Back to Journals » The Application of Clinical Genetics » Volume 18
The Impact of FSHR Polymorphisms (rs6165 and rs6166) on Ovarian Response to Stimulation in Infertile Women with Diminished Ovarian Reserve
Authors Hoang TTT , Trinh ST , Nguyen NN , Ho MN, Pham MD, Hoang NT, Trieu ST , Ho HS
Received 4 April 2025
Accepted for publication 7 July 2025
Published 12 July 2025 Volume 2025:18 Pages 119—129
DOI https://doi.org/10.2147/TACG.S528567
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Martin Maurer
Thuy Thi Thanh Hoang,1,2 Son The Trinh,3 Nhat Ngoc Nguyen,3 Minh Nguyet Ho,4 Minh Dinh Pham,5 Nhung Thi Hoang,5 Sang Tien Trieu,6 Hung Sy Ho1,2
1Department of Obstetrics and Gynecology, Hanoi Medical University, Hanoi, 100000, Vietnam; 2National Assisted Reproductive Technology Center, National Hospital of Obstetrics and Gynecology, Hanoi, 100000, Vietnam; 3Military Institute of Clinical Embryology and Histology, Vietnam Military Medical University, Hanoi, 12108, Vietnam; 4Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, 3800, Australia; 5Research and Development department, Genetic Testing Service Joint Stock Company, Hanoi, 11622, Vietnam; 6Department of Biology and Genetics, Vietnam Military Medical University, Hanoi, 12108, Vietnam
Correspondence: Hung Sy Ho, Department of Obstetrics and Gynecology, Hanoi Medical University, Hanoi, 100000, Vietnam, Email [email protected]; [email protected]
Background: Diminished ovarian reserve (DOR) remains a significant challenge in IVF, as it is closely associated with poor ovarian response. Beyond well-established predictive of ovarian response, genetic polymorphisms in the FSH receptor (FSHR) gene rs6165 and rs6166 have been reported as potential markers.
Purpose: Evaluating the expression of FSHR rs6165 and rs6166 in DOR patients and their impact on ovarian response to stimulation.
Materials and Methods: This prospective cross-sectional included 79 DOR patients (AMH < 1.2 ng/mL and/or AFC < 5) undergoing IVF treatment at the National Hospital of Obstetrics and Gynecology, Vietnam. GnRH antagonist protocol was applied, using alpha follitropin with individualized dosages combined with clomiphene citrate, followed by dual-trigger ovulation induction. FSHR rs6165 and rs6166 were genotyped by Next-Generation Sequencing (NGS) assays, with Sanger sequencing for validation. Ovarian response was assessed based on follicular development and oocyte retrieval.
Results: The overall prevalence of the rs6165 and rs6166 polymorphisms was 10.1% (8/79), with strong linkage disequilibrium observed between the two loci (OR = 490, p < 0.0001). No significant differences in age, AMH, baseline FSH, and AFC were found in all genotypes (AA, AG, GG) of rs6165 and rs6166. In the rs6165 dominant model, patients with G alleles (AG/GG) had lower total oocyte retrieval, FOI and FORT than the AA genotype. In rs6166 codominant, dominant, and recessive models, the GG phenotype retrieved fewer oocytes (p1 = 0.02, p2 = 0.03, p3 = 0.01). FORT was significantly lower in G allele carriers (AG/GG) than AA (p = 0.04).
Conclusion: In the diminished ovarian reserve patients, FSHR rs6165 and rs6166 were associated with ovarian response to stimulation in IVF treatment. Specifically, the presence of G alleles in both rs6165 and rs6166 was correlated with reduced oocyte retrieval, independent of baseline ovarian reserve markers.
