Back to Journals » Journal of Hepatocellular Carcinoma » Volume 12
The Impact of Microwave Ablation on Recurrence and Metastasis of Hepatocellular Carcinoma: Insights From Animal Studies and Cytokine Profiling
Authors Wang Y, Tan H, Wen C, Chen S, Zheng G, Qi H , Xie L, Shen L, Cao F, Fan W
Received 5 January 2025
Accepted for publication 15 April 2025
Published 26 April 2025 Volume 2025:12 Pages 825—835
DOI https://doi.org/10.2147/JHC.S515779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Yujia Wang,1,2,* Hongtong Tan,1,2,* Chunyong Wen,1,2 Shuanggang Chen,1,2 Guanglei Zheng,1,2 Han Qi,1,2 Lin Xie,1,2 Lujun Shen,1,2 Fei Cao,1,2 Weijun Fan1,2
1Department of Minimally Invasive & Interventional Therapy, Sun Yat-Sen University Cancer Center, Guangzhou, 510060, People’s Republic of China; 2State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-Sen University Cancer Center, Guangzhou, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weijun Fan, Department of Minimally Invasive & Interventional Therapy, Sun Yat-Sen University Cancer Center, 651 Dongfeng East Road, Yuexiu District, Guangzhou, Guangdong, 510060, People’s Republic of China, Tel +86 138 2615 4530 ; +86-20-87343272, Fax +86-20-87343272, Email [email protected] Fei Cao, Department of Minimally Invasive & Interventional Therapy, Sun Yat-Sen University Cancer Center, 651 Dongfeng East Road, Yuexiu District, Guangzhou, Guangdong, 510060, People’s Republic of China, +86-13480211104, Email [email protected]
Background: Microwave ablation (MWA) is commonly used to treat hepatocellular carcinoma (HCC), but its effects on normal liver tissue and tumors remain unclear. While MWA causes direct tumor destruction, it also induces inflammatory responses in the surrounding liver tissue, which may influence tumor progression, metastasis, and recurrence. The role of cytokine alterations in this post-ablation inflammatory microenvironment is crucial for understanding how MWA impacts tumor behavior.
Purpose: This study aims to investigate the impact of post-ablation inflammatory responses on HCC recurrence and metastasis through animal experiments and cytokine profiling, with the goal of identifying potential biomarkers or therapeutic targets.
Materials and Methods: This study involved 35 male C57BL/6 mice (6– 8 weeks old) to establish metastatic and orthotopic cancer models. The effects of normal liver tissue ablation and HCC ablation on tumor metastasis and recurrence were investigated. Cytokine expression changes were assessed using the Proteome Profiler Mouse XL Cytokine Array, and prognostic implications were analyzed using the TCGA database. Multiple group comparisons assessed using the Mann–Whitney U-test. Statistical significance was defined as a two-tailed p-value less than 0.05.
Results: Microwave ablation of normal liver tissue promotes intrahepatic metastasis of HCC. Incomplete ablation of liver tumors accelerates intrahepatic or pulmonary metastasis. Post-ablation, increased expression of MMP-9, OPN, VEGF, CHI3L1, AREG, CXCL2, and IL-1α in the peritumoral region suggests a shift toward a pro-inflammatory and pro-metastatic microenvironment, potentially facilitating tumor cell invasion, angiogenesis, and immune evasion.
Conclusion: HCC recurrence and metastasis following ablation may be driven by cytokine-mediated changes in the tumor microenvironment. Targeting key cytokines such as MMP-9, OPN, and CHI3L1 could provide new strategies for improving post-ablation outcomes and reducing recurrence rates in clinical settings.
Summary Statement: Microwave ablation of normal liver tissue significantly promotes metastasis of hepatocellular carcinoma in mice, accompanied by increased cytokine expression, including MMP-9 and VEGF, within the periablation microenvironment.
Keywords: hepatocellular carcinoma, microwave ablation, matrix metallopeptidase 9, tumor recurrence, inflammatory response
Key Results
Ablation significantly increased the number of metastatic tumor cells in the liver and lung in the experimental group compared to the control group.
Cytokines associated with metastasis, including MMP-9, OPN, and VEGF, were significantly upregulated in periablation zones.
MMP-9 enzymatic activity was confirmed through fluorescence imaging, indicating its role in promoting tumor metastasis post-ablation.
