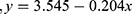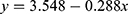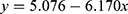Back to Journals » Clinical Ophthalmology » Volume 19
The Impact of Air Temperature and Pollution on Admissions for Acute Ophthalmic Inflammation at the Emergency Eye Department in Katowice, Poland, from 2011 to 2023
Authors Sarnat-Kucharczyk M, Wyględowska-Promieńska D, Patel S
Received 14 January 2025
Accepted for publication 1 April 2025
Published 10 April 2025 Volume 2025:19 Pages 1247—1261
DOI https://doi.org/10.2147/OPTH.S515938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Monika Sarnat-Kucharczyk,1,2 Dorota Wyględowska-Promieńska,1,2 Sudi Patel3
1Department of Ophthalmology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 2Professor Kornel Gibinski University Clinical Centre, Katowice, Poland; 3Department of Cataract and Refractive Surgery, University Eye Clinic Svjetlost, Zagreb, 10000, Croatia
Correspondence: Monika Sarnat-Kucharczyk, Department of Ophthalmology, Faculty of Medical Sciences in Katowice, Medical University of Silesia Katowice, ul. Ceglana 35, 40-514, Katowice, Poland, Tel +48 32 35 81 227, Fax +48 32 25 18 437, Email [email protected]
Purpose: To determine if significant correlations occur between recorded values for a) annual temperature, b) air pollution levels, and the prevalence of acute ophthalmic inflammation among patients attending an eye emergency department in an urban setting between 2011 and 2023.
Patients and Methods: A data bank of cases that attended an eye emergency unit (Medical University of Silesia, Katowice, Poland) between 1/1/2011 and 31/12/2023 was accessed. Cases were classified into inflammatory or noninflammatory ophthalmic groups. The former were then subdivided into subgroups for blepharitis, orbital inflammation, lacrimal system inflammation, conjunctivitis, scleritis, keratitis, uveitis with retinitis, endophthalmitis, and optic neuritis. Data on local temperatures and air pollution levels were obtained from available official publications.
Results: Reporting key results (p < 0.05).Total attending the emergency unit increased from 8,172 to 14,261 (8854 during pandemic lockdown in 2020), prevalence of all acute ophthalmic inflammation (y) decreased from 64.70% to 55.40% and prevalence of conjunctivitis within this group decreased from 53.96% to 36.23%.Annual average (±SD) temperature (x, °C) in Silesia increased from 8.33°C (± 9.18°) in 2010 to 10.64°C (± 6.83°C) in 2020 (paired t-test, p = 0.04). Curvi-linear regression revealed, y = 1.915x3-55.624x2+534.09x-1631.2, (n = 10, r2= 0.489).Prevalence of conjunctivitis was directly correlated with atmospheric concentrations of sulphur dioxide, carbon monoxide and particulate matter in suspended dust with a diameter≤ 10 microns.After factoring all cases of conjunctivitis, a) prevalence of endophthalmitis, uveitis with retinitis, and scleritis were negatively correlated with temperature, b) atmospheric concentrations of certain pollutants were positively correlated with the prevalence of endophthalmitis, uveitis with retinitis, scleritis and keratitis; and negatively correlated with the prevalence of orbital and lacrimal inflammation, blepharitis, and optic neuritis.
Conclusion: Air pollutants and temperature are linked to the prevalence of certain acute ophthalmic inflammations. Some correlations are negative suggesting some protection against the development of certain conditions. However, negative correlational effects do not necessarily imply negative causal effects.
Keywords: air pollution, ophthalmic inflammation, particulate matter, sulphur dioxide, carbon monoxide
Introduction
Global warming (GW) and climate change (CC) are interrelated terms and are often used interchangeably. However, GW refers specifically to the Earth’s rising surface temperatures, while CC refers to shifts in weather patterns.1 The increasing average temperature of the atmosphere near the Earth’s surface and the troposphere is believed to contribute to changes in global climate patterns.