Keywords: diminished ovarian responder, FSHR, rs6165, rs6166
Introduction
Diminished ovarian reserve (DOR) remains a significant challenge in IVF, ranging from 6% to 64%, as it is closely associated with poor ovarian response.1,2 Diminished ovarian reserve (DOR) is a condition characterized by a decline in the ovarian follicle pool, often accompanied by menstrual irregularities, ovulatory dysfunction, and, in severe cases, impaired organ and systemic function due to low estrogen levels.3 Ovarian reserve assessment typically includes measurements such as antral follicle count (AFC) and serum levels of anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), and inhibin B. In 2020, the Practice Committee of the American Society for Reproductive Medicine (ASRM) identified AMH and AFC as the most sensitive and reliable biomarkers for evaluating ovarian reserve.4 In addition to factors affecting ovarian reserve, such as age, genetic abnormalities, and ovarian-damaging conditions like endometriosis and pelvic infections, iatrogenic factors including ovarian surgery, chemotherapy, and pelvic radiation also play a significant role. Moreover, environmental and lifestyle factors, such as smoking,5–8 have been linked to diminished ovarian reserve. Notably, genetic variations in the FSHR gene, particularly the rs6165 and rs6166 polymorphisms, have been investigated for their potential impact on ovarian response.9
Follicle-stimulating hormone (FSH) is a glycoprotein that is synthesized and released from the anterior pituitary gland and regulated by gonadotropin-releasing hormone (GnRH). FSH acts directly by binding to FSH receptors (FSHR) on the plasma membrane of granulosa cells in the ovaries. After that, hormonal signals enter the cells, stimulate follicular development, and play a crucial role in regulating the ovarian cycle.10 Recent research has also emphasized the significant impact of FSHR polymorphisms on ovarian functions, including follicle maturation and steroidogenesis.11,12 In situ hybridization experiments have demonstrated that the human FSH gene is located on chromosome 2p21-p16.13,14 Although various mutations such as Ile160Thr, Ala189Val, and Asn191Ile have been studied to predict the ability of FSH inhibition, two common single-nucleotide polymorphisms (SNPs) located in the coding region (exon 10) of the FSHR gene have been associated with ovarian response to FSH stimulation.15 The first SNP is rs6165, which has allele G at position 919 (c.919G>A), leading to a codon 307 substitution from threonine (ACT) to alanine (GCT), whereas the second variant is rs6166 with allele G at position 2039 (c.2039G>A), resulting in a codon 680 substitution from serine (AGT) to asparagine (AAT).16
As has been reported, previous authors have favored the GnRH antagonist protocol with high doses of FSH (300–450 IU/day) to optimize the number of retrieved oocytes.17,18 However, the OPTIMIST trial reported that a higher FSH dose (450 IU vs 150 IU) does not improve live birth rates, while it increases costs for poor ovarian reserve women.19 Studies related to DOR and FSHR gene polymorphism in ovarian stimulation are limited to date. Hence, this study aims to evaluate the expression of FSHR polymorphisms rs6165 and rs6166 in DOR patients and their influence on ovarian stimulation response with the hypothesis that carriers of the G allele in either polymorphism (rs6165 or rs6166) will exhibit a reduced ovarian response to stimulation, independent of baseline ovarian reserve markers.
Materials and Methods
Study Design and Subjects
This cross-sectional study investigates specific FSHR gene polymorphisms and characterizes the study population. Eligible participants were DOR, defined by AFC < 5 and/or AMH < 1.2 ng/mL, who were provided informed consent.
Conducted from March 2023 to March 2025, the study enrolled 79 patients undergoing in vitro fertilization (IVF) at the National Hospital of Obstetrics and Gynecology, Hanoi, Vietnam. Patients with a history of ovarian surgery, ovarian pathologies (endometriosis, ovarian tumors), uterine abnormalities (fibroids, endometrial polyps), or chronic conditions (autoimmune diseases, renal/endocrine disorders) were excluded.
The genotypic analysis confirmed that rs6165 and rs6166 polymorphisms adhered to Hardy-Weinberg equilibrium (HWE) (p > 0.05), indicating a genetically stable distribution in this cohort.
Ovarian Stimulation Protocol
From day two of menstrual cycles, patients underwent ovarian stimulation using alpha follitropin (Gonal-f, Merck Serono, Italy) with a starting dosage from 150 UI to 225 IU per day. Besides, clomiphene citrate 100 mg/day (Vacitus, Incepta Pharmaceuticals, Bangladesh) was administered for the initial five days of the stimulation period.