Introduction
Some patients with hepatocellular carcinoma who undergo ablation therapy experience local recurrence and distant metastasis postoperatively. Ablation therapy is a widely used treatment for hepatocellular carcinoma (HCC), but some patients experience postoperative local recurrence and distant metastasis, posing a significant clinical challenge.1 Recent studies have revealed that the inflammatory response following ablation alters the tumor microenvironment, leading to the accelerated growth of residual tumors and increased risk of recurrence and metastasis. Inflammatory cytokines released post-ablation can shape an immunosuppressive and tumor-promoting environment, yet the precise mechanisms remain insufficiently understood.2,3
Previous studies have focused on how the inflammatory response post-ablation promotes the growth of residual or occult tumors, leading to local recurrence at the ablation site or distant recurrence both within and outside the liver. Notably, emerging evidence suggests that ablation may facilitate the seeding and colonization of circulating tumor cells (CTCs) in the peritumoral region, thereby accelerating intrahepatic metastasis.4 Researchers found that when 5×10^4 units of MC38 murine colorectal cancer cells were injected via the spleen, immediate thermal ablation created a superficial lesion approximately 3 mm in diameter in the mouse liver. Upon euthanizing the mice on the seventh day post-ablation, significant tumor metastases were observed in the surrounding areas of all ablation sites. These findings indicate that the ablation of normal liver tissue can enhance the invasive and metastatic capacity of colorectal cancer cells, particularly promoting their growth in the regions adjacent to the ablation site.5
Although studies have found that the number of CTCs increases in HCC patients after ablation therapy, there is currently no evidence to suggest that ablation treatment promotes the local implantation and intrahepatic metastasis of CTCs at the ablation site.6 Notably, some studies suggest that the inflammatory response induced by ablation can trigger recurrence and metastasis of HCC, although the specific mechanisms remain contentious. This process may be mediated by the accumulation of neutrophils, fibroblasts, and macrophages around the ablation site, which secrete cytokines such as IL-6, HGF, VEGF, and HSP70. These cytokines collectively promote the growth of residual or distant tumors, leading to recurrence. However, it remains unclear which specific cytokines are involved in tumor metastasis post-ablation.2,4,7,8
Therefore, this study aims to investigate the impact of normal liver tissue ablation on the surrounding area of the ablation site and intrahepatic metastasis of hepatocellular carcinoma cells through a designed animal experiment. By employing a cytokine antibody array to profile changes in inflammatory mediators surrounding the ablation site, this research seeks to provide mechanistic insights into the role of inflammation in post-ablation metastasis. A deeper understanding of the cytokine-driven mechanisms underlying tumor progression could facilitate the development of targeted interventions to mitigate recurrence and improve patient outcomes.
Materials and Methods
Animal Models
All relevant protocols were approved by the Experimental Animal Ethics Committee of Sun Yat-sen University Cancer Center (L102032024070I) and followed the Laboratory Animal - Guideline for Ethical Review of Animal Welfare (GB/T 35892–2018). Male C57BL/6 mice (6–8 weeks old, weighing 18–22 g) were used for all experiments. For the murine model of hepatocellular carcinoma metastasis, a total of 1×10^6 hepa1-6 cells were injected into the spleen along its longest axis. The injection was performed horizontally to a depth of approximately 5 mm using a 29G insulin syringe, with a total injection volume of 100μL. For the murine orthotopic liver tumor model, a suspension containing 1×10^6 hepa1-6 cells mixed with matrix gel at a 1:1 volume ratio was prepared and inoculated beneath the liver capsule of the left liver lobe. The specific establishment of animal models and experimental groups are shown inFigures S1-8.
Assay of Cytokines
Tumor tissue protein was extracted and quantified using the Pierce™ BCA Protein Assay Kit (Cat: 23227, Thermo Scientific™, USA). The cytokine profile was analyzed using the Proteome Profiler Mouse XL Cytokine Array (ARY028, R&D Systems, USA), which enables the simultaneous detection of 111 cytokines and chemokines in an unbiased manner.
The array membrane was blocked for 1 hour at room temperature using Array Buffer 6 (R&D Systems). After blocking, 200 μg of total protein was incubated overnight at 2–8°C with the membrane. After three washes with 1× Wash Buffer, the membrane was incubated with the Detection Antibody Cocktail for 1 hour at room temperature, followed by Streptavidin-HRP incubation for 30 minutes. Chemiluminescence signals were developed using the Chemi Reagents 1 and 2 (R&D Systems) and detected using a Bio-Rad ChemiDoc imaging system. Cytokine spot intensities were quantified using Fiji/ImageJ 2.9.0 (NIH, USA).