According to climate change models for Egypt, cases of keratomycosis are projected to increase over the next few years alongside anticipated increases in CO2 emissions and surface temperatures.2 Models suggest that the warming climate will create more favourable conditions for pathogenic diseases, highlighting a direct correlation between environmental changes and consequential impact on public health.2
It is widely accepted that climate change could have a profound effect on the prevalence and spread of human pathogenic diseases. Despite this recognition, the full extent of the associated risks has not been adequately quantified.3 Climate change is more than a global environmental issue; it directly threatens eye health, and this is expected to impact the delivery of essential eyecare services.4
Climate change is expected to worsen a variety of ocular conditions. Increased UV exposure is likely to accelerate cataract formation, heighten risks of severe allergic eye diseases, glaucoma, age-related macular degeneration, infections (such as trachoma), ocular surface diseases, and ocular injuries are all anticipated to become more prevalent.5 Uncontrolled air pollution is a significant deleterious environmental health issue, particularly in megacities. Pollutants can directly affect the ocular surface and predispose individuals to or exacerbate other ocular conditions. Urban air pollution may trigger a local imbalance of innate and adaptive immune responses, exacerbating the severity of eye diseases.6
Therefore, interventions designed to reduce concentrations of atmospheric pollutants should reduce the incidence of some ocular inflammatory conditions. Conversely, rising average temperatures are expected to increase the incidence of certain ocular inflammatory conditions. The aim of this study was to determine whether changes in the average annual atmospheric temperature values and concentrations of air pollutants were associated with the prevalence of specific ophthalmic inflammatory conditions.
Materials and Methods
Study Design
Inflammatory Conditions
An analysis of acute ophthalmic inflammation was carried out on patients who attended the eye emergency department at the Professor K. Gibinski University Clinical Center of the Medical University of Silesia in Katowice, Poland, between January 1, 2011, and December 31, 2023.
This center is one of the largest public health care ophthalmological units in Poland. Over 14,200 people attended the eye emergency department in 2023 alone. The records were reviewed for each calendar year, and an audit was conducted as follows. Firstly, the total number of cases that attended was recorded. Secondly, the number of cases presenting with an acute inflammatory condition was identified. Thirdly, the inflammatory cases were then divided into the following subgroups: blepharitis, orbital inflammation, lacrimal system inflammation, conjunctivitis, keratitis, uveitis with retinitis, endophthalmitis, optic neuritis, and scleritis.
The inclusion criteria were based on diagnoses categorized under the International Classification of Diseases, 10th Revision (ICD-10), as presented in Table 1 and detailed assessments made by the attending physicians at the eye emergency department.7
 |
Table 1 International Classification of Disease (ICD) Codes and Descriptions During the Study Period.7 |
Temperature
Annual average temperature values were obtained from the official online publications for the whole of Poland [https://www.iea.org/articles/poland-climate-resilience-policy-indicator] and region of Silesia [https://weatherandclimate.com/poland/silesia#google_vignette].8,9
Pollution
Air pollution levels for the region of Silesia were extracted from the official online publications of the Chief Inspectorate of Environmental Protection [Główny Inspektorat Ochrony Środowiska – GIOŚ, https://www.gov.pl/web/gios-en] for the period 2010 to 2023.10 The pollutants noted in this publication and considered for inclusion in this study are listed in Table 2.
 |
Table 2 Pollutants Considered and Listed by Chief Inspectorate of Environmental Protection.10 |
Data and Statistical Analysis
Data were stored in Excel spreadsheets (Microsoft, Redmond, WA) and analyzed to determine the significance of any:
- Changes in the annual prevalence of all acute inflammatory conditions and each subgroup (blepharitis, orbital inflammation, lacrimal system inflammation, conjunctivitis, scleritis, keratitis, uveitis with retinitis, endophthalmitis, and optic neuritis) over the period, analyzed using Pearson correlation.
- Changes in annual average temperature and mean concentrations of atmospheric pollutants over the study period.