Serial transvaginal ultrasonography (TVUS) was performed to monitor ovarian response, which allows adjusting as needed. Gonadotropin-releasing hormone antagonist (Cetrotide, Merck Serono, Italy) was utilized when having follicles with a diameter exceeding 12 mm. A cycle cancellation was defined as the absence of follicles with a diameter greater than 12 mm by day 8 of gonadotropin administration.
Dual-trigger protocol including subcutaneous administration of 0.25 mg recombinant human chorionic gonadotropin (Ovitrelle, Merck Serono, Italy) and 0.2 mg Gonadotropin-releasing hormone agonist (Diphereline, Ipsen Pharma, France) was used for oocyte maturation when having one to two follicles with a diameter exceeding 17 mm. Oocyte retrieval was performed under transvaginal ultrasound guidance 36 hours after trigger.
The aspirated follicular fluid was immediately transferred to the IVF laboratory to collect cumulus-oocyte complexes (COCs), which were isolated and cultured in fertilization media under controlled conditions. Mature oocytes underwent intracytoplasmic sperm injection (ICSI), followed by incubation for fertilization assessment. Embryos were cultured to the blastocyst stage and assessed for quality according to the Istanbul consensus (2011) before transfer or cryopreservation.
The study was approved by the Ethics Committee of Hanoi Medical University and the hospital under decision IRB-VN01.001/IRB00003121/FWA00004148, issued on March 23, 2023.
Genetic Analysis
Blood samples were collected before oocyte retrieval following standard protocols, with no additional venipunctures. Samples were stored at −80°C until genotyping. Genomic DNA was extracted from EDTA-treated whole blood using the QIAamp DNA Blood Mini Kit (QIAGEN, Germany).
Target FSHR SNPs (rs6165, rs6166) were genotyped using NGS assays, with Sanger sequencing for validation. Specific primers (Table 1) were designed via PRIMER-BLAST and PCR was performed under standard thermal cycling conditions. Amplified products were sequenced on the ABI3500 system, and genotyping was analyzed using MiSeq Reporter software.
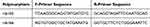 |
Table 1 Specific Primers for Detecting rs6165, rs6166 |
Genetic Parameters and Clinical Outcomes
Genetic Parameter
The prevalence of rs6165 and rs6166 variants in Vietnamese infertile women undergoing in vitro fertilization (IVF) in the DOR patients groups.
Clinical Outcome
The impact of rs6165 and rs6166 variants on ovarian response in this cohort, assessed by: The impact of rs6165 and rs6166 polymorphisms on ovarian response in this cohort, assessed by: Duration of ovarian stimulation; total FSH dose administered; total number of oocytes retrieved; follicle Output Rate (FORT): A dynamic marker reflecting ovarian responsiveness to controlled ovarian stimulation. FORT is calculated as the ratio between pre-stimulation AFC and the number of growing follicles on the trigger day. [FORT = (Number of follicles ≥ 11 mm on trigger day) / (Baseline AFC)]x100. This index has been proposed as a predictor of ovarian sensitivity to gonadotropins, especially in poor responders.20,21 Follicular Oocyte Index (FOI): A measure of follicular recruitment efficiency, defined as the ratio between the total number of oocytes retrieved and the number of follicles ≥ 11 mm on the day of trigger [FOI = (Total oocytes retrieved)/(Follicles ≥ 11 mm on trigger day)] x 100. FOI helps evaluate the efficacy of follicular-oocyte transition, which may be affected by genetic factors, including FSHR polymorphisms.22,23
Statical Analysis
Statistical analyses were performed using Stata 17.0. Normal distribution was assessed using the skewness-kurtosis test. Continuous variables were reported as mean ± standard deviation (SD) if normally distributed and median (interquartile range, IQR) otherwise. Categorical variables were summarized as frequencies and percentages. Group comparisons were conducted using the t-test or Wilcoxon rank-sum test for two groups and one-way ANOVA or the Kruskal–Wallis H-test for three or more groups. A p-value < 0.05 was considered statistically significant.