Hematoxylin and eosin Staining
First, tissue sections embedded in paraffin were respectively deparaffinized in xylene and ethanol. After processing the samples, they were stained with hematoxylin staining solution for 10 minutes, followed by rinsing off the excess staining solution, and then stained with eosin staining solution for 2 minutes. Finally, tissue sections were dehydrated and cleared in ethanol and xylene, mounted with neutral resin, and observed under a microscope.
Immunohistochemical Staining
Paraffin-embedded tissue sections were heated at 60°C for 2 hours, then deparaffinized with xylene and alcohol. The sections were immersed in sodium citrate buffer for antigen retrieval. Use an immunohistochemical pen to delineate the area on the slide, apply hydrogen peroxide enzyme enhancer, and incubate in darkness for 10 minutes at room temperature. Apply serum blocking solution and incubate at room temperature for 1 hour. Incubate the tissue with mouse MMP-9 enzyme antibody for 1 hour, then with HRP-conjugated polymer for 30 minutes at room temperature. Incubate the primary antibody overnight at 4°C. Finally, apply the DAB chromogen working solution and incubate at room temperature. We performed semi-quantitative analysis of the immunohistochemical results by randomly selecting three equal-sized regions in both the tissue ablation and non-ablation areas. The optical density (OD) values of MMP-9 were measured using Fiji/ImageJ 2.9.0 (NIH, USA). The results were analyzed and the average OD values of each region were calculated to compare the differences between the areas.
Validation of MMP-9 Enzymatic Activity in Liver Cancer Ablation
To validate MMP-9 activity post-liver cancer ablation, a fluorescent probe with an MMP-9 cleavage site (PerkinElmer) was used. The probe’s fluorescence is quenched when uncleaved, but in vivo MMP-9 cleaves the substrate, allowing fluorescence upon laser excitation. Microwave ablation was performed on the normal liver of mice in the experimental group, while the control group had only the antenna insertion without ablation. One hour later, both groups were injected with the fluorescent probe. Six hours post-injection, fluorescence was monitored using an in vivo small animal fluorescence imaging system.
Statistical Analysis
The results of the cytokine antibody microarray were analyzed using Image J software (NIH, USA), while data processing and plotting were performed using GraphPad Prism 7.0 software (GraphPad Software, USA). Statistical analysis of the experimental data was conducted using SPSS 24.0 software (IBM, USA), with multiple group comparisons assessed using the Mann–Whitney U-test. Statistical significance was defined as a two-tailed p-value less than 0.05.
Results
The Effect of Ablation on Metastasis of Hepa1-6 Cells
First, we investigated the impact of normal liver tissue ablation on the intrahepatic metastasis of hepatocellular carcinoma cells. H&E staining examination revealed that on the first day postoperatively, there was a significant aggregation of inflammatory cells surrounding the ablation zones in the ablation group, with no definite tumor cells or tissues observed. By the third day, besides abundant infiltration of inflammatory cells around the ablation zones, a few tumor cells were observed at the periphery. On the sixth day, a substantial aggregation of inflammatory cells persisted around the ablation zones in the ablation group, accompanied by the formation of clustered tumor metastases. In contrast, no definite tumor cells or tissues were observed in the control group, which underwent only needle puncture without ablation, throughout the experiment (Figure 1a and b).9 On the ninth day postoperatively, visible tumor tissues were observed surrounding the ablation zones in all six mice of the ablation group. The tumor tissues appeared pinkish, with most of them growing around the ablation zones. One mouse exhibited distant metastasis to the liver lobes. In contrast, no tumor tissues were found in the livers of mice in the control group (Figure 1c). Subsequent HE staining of the tissues revealed circumferential tumor tissues around the ablation zones in mice of the ablation group, whereas only a small amount of clustered tumor cells were observed beneath the liver capsule in mice of the control group (Figure 1d). We then investigated the impact of incomplete ablation on the distant metastasis of hepatocellular carcinoma. It was found that ablation significantly stimulated the spread of Hepa1-6 cells between different liver lobes and lung metastasis, which was not observed in the control group (Figure 1e and f).