Data sets were subjected to linear or curvilinear regression analysis, as appropriate. Depending on the outcomes of the analysis, the data were further scrutinized to determine ‘if the annual prevalences of acute inflammatory conditions were associated with mean annual temperature or the mean concentration of any atmospheric pollutants. Appropriate non-parametric tests were applied when data were not normally distributed (Kolmogorov–Smirnov test of normality). Changes and differences were considered statistically significant at p ˂ 0.05.
Results
The number of patients who attended the eye emergency department between 2011 and 2023 is shown in Table 3. The figures were divided into those attending for an inflammatory or a noninflammatory ophthalmic condition. The inflammatory group is further subdivided into nine subgroups.
 |
Table 3 Audit of Cases Presenting to the Eye Emergency Department, 2011 to 2023 |
The eye emergency department examined 140,712 patients between 2011 and 2023. Of these, 86,786 were reported as requiring treatment for an acute inflammatory ophthalmic condition. The total number of patients presenting with conjunctivitis, keratitis, or blepharitis was 74,140. These three subgroups accounted for more than 85.4% of all inflammatory conditions reported at the emergency department.
The total numbers increased from 2011 to 2019, decreased, and then gradually rose again between 2020 and 2023. The decline can be attributed to the lockdown in response to COVID-19 and subsequent restrictions on population movement and access. Linear regression revealed the following associations between the total number presenting with an acute inflammatory ophthalmic condition (y) and year (x) over the periods 2011-to-2019 and 2020-to-2023.
Prevalence of Acute Ophthalmic Inflammatory Conditions Over the Period 2011–2023
The prevalence of all acute inflammatory cases (calculated as 100 × [number of acute inflammatory cases/total number of all patients reported at emergency department]) and of conjunctivitis among these inflammatory cases (calculated as 100 × [number of conjunctivitis cases/total number of acute inflammatory cases]) decreased significantly (r² = 0.635 and 0.866 respectively), as shown in Figure 1.
Significant fluctuations were observed in the prevalence of blepharitis, orbital inflammation, lacrimal system inflammation, keratitis, uveitis with retinitis, endophthalmitis, and optic neuritis among the total number of reported inflammatory cases. The best-fit expressions describing the shifts in the prevalences are shown in Table 4. No significant change was detected in the prevalence of scleritis among the reported cases.
 |
Table 4 Expressions Describing the Annual Prevalence of Ophthalmic Inflammatory Conditions Among Cases Attended Between 2011 and 2023 |
Prevalence of Ophthalmic Inflammation and Temperature in Silesia
Published data on annual average temperatures for the region of Silesia and the whole of Poland were available up to and including 2020. Linear regression analysis revealed the following associations between average annual temperature (y, °C) and year (x) over the period 2010–2020.
In Silesia, the annual mean [±SD] temperature (°C) increased significantly from 8.33°C [±9.18°C] to 10.64°C [±6.83°C] over this decade (paired t-test, p = 0.04).
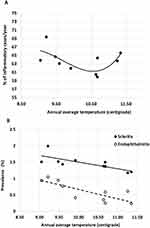
For the period 2011–2020, significant associations (p < 0.05) were identified between annual average temperature and the prevalence of acute inflammatory conditions, particularly the prevalence of endophthalmitis. As shown in Table 3, conjunctivitis was the predominant inflammatory condition, accounting for more than 45% of cases presenting at the emergency department. Fluctuations in the prevalence of conjunctivitis could influence the prevalence of other conditions. Hence, it was decided to repeat the analysis for data covering the period 2011–2020 after excluding all cases of conjunctivitis. This refined analysis revealed significant associations between annual average temperature and the prevalence of scleritis, uveitis with retinitis, and endophthalmitis. The best-fit expressions describing these associations are shown in Table 5.
