding.20
Thus, it is possible, similar to its effect on horizontal fusional vergence, the order of prism presentation may influence the vertical fusional vergence range. A previous study, which reported no significant differences between distance and near vertical fusional vergence ranges, randomized the direction of prism introduced in front of the eyes during the measurements.23 However, the effect of prism order on vertical fusional vergence ranges has not been previously investigated and could have implications on the measured outcomes.
Horizontal fusional vergence ranges are also affected by the testing method (smooth vs step). Mean fusional vergence ranges measured with prism bar were found to be significantly higher (by 0.60Δ- 3.8Δ) than rotary prism ranges.15 The intrasession variability of the step vergence testing method was found to be significantly higher than the smooth vergence testing method.24 The effect of testing method on vertical fusional vergence ranges has also not been previously evaluated.
Therefore, this study examined if the order of prism presentation (Base-Up vs Base-Down) affects vertical fusional reserves measurement in a particular testing method (smooth or step).
Methods
Participants
Students and staff from Hadassah Academic College were recruited. Exclusion criteria included strabismus, horizontal fusional ranges that did not meet Sheard’s criteria,25 visual acuity worse than 0.0 logMAR for distance and near, remote near point of convergence with an accommodative target, or amplitude of accommodation lower than age expected normative values.7 Stereopsis was measured using the Special Edition Randot Test (Bernell, USA) to ensure a threshold of 20.
The study conformed to the tenets of the Declaration of Helsinki and was approved by the Hadassah Academic College internal review board. A statement of informed consent was signed after participants received an oral explanation about the study.
All examinations took place in the eye clinics at the Department of Optometry, Hadassah Academic College, with participants wearing their habitual correction.
Experimental Procedures
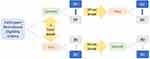
Tests were performed by two fourth year optometry students under the supervision of a licensed Israeli Optometrist. There were two methods of testing fusional vergence reserves: smooth and step. The smooth method was measured using a phoropter. The step method was measured using a prism bar. For both methods the same visual target (J2 sized letter, approximately 0.8 in Snellen decimal, 0.73 mm height) was used at a fixed distance of 40 cm verified with a meterstick. Each method of testing was measured with four prism orders (Base-Up (BU) first or second; measuring infravergence,26 Base-Down (BD) first or second; measuring supravergence26), yielding a total of eight testing conditions, as described in the flow chart in Figure 1. The prism testing order (Base-Up first or Base-Up second) and testing method (Smooth or Step) were counterbalanced across participants. The rate of progressing the prisms in the prism bar was approximately 1 prism-diopter per second and was approximately the same for all measurements. Each condition was measured twice in one randomly determined eye. Therefore, for each participant, the vertical fusional reserves were measured 16 times. As an example, a participant that was allocated to the Base-Up prism first and the smooth followed by step methods, was measured as follows: smooth method with Base-Up, followed by smooth method with Base-Down, and then the step method with Base-Up, followed by the step method with Base-Down. Then, at least one hour later, the same participant was measured with the step method with Base-Down followed by the step method with Base- Up, and then the smooth method with Base-Down followed by the smooth method with Base Up. A break of at least ten seconds was given between each measurement condition. Thus, after the participant above was measured using the smooth method with Base-Up prism and then with Base-Down prism, at least ten seconds later the same participant was measured using the step method with the same prism order (Base-Up followed by Base-Down).
Vertical fusional ranges were measured by increasing the vertical prism power until fusion was interrupted and the patient reported seeing double (“break” point). Prisms were then reduced until fusion was regained and the patient reported seeing one object (“recovery” point).7 As described above, this procedure was repeated two consecutive times for each experimental condition (testing method X prism) and the results were averaged for the analysis.
Statistical Analysis
Break and recovery points were recorded for each condition for each participant. Data were not normally distributed based on the Shapiro–Wilks test, and the effect of prism order (BU first vs BD first) on vertical fusional ranges measured with two testing methods (smooth vs step) was assessed using the Friedman test, with Bonferroni-corrected post-hoc comparisons. Spearman correlation tests and non- parametric Bland and Altman analysis27,28 were also employed to examine the interchangeability of the prism testing orders (BU first vs BD first). Using the non-parametric Bland and Altman analysis, an interval range of ±1Δ was employed based on the instrumental error of the step vergence method17 and a previous study.23
All statistical tests were calculated using SPSS statistical package version 24 (SPSS Inc, Chicago, IL, USA). Bland-Altman plots were created using Excel (Microsoft 365, Redmond, WA, USA).