Results
Demographics
This study enrolled 79 female participants who fulfilled the inclusion and exclusion criteria. The study exhibited a mean age of 35.50 ± 4.66 years and a mean body mass index (BMI) of 22.15 ± 4.89 kg/m². Regarding ovarian reserve, the mean level of baseline FSH, AMH, and AFC were 8.64 ± 3.08 IU/L, 0.73 ± 0.32 ng/mL, and 7.29 ± 3.26, respectively. The average FSH total dose was 1800.25 ± 456.04 IU with 86.94 IU per day. Statistical analysis demonstrated no significant differences in age, BMI, or ovarian reserve parameters (FSH, AMH, AFC) across the AA, AG, and GG genotypes, in both codominant, dominant, and recessive genetic models.
Genotypic Distribution of rs6165 and rs6166 and Their Correlation
The genotypic distribution of rs6165 and rs6166 across the investigated genetic models was shown in Figure 1A. The AA/AG genotypes of the both rs6165, rs6166 were more abundant than the GG genotype (89.9% vs 10.1%). A strong genetic linkage was identified between these polymorphisms. Notably, individuals with the GG genotype in rs6165 were 490 times more likely to also carry the GG genotype in rs6166 (p < 0.0001), and the same association was observed in the reverse direction (Figure 1B).
 |
Figure 1 Genotypic distribution (A) and correlation of rs6165 and rs6166 (B). |
The rs6165 Polymorphism in Diminished Ovarian Reserve Patients Undergoing Ovarian Stimulation
Table 2 describes the demographic characteristics and the association between genotypes of rs6165 and ovarian response in the cohort undergoing ovarian stimulation. In the dominant genetic model, the number of oocytes retrieved for the AA genotype was significantly higher than the AG/GG genotypes (5.73 ± 2.72 vs 4.63 ± 2.81, p = 0.04). Consistent with the finding, the Follicular Output Rate (FORT) and Follicular Oocyte Index (FOI) were higher in the AA genotype (73.27 ± 30.95 and 84.28 ± 35.00, respectively) than in the AG/GG genotypes (60.13 ± 34.29 and 67.18 ± 40.41, respectively) (p = 0.05 and p = 0.03). Additionally, the mean duration of ovarian stimulation was longer in the AA/AG genotypes (9.76 ± 1.44 days) compared to the GG group (8.63 ± 1.3 days) (p = 0.05). However, no significant difference was found between the AA and the AG/GG genotypes concerning the mean antral follicle count (AFC), number of metaphase II (MII) oocytes retrieved, or the ratio of MII oocytes retrieved from AFC.
 |
Table 2 Analysis of rs6165 Polymorphism in DOR Patients Undergoing Ovarian Stimulation Across Three Genetic Models |
The rs6166 Polymorphism in Diminished Ovarian Reserve Patients Undergoing Ovarian Stimulation
As shown in Table 3, the AA genotype retrieved significantly higher total oocytes than the AG and GG genotypes (5.75 ± 2.61 vs 4.93 ± 3.04 vs 2.71 ± 1.11, p = 0.02, respectively) in all three models. In the codominant and recessive models, the duration of stimulation in the GG genotype was significantly shorter than in the AA/AG genotypes (p=0.01). In the dominant and recessive models, the FOI was significantly higher in the AG/AA genotypes when compared to the GG genotype (82.95 ± 33.85 vs 67.23 ± 42.16, p = 0.04 and 78.08 ± 39.01 vs 48.63 ± 21.84, p = 0.03), there were no significant differences regarding the FORT among the three genotypes. Interestingly, the dominant model revealed a higher maturity rate for the GG and AG genotypes (0.71 ± 0.34) compared to the AA genotype (0.62 ± 0.27, p = 0.05).