 |
Table 5 Expressions Describing the Association Between Annual Average Temperature in Silesia and the Prevalence of Ophthalmic Inflammatory Conditions Over the Period 2011–2020 |
Prevalence of Ophthalmic Inflammation and Atmospheric Levels of Pollutants
The reported concentrations of the atmospheric pollutants in Table 2 decreased significantly between 2011 and 2023 (p < 0.05), except for O₃, Ni(PM10), C6H6 and ions (PM2.5). During this period, the prevalence of certain acute ophthalmic inflammatory conditions correlated with the average annual atmospheric concentrations for SO2, NO2, NOX, CO, PM10, PM2.5, Pb(PM10), Ni(PM10), BaP(PM10), WWA(PM10) and formaldehyde; and the prevalence of conjunctivitis amid the inflammatory cases correlated with the average annual atmospheric levels of SO2, NO2, NOX, CO, PM10, PM2.5, Pb(PM10), As(PM10), BaP(PM10), WWA(PM10) and formaldehyde. These results may be skewed by the abrupt decline in attendance during COVID-19 lockdown, as noted in Table 2. Between 2019 and 2020, the total number of attendees fell from 12,205 to 8,854, while the number of those reported with an acute ophthalmic inflammatory condition decreased from 8,035 to 4,598. Accordingly, it was decided to focus the analysis on the period 2011–2019. The significant correlations between the average annual atmospheric concentrations of pollutants and the prevalence of subgroup inflammatory conditions are listed in Tables 6 and 7, for data including and excluding conjunctivitis, respectively. In Table 6, 24 of the 35 significant correlations are negative indicating the prevalence of certain conditions was relatively low when the average annual atmospheric levels of certain pollutant levels were relatively high. In Table 7, the number of negative correlations is 19.
Discussion
We evaluated the possible links between annual mean temperature, air quality and the prevalence of acute inflammatory ophthalmic diseases reported at a single eye emergency department in Katowice, Poland. Previous studies have investigated the associations between air pollution and various systemic conditions, such as ischemic heart disease, stroke and respiratory diseases.11–13 Ophthalmic diseases unrelated to the ocular surface may result from the systemic effects of various air pollutants, as well as from changes in climate and temperature.
Table 3 shows a gradual rise in number of all inflammatory admissions from 2011 to 2019, followed by a decline during the period 2020–2021, and then an increase in 2022–2023. The period 2020–2021 corresponds to the first two years of the COVID-19 pandemic, with the overall decline attributed to restrictions related to the general lockdown.14,15 Nevertheless, equations 1 and 2 model these trends for the periods 2011–2019 and 2020–2023, respectively.
The COVID-19 pandemic led to a significant reduction in the number of patients attending the eye emergency department. This suggests that people with less urgent or milder conditions endured their discomfort and avoided visiting the public hospital as a way of reducing the risk of becoming infected.16 However, the number of sight-threatening cases or cases requiring urgent treatment, such as uveitis and retinitis, increased during the pandemic period compared to pre-pandemic numbers. Figure 1 illustrates that the overall prevalence of all acute inflammatory conditions, including conjunctivitis, tended to decrease between 2011 and 2023. Nevertheless, there was a near-linear increase in the prevalence of all acute inflammatory conditions between 2015 and 2019, just before the lockdown, as well as a rise in the prevalence of conjunctivitis between 2018 and 2019. These trends may be coincidental or potentially indicative of an emerging COVID-19 pandemic. Table 4 lists expressions describing the changes in the prevalence of each condition within the family of acute inflammatory conditions. A closer look at each expression reveals the prevalence of blepharitis peaked in 2018, and then gradually declined. In contrast, the prevalence of lacrimal inflammation, keratitis, orbital inflammation, uveitis with retinitis, endophthalmitis, and optic neuritis tended to increase, particularly after 2019. While this may also be coincidental, it could be a consequence of the COVID-19 pandemic.