Sample Size Calculation
Using G*Power software (version 3.1.9.6)29 the minimum sample size required when comparing the same group of participants for a power (1- β) of 83%, error probability of 5% (α error 0.05) and an effect size (d) of 0.60Δ, was 27 participants.
This effect size (0.60Δ) was selected as the step size in the vertical prism bar is 0.50Δ, and this is the minimal difference which is greater than the vertical prism bar step size. However, this effect size is much lower than previously reported standard deviations of measured vertical fusional amplitudes, which are greater than 1.00Δ.23 When an effect size (d) of 1.00Δ is calculated, then the power increases to 99%.
Results
Participants
The clinical outcome measures of 27 participants (median age: 22.0 (IQ1: 21.0, IQ3: 24.0) mean age: 22.5 ± 2.0, range: 20.0–29.0 years, 20 female, 7 male) are tabulated in Table 1. All participants had good distance and near acuity, and normal binocularity.
 |
Table 1 Clinical Outcome Measures of the 27 Participants. The Means (±standard Deviations), Medians, Range, and First and Third Quartiles (Q1, Q3) for Each Outcome Measure are Listed in the Rows |
Effect of Prism Order
Break Values
The mean vertical break value (see Table 2) for the step method was 4.20±1.15Δ when BU was measured first and 4.20±1.21Δ when BD was measured first. For the smooth method it was 4.31±0.98Δ when BU was measured first, and 4.15±1.14Δ when BD was measured first.
The mean vertical break value for the step method was 3.74 ± 1.02Δ when BU was measured second and 3.85 ± 1.09 when BD was measured second. For the smooth method it was 3.91 ± 0.99Δ when BU was measured second, and 3.81 ± 1.04Δ when BD was measured second.
Although the Friedman test applied to the smooth method showed a significant effect of prism order on the break values (χ2(3) = 16.26, p< 0.001), the post-hoc analysis (Bonferroni-corrected) did not find significant differences for both BU-first compared with BU-second (Z=0.87, p=0.08), or BD-first compared with BD-second (Z=−0.76, p=0.18).
The Friedman test applied to the step method showed a significant effect of prism order on the break values (χ2(3) = 10.25, p< 0.02). However, the post-hoc analysis (Bonferroni-corrected) did not find significant differences for both BU-first compared with BU-second (Z=0.74, p=0.21), or BD-first compared with BD-second (Z=−0.63, p=0.43).
Recovery Values
The mean vertical recovery value (see Table 3) for the step method was 3.07 ± 1.04Δ when BU was measured first and 3.00 ± 0.96Δ when BD was measured first. For the smooth method it was 2.97 ± 0.85Δ when BU was measured first, and 2.70 ± 0.87Δ when BD was measured first.
The mean vertical recovery value for the step method was 2.63 ± 0.93 Δ when BU was measured second and 2.65 ± 1.06 Δ when BD was measured second. For the smooth method it was 2.74 ± 0.93 Δ when BU was measured second, and 2.54 ± 1.04Δ when BD was measured second.
The Friedman test applied to the smooth method showed no significant effect of prism order on the recovery values (χ2(3) = 6.77, p= 0.08). For the step method, the Friedman test showed a significant effect of prism order on the recovery values (χ2(3) = 13.34, p= 0.004), without significant differences for both BU-first compared with BU-second (Z=0.85, p=0.09), and BD-first compared with BD-second (Z=−0. 74, p=0.21) in the post-hoc analysis (Bonferroni-corrected).
The non-parametric Bland-Altman analysis (Figures 2 and 3, Table 4) demonstrated that the median differences (and the mean differences) between the prism orders for all conditions was lower than 0.50Δ with 78% to 93% of the observations falling within the confidence intervals and clinically acceptable limits of agreement (within ±1.00Δ), though the confidence intervals were wide.
 |
Table 4 Correlation and Non-Parametric Bland and Altman Analysis Assessing the Interchangeability Between the Prism Presentation Order for Infravergence (BU) and Supravergence (BD) Measured Using the Smooth and Step Methods. The Columns Depict Median Differences, Upper and Lower Confidence Intervals Calculated Based on the Quartiles, the Percentage of Observations Falling Within the Calculated Confidence Intervals, and the Clinically Acceptable Limits (± 2Δ).17 |
Discussion
This is the first study to examine the effect of prism-type (BU or BD) presentation on the near vertical fusional vergence ranges using the step and smooth methods. Findings show that for both smooth and step vertical fusional vergence measurements, the prism presentation order does not significantly affect the results. These results indicate that, in contrast with earlier reports pertaining to horizontal fusional vergence ranges,18,20,21 the order of prism presentation does not influence the clinical outcome when measuring vertical fusional vergence ranges. The mean differences for all prism orders for both step and smooth methods were smaller than 0.50Δ, which is lower than the minimal step size in the vertical prism bar, lower than the examiner’s resolution,17 and clinically insignificant.17,23 These findings also suggest that clinicians can measure vertical fusional vergence ranges without concern for the order of prism presentation, which simplifies the assessment process.