 |
Table 3 Analysis of rs6166 Polymorphism in DOR Patients Undergoing Ovarian Stimulation Across Three Genetic Models |
Discussions
Compared to Polyzos et al, our cohort had a similar BMI but was significantly older, with lower AMH and AFC levels. This difference is possible that our study included patients DOR in Viet Nam whereas Polyzos et al excluded PCOS patients (Rotterdam criteria) and those with AMH <1.1 ng/mL and AFC <9. Consequently, our outcomes total oocytes retrieved (5.15 ± 2.79), FORT (66.38 ±.33.25), and FOI (75.23 ± 38.41) were lower than those reported by Polyzos et al. However, despite targeting poor responders, FOI and FORT still exceeded 50%, possibly due to subjectivity in AFC assessment via ultrasound, leading to underestimation.24 Comparing other recently published data, the specific combinations of FSHR rs6165 and rs6166 genotypes may influence follicular output index (FOI) and oocyte yield, especially in normo-responders under 38 years old.25 Compared to Hussein et al, our cohort was older, had lower BMI, and exhibited higher AMH and AFC levels, likely due to differing poor responder definitions, while Hussein et al included only patients with AMH <0.5 ng/mL and/or AFC <5, whereas we encompassed a broader DOR population.26 In 2023, a prospective multicenter study demonstrated a significant difference in the ratio of total gonadotropin consumption to the number of oocytes retrieved among three genotypes of FSH receptor (FSHR) rs6165 and FSHR rs6166 carriers (p = 0.05). Specifically, this ratio was lower in homozygous A/A carriers compared to homozygous G/G and heterozygous carriers.27
Hussein et al studied 210 Iraqi women (aged 20–35 years), including 70 poor ovarian responders, and reported an rs6165 TT genotype frequency (equivalent to GG in our study) of 18.4%, comparable to our GG frequency (p = 0.165, Fisher’s exact test). Similarly, Polyzos et al analyzed 168 European and 200 Asian IVF patients, including a Vietnamese cohort, and found significant ethnic differences in rs6165 and rs6166 distribution (p < 0.001). Among Europeans, the AA, AG, and GG frequencies for rs6165 were 26.8%, 50.0%, and 23.2%, while in Asians, they were 20.0%, 35.5%, and 44.5%, respectively (Figure 2A). The rs6166 frequencies were 45.0% (AA), 45.5% (AG), and 9.5% (GG) in Asians (Figure 2B). Our rs6166 genotype frequency aligned with Polyzos’ Vietnamese cohort (p > 0.05), but rs6165 frequency was lower (p = 0.02), likely due to differences in patient selection. Our study focused on DOR patients, whereas Polyzos et al included a broader population.25 In the poor responder subgroup (oocytes <10) from Polyzos’ study, the rs6166 GG genotype frequency was 28.2% in Europeans and 26.3% in Asians, while for rs6165, it was 30.8% and 19.1%, respectively. In contrast, our study reported a GG frequency of 10.1% for both rs6165 and rs6166. The rs6165 frequency was comparable (p = 0.18), but rs6166 was significantly lower (p = 0.03, Chi-square test), likely due to stricter inclusion criteria in our study (DOR patients, AMH <1.2 ng/mL, AFC <5) compared to Polyzos et al, who included a broader poor responder range (AMH <1.1 ng/mL, AFC <9). These findings highlight the variability in rs6165 and rs6166 frequencies due to ethnicity, geography, and patient selection, underscoring the need for population-specific genetic studies in reproductive medicine.
 |
Figure 2 Geographic distribution of rs6165 (A) and rs6166 (B) genotype frequency. |
Analysing of FSHR rs6165 genotype associations with ovarian response, across codominant, dominant, and recessive genetic models, no statistically significant differences were observed in age, BMI, AMH levels, AFC, or baseline FSH levels among the AA, AG, and GG genotypes. In the dominant model, the AA genotype was associated with a higher total oocyte retrieval, Follicular Output Rate (FORT), and Follicular Oocyte Index (FOI) compared to the combined AG/GG genotype group (p < 0.05). However, the number of metaphase II (MII) oocytes and the MII oocyte-to-AFC ratio exhibited no significant intergroup differences. The MII oocyte-to-total oocyte ratio was numerically lower in the AA genotype, likely due to a higher total oocyte count and slightly lower AFC, though this difference was not statistically significant (p = 0.95). Stimulation duration was longer in the AA and AG groups than in the GG group (p = 0.05), suggesting that faster follicular development in the GG genotype may indicate a less favorable ovarian response, potentially due to genetic factors. The rs6165 variant results from a G-to-A substitution at position 49,191,041 on chromosome 2, leading to a threonine-to-alanine change at position 307 of the FSH receptor. This alteration replaces a polar residue with a non-polar, hydrophobic one, potentially disrupting a glycosylation site, reducing FSH binding affinity, and impairing ovarian response.28,29 The 2022 Delphi Consensus reported that the GG genotype (Ala/Ala) was linked to fewer retrieved oocytes, lower MII oocyte counts, and a shorter stimulation duration than the AA (Thr/Thr) and AG (Ala/Thr) genotypes. However, in our cohort of poor ovarian responders (POSEIDON Groups 3 and 4), the AA genotype was associated with a higher total oocyte yield, while the number of MII oocytes did not differ significantly among genotypes. The shorter stimulation duration in the GG group aligns with previous findings.30–32