Equations 3 and 4 show that the annual average temperature (AAT) in Silesia and increased linearly over the decade 2010–2020. Silesia was subjected to a rise in AAT rising from 8.33°C in 2010 to 10.64°C in 2020, mirroring the impact of global warming. Several infectious and non-infectious ophthalmic diseases are influenced by temperature.17 The equations in Table 5 describe the associations between AAT in Silesia and prevalence of acute ophthalmic inflammatory conditions reported at the eye department between 2010 and 2020. These equations should be viewed with caution. Figure 2a shows that the prevalence of all conditions reduced as AAT increased from 8.5°C to 10.5°C then increased as AAT continued to rise. Furthermore, after factoring out all cases of conjunctivitis, the prevalence of endophthalmitis, uveitis with retinitis, and scleritis had an inverse association with AAT. Figure 2b demonstrates the associations for endophthalmitis and scleritis. These are counter-intuitive, suggesting that other more influential factors could be eclipsing any real link between AAT and the prevalence of these conditions.
Table 6 shows the prevalence of conjunctivitis was strongly associated with atmospheric concentrations of CO, SO2, and PM10. Figures 3 and 4 feature the prevalence of selected subtypes, including conjunctivitis (Figures 3a and 4a), in relation to atmospheric concentrations of CO and SO2. Mimura et al discussed the association between the number of outpatient visits for allergic conjunctivitis and the local PM2.5 levels.18 Furthermore, the risk of conjunctivitis associated with particulate matter and SO2 was highest during the transitional season, whereas the risk associated with NO2 peaked in the winter season. These findings underscore the importance of managing atmospheric pollution and provide a reference for public health measures.19 The prevalence of conjunctivitis, as noted in Figure 1, decreased as the atmospheric concentrations of CO, SO2, and PM10 declined. Sendra et al strongly expressed the view that urban air pollution should be regarded as a key factor in the development of ophthalmic inflammatory diseases.6 The results in Table 4 and Table 6 can be interpreted as evidence supporting this view, particularly in the case of conjunctivitis.
Table 6 and Figures 3a and 4a show the overall prevalence of keratitis and other conditions negatively correlated with atmospheric concentrations of CO and SO2. However, as noted in Table 7 and Figures 3b and c, 4b and c some of these correlations were rendered positive for conditions such as keratitis, blepharitis, scleritis, uveitis with retinitis and endophthalmitis after excluding conjunctivitis from the analysis.
The prevalence of keratitis is positively correlated with atmospheric levels of CO and formaldehyde after factoring out conjunctivitis. The prevalence of fungal keratitis is influenced by factors such as geography, climate, age, gender, socioeconomic status, agricultural activity, and the level of urbanization.20–22 This condition is commonly found in tropical and subtropical regions, where warm and humid climates create ideal conditions for the growth and spread of fungi responsible for the infection. Consistent with conjunctivitis, the results in Table 4 and Table 7 provide further support for earlier studies.6
The prevalence of endophthalmitis, uveitis with retinitis, and scleritis is positively linked with atmospheric concentrations of certain pollutants after factoring out conjunctivitis. Autoimmune eye diseases (AEDs) can affect both intraocular and periorbital structures, leading to severe visual impairment. The roles of air pollution and meteorological factors in the initiation and progression of AEDs have become increasingly significant, involving both systemic and local mechanisms. Exposure to excessive air pollution, including PM2.5 and PM10, as well as high temperatures, can disrupt the Th17/Treg balance, regulate macrophage polarization, activate neutrophils, induce systemic inflammation and oxidative stress, reduce retinal blood flow, promote tissue fibrosis, activate the sympathetic nervous system, negatively affect nutrient synthesis, and induce heat stress, all of which may contribute to the worsening of AEDs.23 While previous studies have revealed an association between ocular surface disorders and air pollution, few have focused on links with uveitis. The risk of uveitis appears to increase with increasing NOx levels.24 The corresponding result in Table 7 does not support this. Furthermore, there is a negative correlation between atmospheric concentrations of NOx and the prevalence of orbital inflammation and lacrimal