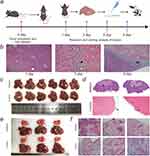 |
Figure 1 The Effect of Ablation of Normal Liver Tissue on Intrahepatic Metastasis of HCC. (a) Scheme of the experimental procedures (Created with BioGDP.com9). (b) For the ablation group, H&E staining was performed on the surrounding tissues of mouse liver ablation zones on days 1, 3, and 6. The white pentagonal star area represents the ablation zone, the white arrow indicates the hyperemic band, the black pentagonal star represents inflammatory cell infiltration, and the yellow and black arrows respectively indicate scattered tumor cells and clustered tumor cell infiltration.(c and d) On the ninth day, anatomical images and H&E staining of both the ablation group and the control group. (d) In the ablation group, black arrows indicate ablation zones, while white arrows indicate tumor growth surrounding the ablation margin. In the control group, blue arrows indicate small tumor tissues. (e and f) Impact of Incomplete Ablation on Distant Metastasis of Hepatocellular Carcinoma. (e) Incomplete ablation stimulates intrahepatic (black arrows) and pulmonary metastasis (red arrows) of hepatocellular carcinoma. (f) H&E staining demonstrated the presence of metastatic tumor foci in the lungs of mice from the ablation group (black arrows). |
Differential Expression of Cytokines
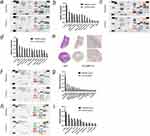
We initially investigated the differential expression of cytokines in the surrounding tissues following the ablation of normal liver tissue. On the first day post-ablation, the surrounding tissues exhibited significantly higher expression of cytokines closely associated with liver cancer metastasis, including MMP-9, OPN, and CHI3L1. Additionally, cytokines related to tumor angiogenesis and progression, such as VEGF and HGF, were also relatively elevated (Figure 2a and b). By the third day post-ablation, MMP-9, OPN, and CHI3L1 remained significantly overexpressed, with a gradual increase in the expression of cytokines such as CXCL2 and IL-1α. However, VEGF and HGF did not show significant differences at this time point (Figure 2c and d). MMP-9, in particular, is closely linked to the invasion and metastasis of liver cancer. Immunohistochemistry was used to further validate the antibody array results, showing substantial MMP-9 expression in the surrounding tissues on the first day post-ablation (Figures 2e and S9).
Next, we examined the impact of complete ablation of hepatocellular carcinoma on cytokine expression. At 24 hours post-ablation, cytokines closely associated with liver cancer metastasis, such as MMP-9, OPN, and CHI3L1, were significantly overexpressed in the ablation group compared to the control group. Additionally, cytokines related to tumor angiogenesis, including VEGF and AREG, were also relatively elevated (Figure 2f and g).
Finally, we explored the effect of complete ablation of hepatocellular carcinoma on cytokine expression in adjacent tumor tissues. The mice were sacrificed one day post-ablation, and the differences in cytokine expression between the ablated and non-ablated tumor tissues were analyzed. Cytokines closely associated with liver cancer metastasis, such as MMP-9 and VEGF, were significantly overexpressed, while OPN, HGF, and AREG also showed slightly elevated expression levels (Figure 2h and i).
Validation of MMP-9 Enzymatic Activity in Liver Cancer Ablation
The activity of MMP-9 enzyme in the ablation zone microenvironment was detected using a fluorescent probe (PerkinElmer) with an MMP-9 cleavage site.(Figure 3a). Initially, microwave ablation was performed on the normal liver of mice in the experimental group, while only the insertion of the microwave ablation antenna without ablation was conducted on the control group (Figure 3b and c). One hour post-procedure, both groups of mice were injected with the aforementioned fluorescent probe. Six hours later, the fluorescence in the mice was monitored using an in vivo small animal fluorescence imaging system. The results showed a peripheral fluorescent signal in the ablation zone of the ablation group (Figure 3d and e), confirming the activation of MMP-9 enzyme around the ablation zone following normal liver tissue ablation, which suggests its potential role in promoting tumor metastasis.