The relationship between horizontal and vertical fusional vergence ranges is equivocal. Based on the finding that only horizontal but not vertical vergence adaptation is reduced in individuals with convergence insufficiency, Braudaset et al30 concluded that horizontal and vertical vergences are independent mechanisms. In addition, Ulyat et al23 did not find correlations between the horizontal and vertical fusional ranges at 33 cm and at 6 meters. Further, Stevenson et al31 found that vertical fusional ranges were not influenced by the instruction to “track” or “fixate” but these instructions dramatically affected the horizontal fusional ranges. Finally, during lexical processing, the horizontal vergence system was found to be automatically activated, whereas the vertical vergence system was not activated. The horizontal and vertical vergence systems were also not found to be correlated, indicating independent mechanisms.3
Conversely to these findings, horizontal vergence position of the eyes has been shown to affect the vertical fusional vergence ranges.32 Specifically, vertical fusional ranges are greater when the eyes are converged at the beginning of the examination, indicating an interaction between the horizontal and vertical vergence systems. Additionally, induced vertical divergence reduces the horizontal fusional vergence range.33 Finally, horizontal vergence training also improves the vertical vergence ranges by a mean value of 0.58Δ.34
Previous studies examining the effect of prism order on horizontal fusional vergence ranges, report that the prism type can induce changes ranging between 0.50Δ.20 and 6Δ.18 In the present study, differences in the vertical fusional vergence ranges obtained with varying prism orders were all lower than 0.50Δ.
The findings of the present study align with those suggesting that the horizontal and vertical fusional vergence systems function as independent mechanisms.3,23,30,31 Thus, based on the findings reported herein, clinicians may consider diagnosing horizontal and vertical vergence disorders as distinct conditions, each potentially exhibiting unique characteristics and responses to therapeutic interventions.
Limitations of the Study
Vertical vergence amplitudes can be improved with practice.13 In the present investigation, each testing condition was measured two consecutive times. Therefore, it is possible that the findings of this study were impacted by a training effect. Further, in the clinical setting, patients are only measured once. To address this, the same analysis was applied to the initial (first) measurement for each experimental condition, and the findings did not change. Specifically, the Friedman test examining the effect of prism order on the break values for the step method did not find a significant effect (χ2(3) = 4.70, p=0.19). When applied to the smooth method there was a significant difference (χ2(3) = 14.37, p=0.002), but post-hoc analysis (Bonferroni-corrected) did not show significant differences for both BU-first compared with BU-second (Z=0.65, p=0.39), or BD-first compared with BD-second (Z=0.79, p=0.14). The Friedman test examining the effect of prism order on the recovery values was not significant for either the smooth (χ2(3) = 5.50, p=0.14) or step methods (χ2(3) = 4.99, p=0.17). Further, all differences were less than 0.50Δ which is clinically insignificant and lower than the examiner’s resolution.17,23
When comparing the BU-first vs BU-second and BD-first vs BD-second for both the step and smooth methods, post-hoc tests showed insignificant differences despite the initial Friedman test demonstrating statistical significance. This discrepancy may indicate that the post-hoc tests lack power.35,36 However, even if a significant statistical difference had been detected, the mean difference between the BU-first and BU-second, as well as the BD-first and BD-second for both methods was less than 0.50Δ, which is clinically insignificant and lower than the examiner’s resolution.17,23
The findings of this study were obtained at a testing distance of 40cm. Vertical fusion ranges have been reported to vary with testing distance.23 Therefore, it is possible that our findings are only applicable to near testing distances and cannot be generalized to other test distances which should be examined in further research studies.
Horizontal fusional vergence ranges are affected by target size.37 This study examined a J2 sized target. Therefore, the reported findings are relevant for the target sizes that were examined. Future studies can examine the effects of target size on the measurements.
The present study examined the vertical fusional ranges subjectively, as is common in clinical practice. Future studies can compare these findings to those obtained with objective means of measurement, as reported by others for horizontal vergence ranges.38–40
Although it is possible to induce undesired vertical prism through an unintended tilt of a large value of horizontal prism when measured using a prism bar,41 the values of the vertical fusional range are much lower and presumably did not introduce unintended horizontal deviations.