inflammation. Tan et al suggested that PM2.5 concentrations above the level of the minimum exposure were responsible for 13% of new cases of uveitis.25 Our findings align with this, providing further evidence supporting the link between fine particulate air pollution and uveitis. Furthermore, numerous industrial chemicals can harm the retina. Current retinal environmental health studies tend to involve animal models, isolated mammalian retinas, animal- or human-derived retinal cells, and retinal organoids.26 Moreover, environmental pollution has the potential to promote the generation of reactive oxygen species (ROS), impair antioxidant defense mechanisms, and induce oxidative stress.27 Endophthalmitis is usually an exogenous infection after ophthalmic surgery, penetrating ocular trauma, or the extension of a corneal infection.28 Endophthalmitis can also be endogenous, arising from bacteremic or fungemic seeding in the eye.29 Figures 3c, 4c and 5 show the prevalence of endophthalmitis is small, in comparison with other conditions, but it is positively linked to air pollution. A search for published reports assessing the environmental factors such as air pollutants and temperature on the onset of endophthalmitis yielded no results. Formaldehyde is an environmental and occupational chemical carcinogen known to cause damage to proteins and nucleic acids.30 Exposure to formaldehyde from different sources can induce eye irritations, heart diseases and brain damage.31–33 Previous studies have primarily focused on the occupational exposure to formaldehyde in humans.34,35 However, as indoor formaldehyde has a negative impact on human health, elevated outdoor levels of this toxin are also likely to induce similar effects. Thus, it is reasonable to expect a link between atmospheric levels of formaldehyde at lower concentrations and particular ophthalmic conditions. However, the associations with optic neuritis, orbital inflammation, blepharitis, and lacrimal inflammation were negative, implying that increasing formaldehyde concentrations may provide some protective effects against the factors triggering these conditions.
Surprisingly, the prevalence of optic neuritis, orbital inflammation, blepharitis, and lacrimal inflammation was negatively associated with levels of certain pollutants. Figures 3b, 4b and c, 5 and 6 indicate that decreases in the atmospheric concentrations of these pollutants were linked to an increase in the prevalence of the four conditions. Could these pollutants be providing some protection against the development of these conditions, or are these associations driven by other factors, creating a false impression of a protective effect? It is important to note that a negative correlation does not necessarily imply a negative causal relationship.
Study Limitations
This study is limited by several factors, including age, gender, the number of cases, pre-existing health conditions of attendees, and psychosocial issues that may have influenced the biological response to air pollutants. Seasonal allergies, influenza outbreaks, and other potential confounding factors may have influenced hospital admissions; however, these variables were not included due to there being a lack of comprehensive data. Future studies should aim to incorporate such factors to provide a more comprehensive understanding of their impact. Additional limitations include methods for sampling air pollutant concentrations, prevailing conditions during sampling, solar radiation, atmospheric stability, and location. While an observational study is useful for exploring potential links between exposure and outcomes, it carries the risk of reverse causality or undetected bias.1 Nevertheless, this study offers valuable insights into the relationship between temperature changes, air pollutant exposure, and the prevalence of acute ophthalmic infections.
Future Research Directions
Future studies should explore the effects of air temperature and pollution on different age groups and patients with congenital diseases to provide more detailed insights. However, due to data limitations, such subgroup analyses were not feasible in the present study.
Comparative studies examining the impact of air temperature and pollution on ophthalmic inflammation across different Polish cities with varying pollution levels would provide valuable insights. However, due to data availability constraints, our study focused solely on the population of Katowice.
Conclusion
Exposure to typical concentrations of air pollutants commonly found in an urban setting, along with rising air temperatures, contributes to the prevalence of acute inflammatory eye diseases.