 |
Figure 3 Validation of MMP-9 Enzymatic Activity in Liver Cancer Ablation.(a) Schematic diagram of MMP-9 visualization imaging process (Created in BioRender. Yang, J (2025) https://BioRender.com/k92d109). (b and c) Schematic diagram of mouse liver microwave ablation. (d and e) Monitoring fluorescence in mice using an in vivo small animal fluorescence imaging system. The correlation between various cytokines and patient prognosis in the TCGA liver cancer database. Specifically, patients with low expression of MMP-9 (f), IL-1α (g), AREG (h), CXCL2 (i), VEGFA (j), and OPN (SPP1) (k) have a more favorable prognosis than those with high expression of these cytokines. |
The Relationship Between the Expression of Various Factors and Patient Prognosis in the TCGA Liver Cancer Database
In the analysis of the TCGA liver cancer database, the expression levels of various cytokines were found to correlate with patient prognosis. Kaplan-Meier survival curves indicated that patients with low expression of MMP-9 (HR = 1.63, 95% CI: 1.15–2.31, P = 0.006), IL-1α (HR = 1.49, 95% CI: 1.05–2.11, P = 0.026), AREG (HR = 1.44, 95% CI: 1.02–2.04, P = 0.038), VEGFA (HR = 1.52, 95% CI: 1.07–2.16, P = 0.019), and OPN (SPP1) (HR = 2.15, 95% CI: 1.50–3.08, P < 0.001) had significantly better overall survival compared to those with high expression. Although the expression of CXCL2 did not reach statistical significance (HR = 0.72, 95% CI: 0.51–1.02, P = 0.065), a trend towards better survival with low expression was observed (Figure 3f–k). These findings suggest that the expression levels of these cytokines could serve as potential prognostic biomarkers in liver cancer (Figure 3f–k).
Discussion
Studies have found that the inflammatory response following ablation can induce tumor implantation and metastasis, leading to intrahepatic recurrence of tumors. Jone et al5 discovered that ablation of normal liver tissue significantly promotes intrahepatic metastasis of colorectal cancer cells, particularly around the ablation zone. In this study, hepatocellular carcinoma cells were injected into the spleens of mice, followed by immediate ablation of the superficial liver region. Compared to the control group, the ablation group showed significant tumor implantation and metastasis around the ablation site, thus validating the hypothesis.
Since the aforementioned studies were conducted by injecting tumor cells into the spleen and ablating normal liver tissue, whereas in clinical practice, ablation is typically performed on primary liver tumors, affecting only a small amount of adjacent normal tissue, we further developed a mouse model of incomplete ablation of orthotopic liver cancer. In this model, we performed ablation and sham ablation treatments on intrahepatic tumors in two groups of mice. On the 7th day post-treatment, all mice in the ablation group exhibited metastases to other liver lobes and the lungs, while the control group showed no metastases to distant liver lobes or the lungs. These findings suggest that incomplete ablation of liver cancer can enhance the invasive and metastatic potential of residual tumor tissue, thereby promoting metastasis.
However, the mechanisms by which ablation induces liver cancer metastasis remain unclear. The IL-6/STAT3-induced inflammatory response may play a crucial role in the development and progression of liver cancer. Rozenblum et al4 demonstrated the critical role of IL-6 in promoting intrahepatic tumor recurrence and metastasis after ablation by using IL-6 knockout mice. Additionally, studies have shown that the PI3K/mTOR/AKT pathway, COX-2, and HSP70 play significant roles in the recurrence and metastasis of hepatocellular carcinoma following ablation.8,10,11
To further investigate the impact of post-ablation inflammatory responses on tumor progression, this study compared the expression differences of 111 cytokines. Significant differences in cytokine expression were observed between the tissue surrounding the ablation zone and the distant untreated liver tissue one day and three days after ablating normal liver tissue in mice. Cytokines closely related to liver cancer metastasis, such as MMP-9, OPN, and CHI3L1, were significantly upregulated in the tissue near the ablation zone. Additionally, cytokines associated with tumor angiogenesis and growth, including VEGF and HGF, were also relatively upregulated, while the expression of CXCL2, IL-1α, and other factors closely related to tumor metastasis gradually increased. To further simulate clinical scenarios, we established orthotopic tumors in the livers of mice and performed complete ablation. Twenty-four hours post-ablation, we compared cytokine expression between the tissue surrounding the ablation zone and the distant untreated liver tissue. The results revealed significant upregulation of MMP-9, OPN, and CHI3L1, along with a relative increase in cytokines associated with tumor angiogenesis, such as VEGF and AREG.