Across codominant, dominant, and recessive models, the AA genotype was associated with a significantly higher total oocyte yield than the AG and GG groups, indicating that the A allele favors better ovarian response. In both dominant and recessive models, FOI was higher in A-containing genotypes, whereas AFC and FORT showed no significant differences. In the dominant model, the MII oocyte rate was higher in G carriers, likely due to a lower AFC in the AA group, though this difference was not statistically significant. As previously described, the AG/GG group had a lower total oocyte yield. In both codominant and recessive models, the GG genotype was linked to shorter stimulation duration and fewer follicles retrieved, though oocyte yield remained consistent. These findings align with the Delphi Consensus, which reported higher basal gonadotropin levels, increased r-hFSH consumption, lower estradiol levels on hCG day, fewer retrieved oocytes, and a higher incidence of hypo-responders in Ser/Ser (GG) carriers versus Asn/Asn (AA). However, in our study, baseline gonadotropin levels and total FSH consumption did not significantly differ, possibly due to the exclusive focus on DOR patients or a limited sample size.31,33–35 Overall, the A genotype was associated with a better ovarian response, while the GG genotype correlated with a shorter stimulation duration in both codominant and recessive models. This suggests that an excessively short stimulation duration may predict poor ovarian response. The rs6166 variant (Asn680Ser) results from an A-to-G substitution at position 49,189,921 on chromosome 2, replacing asparagine with serine in the FSHR intracellular domain. This alteration affects a potential phosphorylation site, impacting FSHR binding and ovarian response. Despite these genetic variations, total exogenous FSH dose and daily FSH dose did not differ significantly across models, likely due to the study’s exclusive focus on POSEIDON 3 and 4 patients, who received an already optimized low-dose stimulation protocol.28,29
This work had several limitations, such as a relatively small sample size, the population-specific nature of the cohort, and potential residual confounding, such as ultrasound variability in AFC assessment; however, the finding of this work was useful for clinical relevance. Firstly, genotyping of FSHR rs6165 and rs6166 may support personalized ovarian stimulation protocols by predicting ovarian response in patients with diminished ovarian reserve (DOR), particularly within POSEIDON groups 3 and 4. Secondly, the AA genotype of rs6165 is associated with higher total oocyte yield, FOI, and FORT, whereas the GG genotype tends to correlate with shorter stimulation duration and poorer ovarian response, indicating the need for closer monitoring in these patients.
Conclusions
The study population exhibited an allele frequency of 10.1% for both rs6165 and rs6166 polymorphisms. A strong linkage was observed between the rs6165 and rs6166 FSHR gene polymorphisms. These polymorphisms were found to influence ovarian response in patients with diminished ovarian reserve (DOR) undergoing controlled ovarian stimulation (COS) for in vitro fertilization (IVF). Specifically, the AA genotype of rs6165 was associated with a significantly higher oocyte retrieval, Follicular Oocyte Index (FOI), and Follicular Output Rate (FORT), suggesting an enhanced ovarian response. Conversely, the GG genotypes of both rs6165 and rs6166 were correlated with a shorter stimulation duration. Furthermore, the AG and GG genotypes of rs6166 demonstrated a lower FORT compared to the AA genotype, suggesting a potential impact on follicular output efficiency. Future studies involving larger, prospective cohorts are warranted to validate these findings and to determine their impact on clinically meaningful outcomes, including live birth rates.
Data Sharing Statement
All data supporting the findings of this study are available from the corresponding author on request.
Ethics Approval
The study was approved by the Ethics Committee of Hanoi Medical University and the hospital (under decision IRB-VN01.001/IRB00003121/FWA00004148, issued on March 23, 2023) and complied with the Declaration of Helsinki.