Abbreviations
CC: Climate change; ICD-10: International Classification of Diseases, Tenth Revision; PM10: Particulate matter ≤10 microns in diameter; PM2.5: Particulate matter ≤2.5 microns in diameter; SO2: Sulphur dioxide; NOₓ: Nitric oxide (NO) and nitrogen dioxide (NO2); BaP (PM10): Benzo(a)pyrene measured in particulate matter ≤10 microns in diameter; O₃: Ozone; CO: Carbon monoxide; NO2: Nitrogen dioxide; AEDs: Autoimmune eye diseases.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Adepoju OA, Afinowi OA, Tauheed AM. et al. Multisectoral perspectives on Global Warming and Vector-borne Diseases: a Focus on Southern Europe. Curr Trop Med Rep. 2023;10(2):47–70. doi:10.1007/s40475-023-00283-y
2. Saad-Hussein A, El-Mofty HM, Hassanien MA. Climate change and predicted trend of fungal keratitis in Egypt. East Mediterr Health J. 2011;17(6):468–473.
3. Mora C, McKenzie T, Gaw IM, et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat Clim Chang. 2022;12(9):869–875. doi:10.1038/s41558-022-01426-1
4. Watts N, Amann M, Arnell N, et al. The 2019 report of the lancet countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019;394(10211):1836–1878. doi:10.1016/S0140-6736(19)32596-6
5. McCance E, Taylor HR, Acharya NR, Thiel CL, Resnikoff S, Bourne R. National Eye Institute’s (NEI) coordination efforts and current opportunities for sustainability, adaptation, and climate resilience in global eye health - ARVO 2023 session commentary. Eye. 2024;38(11):1984–1985. doi:10.1038/s41433-023-02854-9
6. Sendra VG, Tau J, Zapata G, et al. Polluted air exposure compromises corneal immunity and exacerbates inflammation in acute herpes simplex keratitis. Front Immunol. 2021;12:618597. doi:10.3389/fimmu.2021.618597
7. International Classification of Diseases. Tenth revision international classification of diseases, 10th Revision (ICD-10) code diagnosis and a detailed diagnosis made by the attending physicians at the emergency department. Available from:(https://icd.who.int/browse10/2019/en#/VII.).
8. Poland climate resilience policy indicator. Available from:. https://www.iea.org/articles/poland-climate-resilience-policy-indicator.
9. Silesia. Poland Climate. (https://weatherandclimate.com/poland/silesia#google_vignette.
10. chief inspectorate of environmental protection, Poland (Główny Inspektorat Ochrony Środowiska – GIOŚ, Available from:. https://www.gov.pl/web/gios-en.
11. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
12. Mo Z, Fu Q, Lyu D, et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: a case-crossover study. Environ Pollut. 2019;246:183–189. doi:10.1016/j.envpol.2018.11.109
13. Akinbami LJ, Lynch CD, Parker JD, Woodruff TJ. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001-2004. Environ Res. 2010;110(3):294–301. doi:10.1016/j.envres.2010.01.001
14. Franzolin E, Longo R, Gusson E, Ficial B, Marchini G. Pediatric eye emergency department activity during the first wave of Covid-19 pandemic. Ital J Pediatr. 2021;47(1):217. doi:10.1186/s13052-021-01167-5
15. Boserup B, McKenney M, Elkbuli A. The impact of the COVID-19 pandemic on emergency department visits and patient safety in the United States. Am J Emerg Med. 2020;38(9):1732–1736. doi:10.1016/j.ajem.2020.06.007
16. Franzolin E, Casati S, Albertini O, et al. Impact of Covid-19 pandemic on ophthalmic emergency department in an Italian tertiary eye centre. Eur J Ophthalmol. 2022;32(1):680–687. doi:10.1177/1120672121998223
17. Qassim A, Viki M, Ng SK, Jersmann H, Casson RJ. Climate and season: the effects on ophthalmic diseases. Clin Exp Ophthalmol. 2017;45(4):385–392. doi:10.1111/ceo.12883