MMP-9, a member of the matrix metalloproteinase family, plays a critical role in tumor invasion and metastasis. The injury caused by liver ablation induces the accumulation of inflammatory cells, which express MMP-9, allowing them to traverse blood vessels and reach the peri-ablation area.12,13 Frich et al14 found that the concentrations of activated MMP-2 and MMP-9 enzymes in the peri-ablation area of normal liver tissue in mice were three times higher than in distant non-ablated normal liver tissue after RFA. They inferred that MMP-2 and MMP-9 were primarily secreted by macrophages surrounding the ablation site. Osteopontin (OPN) is considered one of the most crucial molecules in the process of hepatocellular carcinoma (HCC) metastasis.15 Zhao et al16 found that downregulation of osteopontin both in vivo and in vitro can inhibit tumor growth and metastasis, potentially by preventing tumor cell apoptosis, thereby promoting tumorigenesis and metastasis. Chitinase 3-like 1 (CHI3L1) has been shown to be significantly overexpressed in various solid tumors and is closely associated with metastasis and poor prognosis in patients.17 Studies have indicated that CHI3L1 can inhibit the expression of E-cadherin and increase the expression of MMP-9, thereby enhancing cell motility.18 Seventy-two hours after ablation of normal liver tissue, IL-1α and CXCL2 were significantly upregulated in the tissue surrounding the ablation zone. IL-1α is released from hepatocytes in a perinecrotic state at the margins of the ablation site. On one hand, IL-1α stimulates a local inflammatory response and induces compensatory proliferation of liver cells. Concurrently, it also induces IL-6 activation of STAT3, promoting liver regeneration and tumor growth.19,20 Song et al21 found that CXCL2 levels were significantly elevated in the peripheral blood of hepatocellular carcinoma (HCC) patients compared to healthy individuals, and its expression was markedly upregulated in HCC cells compared to normal hepatocytes. Additionally, miR-532-5P can inhibit HCC growth and metastasis by reducing CXCL2 expression. In our intrahepatic dual-tumor model, in addition to MMP-9 and OPN, VEGF and AREG (Amphiregulin) were also significantly upregulated. VEGF is an inducer of angiogenesis, promoting the formation of tumor vasculature and increasing vascular permeability, thus playing a critical role in tumor growth, invasion, and metastasis. Ahmed et al2 found through animal experiments that the ablation of normal liver tissue accelerated the metastasis of distant tumors. The researchers used c-MET and VEGF receptor inhibitors, which significantly reduced the ablation-induced growth of distant tumors. This confirmed that the growth of distant tumors induced by ablation is mediated through the activation of the HGF/c-Met pathway and VEGF. AREG plays a significant role in tumor metastasis. It has been found in various types of cancers that AREG can upregulate the expression of multiple genes related to tumor metastasis, such as matrix metalloproteinases, thereby enhancing the invasive capability of the tumor.22 In addition, this study successfully validated the activity of MMP-9 enzyme in the microenvironment of ablation foci following liver cancer ablation using a fluorescent probe containing MMP-9 cleavage sites. The experimental results demonstrated significant peripheral fluorescent signals around the ablation foci in normal liver tissue subjected to microwave ablation, indicating that MMP-9 enzyme was activated post-ablation. This finding holds substantial clinical significance, suggesting that the activation of MMP-9 enzyme post-ablation may play a crucial role in tumor metastasis and recurrence.
Currently, numerous studies suggest that IL-6 is a major factor contributing to tumor growth or metastasis following ablation. Research indicates that IL-6 is an early inflammatory cytokine response post-ablation. The elevation of IL-6 and other cytokines induces the aggregation and migration of hepatic stellate cells to the ablation zone or the residual tumor tissue through the NF-κB or JAK-STAT3 pathways. Once activated, these stellate cells promote tumor cell growth by secreting IL-6, HGF, TGF-β1, and VEGF. Additionally, they secrete matrix metalloproteinases, which reconstruct the extracellular matrix surrounding the tumor, thereby accelerating tumor metastasis.23,24 In our study, although IL-6, HGF, and VEGF did not show significant differences in expression post-ablation, this does not exclude their involvement in long-term tumor progression. Further studies are needed to determine their dynamic changes over time. This could be attributed to the timing of our assessments, which were conducted at 24 and 72 hours post-ablation, potentially missing the optimal detection window. Moreover, the concentration of IL-6 in the tissue surrounding the ablation zone was found to be low, suggesting that the Cytokine Array may not accurately measure IL-6 levels under these conditions. Further analysis of TCGA database has also confirmed that elevated expression of all factors except CXCL2 is associated with poor prognosis in patients. These findings highlight the potential risks of incomplete ablation in hepatic cancer therapy. Residual tumors and peri-ablation tissues may facilitate tumor dissemination by increasing MMP-9 and OPN expression, leading to enhanced tumor cell motility and vascular permeability. Ultimately, this process may lead to intrahepatic spread or distant metastasis of the tumor. Therefore, targeting these pathways post-ablation could be a promising strategy to reduce recurrence and metastasis. Furthermore, local delivery of MMP-9 inhibitors or nanotechnology-based drug delivery systems may provide a more precise and effective intervention. Future research should integrate clinical data with experimental models to evaluate the efficacy of these interventions and explore personalized therapeutic approaches to optimize liver cancer ablation outcomes.