It is possible that the participant’s responses are affected by the previously tested method and prism. However, this is unlikely given that vergence adaptation typically requires several minutes and not seconds.42 In addition, in the present study, there were at least 10 seconds between the testing methods. Further, these conditions are similar to the standard testing conditions in the optometric clinic. Under the standard circumstances, fusional vergence testing is typically part of a full battery of testing. This testing battery may include heterophoria measurements, horizontal fusional vergence measurements, fixation disparity, etc. which employ prisms. These tests could also potentially affect the vertical fusion ranges. Therefore, the present study resembles standard clinical testing conditions.
Finally, the conclusions of this study are applicable only to populations with characteristics similar to those of the cohort examined.
Conclusions
The order of prism presentation does not affect near vertical fusional vergence reserves using the smooth and step methods in young adults. Therefore, clinicians may choose their preferred method and prism presentation order to examine patients in the clinic.
Acknowledgments
The authors thank Chanie Loring Rand, Adina Guttermann, and Sara Elana Friedman their assistance with the experiment and Halit Cantor for statistical assistance. The authors are also grateful to the late Prof. Michel Millodot for his assistance with the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pratt-Johnson JA. Central disruption of fusional amplitude. Br j Ophthalmol. 1973;57(5):347. doi:10.1136/bjo.57.5.347
2. Rashbass C, Westheimer G. Disjunctive eye movements. J Physiol. 1961;159(2):339–360. doi:10.1113/jphysiol.1961.sp006812
3. Nikolova M, Jainta S, Blythe HI, Jones MO, Liversedge SP. Vergence responses to vertical binocular disparity during lexical identification. Vision Res. 2015;106:27–35. doi:10.1016/j.visres.2014.10.034
4. Costa Lança C, Rowe FJ. Variability of fusion vergence measurements in heterophoria. Strabismus. 2016;24(2):63–69. doi:10.3109/09273972.2016.1159234
5. Crone RA. A new theory about heterophoria. Ophthalmologica. 1971;162(4–5):199–204. doi:10.1159/000306273
6. Yammouni R, Evans BJ. Is reading rate in digital eyestrain influenced by binocular and accommodative anomalies? J Optometry. 2021;14(3):229–239. doi:10.1016/j.optom.2020.08.006
7. Scheiman M, Wick B. Clinical Management of Binocular Vision.
8. Cacho-Martínez P, García-Muñoz Á, Ruiz-Cantero MT. Do we really know the prevalence of accomodative and nonstrabismic binocular dysfunctions? J Optometry. 2010;3(4):185–197. doi:10.1016/S1888-4296(10)70028-5
9. Fu T, Wang J, Levin M, Su Q, Li D, Li J. Fusional vergence detected by prism bar and synoptophore in Chinese childhood intermittent exotropia. J Ophthalmol. 2015;2015:1–6. doi:10.1155/2015/987048
10. Scheiman M, Herzberg H, Frantz K, Margolies M. A normative study of step vergence in elementary schoolchildren. J Am Optom Assoc. 1989;60(4):276–280.
11. Álvarez CP, Puell MC, Sánchez–Ramos C, Villena C. Normal values of distance heterophoria and fusional vergence ranges and effects of age. Graefes Arch Clin Exp Ophthalmol. 2006;244(7):821–824. doi:10.1007/s00417-005-0166-5
12. Chen AH, Abidin AHZ. Vergence and accommodation system in Malay primary school children. Malays J Med Sci. 2002;9(1):9.
13. Luu CD, Green Julie F, Abel L. Vertical fixation disparity curve and the effects of vergence training in a normal young adult population. Optometry Vision Sci. 2000;77(12):663–669. doi:10.1097/00006324-200012000-00013
14. Feldman JM, Cooper J, Carniglia P, Schiff FM, Skeete JN. Comparison of fusional ranges measured by Risley prisms, vectograms, and computer orthopter. Optom Vis Sci. 1989;66(6):375–382. doi:10.1097/00006324-198906000-00007
15. Goss DA, Becker E. Comparison of near fusional vergence ranges with rotary prisms and with prism bars. Optometry. 2011;82(2):104–107. doi:10.1016/j.optm.2010.09.011
16. O’Connor A, Stephenson G Comparison of tonic and phasic measures on motor fusion. 2008.
17. Lanca CC, Rowe FJ. Measurement of fusional vergence: a systematic review. Strabismus. 2019;27(2):88–113. doi:10.1080/09273972.2019.1583675
18. Sassonov O, Sassonov Y, Koslowe K, Shneor E. The effect of test sequence on measurement of positive and negative fusional vergence. Optom Vis Dev. 2010;41(1):24–27.