18. Mimura T, Ichinose T, Yamagami S, et al. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci Total Environ. 2014;487:493–499. doi:10.1016/j.scitotenv.2014.04.057
19. Yan K, Wang M, Cheng Y, et al. An update on the association between ambient short-term air pollution exposure and daily outpatient visits for conjunctivitis: a time-series study in Hangzhou, China. Environ Sci Pollut Res Int. 2023;30(46):102790–102802. doi:10.1007/s11356-023-29647-7
20. Tuli SS. Fungal keratitis. Clin Ophthalmol. 2011;5:275–279. doi:10.2147/OPTH.S10819
21. Brown L, Leck AK, Gichangi M, Burton MJ, Denning DW. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021;21(3):e49–e57. doi:10.1016/S1473-3099(20)30448-5
22. Sharma N, Bagga B, Singhal D, et al. Fungal keratitis: a review of clinical presentations, treatment strategies and outcomes. Ocul Surf. 2022;24:22–30. doi:10.1016/j.jtos.2021.12.001
23. Cao F, Liu ZR, Ni QY, et al. Emerging roles of air pollution and meteorological factors in autoimmune eye diseases. Environ Res. 231(Pt 1):116116. doi:10.1016/j.envres.2023.116116
24. Bai YC, Wang CY, Lin CL, Lai JN, Wei JC. Association between air pollution and the risk of uveitis: a nationwide, population-based cohort study. Front Immunol. 2021;12:613893. doi:10.3389/fimmu.2021.613893
25. Tan H, Pan S, Zhong Z, Su G, Kijlstra A, Yang P. Association between fine particulate air pollution and the onset of uveitis in Mainland China. Ocul Immunol Inflamm. 2022;30(7–8):1810–1815. doi:10.1080/09273948.2021
26. Wang Y, Yin N, Yang R, Faiola F. Pollution effects on retinal health: a review on current methodologies and findings. Toxicol Ind Health. 2023;39(6):336–344. doi:10.1177/07482337231174072
27. Böhm EW, Buonfiglio F, Voigt AM, et al. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023;68:102967. doi:10.1016/j.redox.2023.102967
28. Jian H-J, Chiou Y-R, Wu W-Y, Huang Y-F, Chen W-R, Chien Y-C. Carbon-in-carbon: hybrid carbonized nanomaterials with multifunctional activities for the treatment of endophthalmitis. Chemical Engineering Journal. 2024;491:151997. doi:10.1016/j.cej.2024.151997
29. Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19(3):227–234. doi:10.1111/1469-0691.12118
30. Gonzalez-Rivera JC, Sherman MW, Wang DS, Chuvalo-Abraham JCL, Hildebrandt Ruiz L, Contreras LM. RNA oxidation in chromatin modification and DNA-damage response following exposure to formaldehyde. Sci Rep. 2020;10:16545. doi:10.1038/s41598-020-73376-7
31. Vazquez-Ferreiro P, Carrera Hueso FJ, Alvarez Lopez B, Diaz-Rey M, Martinez-Casal X, Ramón Barrios MA. Evaluation of formaldehyde as an ocular irritant: a systematic review and Meta-analysis. Cutan Ocul Toxicol. 2019;38(2):169–175. doi:10.1080/15569527.2018.156170
32. Zhang Y, Yang Y, He X, et al. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. J Cell mol Med. 2021;25(12):5358–5371. doi:10.1111/jcmm.16602
33. Rana I, Rieswijk L, Steinmaus C, Zhang L. Formaldehyde and brain disorders: a meta-analysis and bioinformatics approach. Neurotox Res. 2021;39(3):924–948. doi:10.1007/s12640-020-00320-y
34. Dally KA, Hanrahan LP, Woodbury MA, Kanarek MS. Formaldehyde exposure in nonoccupational environments. Arch Environ Health. 1981;36(6):277–284. doi:10.1080/00039896.1981.10667638
35. Nielsen GD, Larsen ST, Wolkoff P. Recent trend in risk assessment of formaldehyde exposures from indoor air. Arch Toxicol. 2013;87(1):73–98. doi:10.1007/s00204-012-0975-3
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.