In conclusion, this study found that ablation of normal liver tissue promotes intrahepatic metastasis of liver cancer cells, potentially through inflammation and immune responses triggered by the ablation process. Incomplete ablation of liver cancer significantly accelerates intrahepatic or pulmonary metastasis. Comprehensive antibody array analysis identified key cytokine alterations in the ablation microenvironment, shedding light on potential therapeutic targets that could mitigate ablation-induced tumor progression. These factors may be closely related to the recurrence and metastasis of liver cancer induced by ablation.
Abbreviations
HCC, Hepatocellular Carcinoma; MMP-9, Matrix Metallopeptidase 9; VEGF, Vascular Endothelial Growth Factor; OPN, Osteopontin; CHI3L1, Chitinase 3-like 1; HGF, Hepatocyte Growth Factor; CXCL2, Chemokine (C-X-C motif) ligand 2; IL-1α, Interleukin 1 alpha; AREG, Amphiregulin; TCGA, The Cancer Genome Atlas.
Data Sharing Statement
All data generated or analyzed during this animal study are available from the corresponding author, Weijun Fan, upon reasonable request. The data include all experimental results related to the microwave ablation and its effects on hepatic cancer recurrence and metastasis, as well as cytokine profiling from the animal models. Additional, related documents, such as the detailed experimental protocols and statistical analyses, will also be available. Data will become accessible immediately upon publication and will remain available for five years. Access to the data will be granted upon request, subject to approval by the authors, and will follow standard academic sharing practices.
Acknowledgment
Yujia Wang and Hongtong Tan are joint first authors.
Fei Cao and Weijun Fan are listed as co-corresponding authors, with Fan serving as the senior corresponding author.
Funding
This study was supported by the Science and Technology Project of Guangzhou City (202201011375); Fostering Program for NSFC Young Applicants (Tulip Talent Training Program) of Sun Yat-sen University Cancer Center (TTP-SYSUCC-202314); Youth Teacher Cultivation Project for Basic Scientific Research Funding at Sun Yat-sen University (24qnpy295); Open Project of the Key Laboratory of Personalized Active Immunotherapy for Tumors in Fujian Province (FJZLJZ-202401).
Disclosure
The authors of this manuscript declare no relationships with any companies. The authors report no conflicts of interest in this work.
References
1. Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908–919. PubMed PMID: 25688888. doi:10.1148/radiol.15141483
2. Ahmed M, Kumar G, Moussa M, et al. Hepatic radiofrequency ablation-induced stimulation of distant tumor growth is suppressed by c-Met inhibition. Radiology. 2016;279(1):103–117. PubMed PMID: 26418615; PubMed Central PMCID: PMCPMC4819900. doi:10.1148/radiol.2015150080
3. Liao H, Ahmed M, Markezana A, et al. Thermal ablation induces transitory metastatic growth by means of the STAT3/c-met molecular pathway in an intrahepatic colorectal cancer mouse model. Radiology. 2020;294(2):464–472. PubMed PMID: 31845846; PubMed Central PMCID: PMCPMC6996862. doi:10.1148/radiol.2019191023
4. Rozenblum N, Zeira E, Bulvik B, et al. Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology. 2015;276(2):416–425. PubMed PMID: 25822472. doi:10.1148/radiol.15141918
5. Jones EL, Halpern AL, Carmichael H, et al. Hepatic ablation promotes colon cancer metastases in an immunocompetent murine model. Ann Surg. 2019;270(4):675–680. PubMed PMID: 31348044. doi:10.1097/sla.0000000000003474
6. Jiao LR, Apostolopoulos C, Jacob J, et al. Unique localization of circulating tumor cells in patients with hepatic metastases. J Clin Oncol. 2009;27(36):6160–6165. PubMed PMID: 19884529. doi:10.1200/jco.2009.24.5837
7. Kumar G, Goldberg SN, Gourevitch S, et al. Targeting STAT3 to suppress systemic pro-oncogenic effects from hepatic radiofrequency ablation. Radiology. 2018;286(2):524–536. PubMed PMID: 28880787; PubMed Central PMCID: PMCPMC5790305. doi:10.1148/radiol.2017162943