Acknowledgment
This work was supported by the National Hospital of Obstetrics and Gynecology, Hanoi, Vietnam.
Author Contributions
The corresponding author provided the conceptual framework for this study. Besides, all authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
All authors have no conflicts of interest in this study and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35(1):17–23. doi:10.1007/s10815-017-1058-4
2. Hu S, Xu B, Jin L. Perinatal outcome in young patients with diminished ovarian reserve undergoing assisted reproductive technology. Fertil Steril. 2020;114(1):118–124.e1. doi:10.1016/j.fertnstert.2020.02.112
3. Li N, Xu W, Liu H, et al. Whole exome sequencing reveals novel variants associated with diminished ovarian reserve in young women. Front Genet. 2023:14. doi:10.3389/fgene.2023.1154067
4. Penzias A, Azziz R, Bendikson K, et al. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114(6):1151–1157. doi:10.1016/j.fertnstert.2020.09.134
5. Rosevear SK, Ford WCL, Wardle PG, Hull MGR, Holt DW, Lee TD. Smoking and decreased fertilisation rates in vitro. Lancet. 1992;340(8829):1195–1196. doi:10.1016/0140-6736(92)92895-M
6. Sharara FI, Beatse SN, Leonardi MR, Navot D, Scott RT. Cigarette smoking accelerates the development of diminished ovarian reserve as evidenced by the clomiphene citrate challenge test. Fertil Steril. 1994;62(2):257–262. doi:10.1016/S0015-0282(16)56875-7
7. Zenzes MT, Reed TE, Casper RF. Effects of cigarette smoking and age on the maturation of human oocytes. Hum Reprod. 1997;12(8):1736–1741. doi:10.1093/humrep/12.8.1736
8. Conforti A, Carbone L, Girolamo RD, et al. Therapeutic management in women with a diminished ovarian reserve: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2025;123(3):457–476. doi:10.1016/j.fertnstert.2024.09.038
9. Kaur M, Singh S, Kaur A. Polymorphisms in FSHR modulating susceptibility to polycystic ovary syndrome: an updated meta-analysis. J Ovarian Res. 2023;16(1):183. doi:10.1186/s13048-023-01238-7
10. Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131–141. doi:10.1016/j.mce.2017.10.015
11. Kaur M, Singh S, Kaur R, Beri A, Kaur A. Analyzing the impact of FSHR variants on polycystic ovary syndrome-a case-control study in Punjab. Reprod Sci Thousand Oaks Calif. 2023;30(8):2563–2572. doi:10.1007/s43032-023-01194-z
12. Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology*. Endocr Rev. 1997;18(6):739–773. doi:10.1210/edrv.18.6.0320
13. Rousseau-Merck MF, Atger M, Loosfelt H, Milgrom E, Berger R. The chromosomal localization of the human follicle-stimulating hormone receptor gene (FSHR) on 2p21-p16 is similar to that of the luteinizing hormone receptor gene. Genomics. 1993;15(1):222–224. doi:10.1006/geno.1993.1041
14. Gromoll J, Ried T, Holtgreve-Grez H, Nieschlag E, Gudermann T. Localization of the human FSH receptor to chromosome 2 p21 using a genomic probe comprising exon 10. J Mol Endocrinol. 1994;12(3):265–271. doi:10.1677/jme.0.0120265
15. Dieamant F, Petersen CG, Vagnini LD, et al. The Ala307Thr polymorphism of the follicle-stimulating hormone receptor (FSHR) gene is associated with the dose of recombinant FSH received during IVF/ICSI treatment. JBRA Assist Reprod. 2023;27(1):78–84. doi:10.5935/1518-0557.20230009
16. Simoni M, Nieschlag E, Gromoll J. Isoforms and single nucleotide polymorphisms of the FSH receptor gene: implications for human reproduction. Hum Reprod Update. 2002;8(5):413–421. doi:10.1093/humupd/8.5.413
17. Loutradis D, Drakakis P, Vomvolaki E, Antsaklis A. Different ovarian stimulation protocols for women with diminished ovarian reserve. J Assist Reprod Genet. 2007;24(12):597–611. doi:10.1007/s10815-007-9181-2