19. Sethi B. Vergence adaptation: a review. Documenta Ophthalmologica. 1986;63(3):247–263. doi:10.1007/BF00160760
20. Rosenfield M, Ciuffreda KJ, Ong E, Super S. Vergence adaptation and the order of clinical vergence range testing. Optom Vis Sci. 1995;72(4):219–223. doi:10.1097/00006324-199504000-00001
21. Fray KJ. Fusional amplitudes: exploring where fusion falters. Am Orthopt J. 2013;63(1):41–54. doi:10.3368/aoj.63.1.41
22. Goss D. Effect of test sequence on fusional vergence ranges. New England J Optometry. 1995;47(2):39–42.
23. Ulyat K, Firth AY, Griffiths HJ. Quantifying the vertical fusion range at four distances of fixation in a normal population. Brit and Irish Orthoptic J. 2004;1:43–45. doi:10.22599/bioj.244
24. Ciuffreda MA, Ciuffreda KJ, Wang B. Repeatability and variability of near vergence ranges. J Behav Optometry. 2006;17(2):39–46.
25. Worrell BE, Hirsch MJ, Morgan MW. An evaluation of prism prescribed by Sheard’s criterion. Am J Optom Arch Am Acad Optom. 1971;48(5):373–376. doi:10.1097/00006324-197105000-00001
26. Millodot M. Dictionary of optometry and visual science. Clin Exp Optometry. 2009;925:465.
27. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi:10.1177/096228029900800204
28. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi:10.1016/S0140-6736(86)90837-8
29. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/BF03193146
30. Brautaset RL, Jennings JAM. Horizontal and vertical prism adaptation are different mechanisms. Ophthalmic Physiol Opt. 2005;25(3):215–218. doi:10.1111/j.1475-1313.2005.00276.x
31. Stevenson S, Lott L, Yang J. The influence of subject instruction on horizontal and vertical vergence tracking. Vision Res. 1997;37(20):2891–2898. doi:10.1016/S0042-6989(97)00109-0
32. Hara N, Steffen H, Roberts DC, Zee DS. Effect of horizontal vergence on the motor and sensory components of vertical fusion. Invest Ophthalmol Visual Sci. 1998;39(12):2268–2276.
33. Richardson GA, Firth AY. The effect of induced vertical divergence on horizontal fusional amplitudes. Br Ir Orthopt J. 2009;60:71–74. doi:10.22599/bioj.13
34. Rutstein RP, Daum KM, Cho M, Eskridge JB. Horizontal and vertical vergence training and its effect on vergences, fixation disparity curves, and prism adaptation. II. Vertical data. Am J Optom Physiol Opt. 1988;65(1):8–13. doi:10.1097/00006324-198801000-00002
35. Johnstone EC, Owens DGC, Lawrie SM, McIntosh AM, Sharpe MD. Companion to Psychiatric Studies E-Book. Elsevier Health Sciences; 2010.
36. Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241–252. doi:10.1080/00401706.1964.10490181
37. Rowe FJ. Fusional Vergence Measures and Their Significance in Clinical Assessment. Strabismus. 2010;18(2):48–57. doi:10.3109/09273971003758412
38. Gao TY, Wong J, Zhou LW, et al. Objective estimation of fusional reserves using infrared eye tracking: the digital fusion-range test. Clin Exp Optom. 2023;106(7):769–776. doi:10.1080/08164622.2022.2134763
39. Rovira-Gay C, Mestre C, Argiles M, Vinuela-Navarro V, Pujol J. Feasibility of measuring fusional vergence amplitudes objectively. PLoS One. 2023;18(5):e0284552. doi:10.1371/journal.pone.0284552
40. Gantz L, Caspi A. Synchronization of a Removable Optical Element with an Eye Tracker: Test Case for Heterophoria Measurement. Trans. Vis. Sci. Tech. 2020;9(7):40. doi: 10.1167/tvst.9.7.40
41. Antona B, Barrio A, Barra F, Gonzalez E, Sanchez I. Repeatability and agreement in the measurement of horizontal fusional vergences. Ophthalmic Physiol Opt. 2008;28(5):475–491. doi:10.1111/j.1475-1313.2008.00583.x
42. Cooper J. Clinical implications of vergence adaptation. Optom Vis Sci. 1992;69(4):300–307. doi:10.1097/00006324-199204000-00008
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.