8. Kumar G, Goldberg SN, Wang Y, et al. Hepatic radiofrequency ablation: markedly reduced systemic effects by modulating periablational inflammation via cyclooxygenase-2 inhibition. Eur Radiol. 2017;27(3):1238–1247. PubMed PMID: 27287478. doi:10.1007/s00330-016-4405-4
9. Jiang S, Li H, Zhang L, et al. Generic Diagramming Platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025;53(D1):D1670–d6. PubMed PMID: 39470721; PubMed Central PMCID: PMCPMC11701665. doi:10.1093/nar/gkae973
10. Ahmed M, Kumar G, Gourevitch S, et al. Radiofrequency ablation (RFA)-induced systemic tumor growth can be reduced by suppression of resultant heat shock proteins. Int J Hyperthermia. 2018;34(7):934–942. PubMed PMID: 29631466; PubMed Central PMCID: PMCPMC6647038. doi:10.1080/02656736.2018.1462535
11. Jondal DE, Thompson SM, Butters KA, et al. Heat stress and hepatic laser thermal ablation induce hepatocellular carcinoma growth: role of PI3K/mTOR/AKT signaling. Radiology. 2018;288(3):730–738. PubMed PMID: 29737948; PubMed Central PMCID: PMCPMC6122226. doi:10.1148/radiol.2018172944
12. Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur J Med Chem. 2020;194:112260. PubMed PMID: 32224379. doi:10.1016/j.ejmech.2020.112260
13. Stetler-Stevenson M, Mansoor A, Lim M, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997;89(5):1708–1715. [PubMed PMID: 9057654]. doi:10.1182/blood.V89.5.1708
14. Frich L, Bjørnland K, Pettersen S, Clausen OP, Gladhaug IP. Increased activity of matrix metalloproteinase 2 and 9 after hepatic radiofrequency ablation. J Surg Res. 2006;135(2):297–304. PubMed PMID: 16934296. doi:10.1016/j.jss.2006.05.010
15. Qin L. Osteopontin is a promoter for hepatocellular carcinoma metastasis: a summary of 10 years of studies. Front Med. 2014;8(1):24–32. PubMed PMID: 24464486. doi:10.1007/s11684-014-0312-8
16. Zhao J, Dong L, Lu B, et al. Down-regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology. 2008;135(3):956–968. PubMed PMID: 18555021. doi:10.1053/j.gastro.2008.05.025
17. Libreros S, Iragavarapu-Charyulu V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J Leukoc Biol. 2015;98(6):931–936. PubMed PMID: 26310833; PubMed Central PMCID: PMCPMC6608021. doi:10.1189/jlb.3VMR0415-142R
18. Scully S, Yan W, Bentley B, Cao QJ, Shao R. Inhibitory activity of YKL-40 in mammary epithelial cell differentiation and polarization induced by lactogenic hormones: a role in mammary tissue involution. PLoS One. 2011;6(10):e25819. PubMed PMID: 21991364; PubMed Central PMCID: PMCPMC3185048. doi:10.1371/journal.pone.0025819
19. Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–8. PubMed PMID: 21339211. doi:10.1136/ard.2010.140145
20. He G, Yu GY, Temkin V, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17(3):286–297. PubMed PMID: 20227042; PubMed Central PMCID: PMCPMC2841312. doi:10.1016/j.ccr.2009.12.048
21. Song X, Wang Z, Jin Y, Wang Y, Duan W. Loss of miR-532-5p in vitro promotes cell proliferation and metastasis by influencing CXCL2 expression in HCC. Am J Transl Res. 2015;7(11):2254–2261. PubMed PMID: 26807173; PubMed Central PMCID: PMCPMC4697705.
22. Nagathihalli NS, Beesetty Y, Lee W, et al. Novel mechanistic insights into ectodomain shedding of EGFR Ligands Amphiregulin and TGF-α: impact on gastrointestinal cancers driven by secondary bile acids. Cancer Res. 2014;74(7):2062–2072. PubMed PMID: 24520077; PubMed Central PMCID: PMCPMC3975694. doi:10.1158/0008-5472.Can-13-2329
23. Ruan Q, Wang H, Burke LJ, et al. Therapeutic modulators of hepatic stellate cells for hepatocellular carcinoma. Int, J, Cancer. 2020;147(6):1519–1527. PubMed PMID: 32010970. doi:10.1002/ijc.32899
24. Zhang R, Lin XH, Liu HH, et al. Activated hepatic stellate cells promote progression of post-heat residual hepatocellular carcinoma from autophagic survival to proliferation. Int J Hyperthermia. 2019;36(1):253–263. PubMed PMID: 30701994. doi:10.1080/02656736.2018.1558459
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.