18. Youssef MA, van Wely M, Al-Inany H, et al. A mild ovarian stimulation strategy in women with poor ovarian reserve undergoing IVF: a multicenter randomized non-inferiority trial. Hum Reprod Oxf Eng. 2017;32(1):112–118. doi:10.1093/humrep/dew282
19. van Tilborg TC, Torrance HL, Oudshoorn SC, et al. Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 1: the predicted poor responder. Hum Reprod Oxf Eng. 2017;32(12):2496–2505. doi:10.1093/humrep/dex318
20. Solernou R, Peralta S, Casals G, et al. The follicular output rate (FORT) as a method to evaluate transdermal testosterone efficacy in poor responders. JBRA Assist Reprod. 2021;25(2):229–234. doi:10.5935/1518-0557.20200086
21. Alviggi C, Conforti A, Esteves SC, et al. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker—the follicle-to-oocyte (FOI) Index. Front Endocrinol. 2018;9:589. doi:10.3389/fendo.2018.00589
22. Alviggi C, Andersen CY; Poseidon Group (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number), et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105(6):1452–1453. doi:10.1016/j.fertnstert.2016.02.005
23. Esteves SC, Alviggi C, Humaidan P, et al. The POSEIDON criteria and its measure of success through the eyes of clinicians and embryologists. Front Endocrinol. 2019:10. doi:10.3389/fendo.2019.00814
24. Polyzos NP, Neves AR, Drakopoulos P, et al. The effect of polymorphisms in FSHR and FSHB genes on ovarian response: a prospective multicenter multinational study in Europe and Asia. Hum Reprod Oxf Eng. 2021;36(6):1711–1721. doi:10.1093/humrep/deab068
25. Neves AR, Garcia S, Vuong LN, Blockeel C, Spits C, Polyzos NP. The additive effect of combinations of FSH receptor gene variants in ovarian response to stimulation. Reprod Sci Thousand Oaks Calif. 2024;31(11):3560–3568. doi:10.1007/s43032-024-01700-x
26. Hussein A, Abdulwahab HH, Oda MH. Role of FSHR gene (rs6165) polymorphism in ovarian stimulation by exogenous FSH of Iraqi infertile women. Int J Health Sci. 2022;6(S6):10597–10605. doi:10.53730/ijhs.v6nS6.12833
27. Alviggi C, Longobardi S, Papaleo E, et al. Genetic variants of gonadotropins and their receptors could influence controlled ovarian stimulation: IVF data from a prospective multicenter study. Genes. 2023;14(6):1269. doi:10.3390/genes14061269
28. Simoni M, Casarini L. MECHANISMS IN ENDOCRINOLOGY: genetics of FSH action: a 2014-and-beyond view. Eur J Endocrinol. 2014;170(3):R91–R107. doi:10.1530/EJE-13-0624
29. Aittomäki K, Lucena JL, Pakarinen P, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82(6):959–968. doi:10.1016/0092-8674(95)90275-9
30. Alviggi C, Conforti A, Santi D, et al. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(5):599–614. doi:10.1093/humupd/dmy019
31. Conforti A, Tüttelmann F, Alviggi C, et al. Effect of genetic variants of gonadotropins and their receptors on ovarian stimulation outcomes: a Delphi consensus. Front Endocrinol. 2022;12:797365. doi:10.3389/fendo.2021.797365
32. Trevisan CM, Peluso C, Cordts EB, et al. Ala307Thr and Asn680Ser polymorphisms of FSHR gene in human reproduction outcomes. Cell Physiol Biochem. 2014;34(5):1527–1535. doi:10.1159/000366356
33. Mayorga MP, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype*. J Clin Endocrinol Metab. 2000;85(9):3365–3369. doi:10.1210/jcem.85.9.6789
34. Alviggi C, Conforti A, Caprio F, et al. In estimated good prognosis patients could unexpected “Hyporesponse” to controlled ovarian stimulation be related to genetic polymorphisms of FSH receptor? Reprod Sci. 2016;23(8):1103–1108. doi:10.1177/1933719116630419
35. Sudo S, Kudo M, Wada SI, Sato O, Hsueh AJW, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8(10):893–899. doi:10.1093/molehr/8.10.893
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

